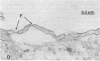
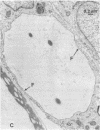
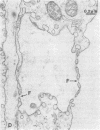
Abstract
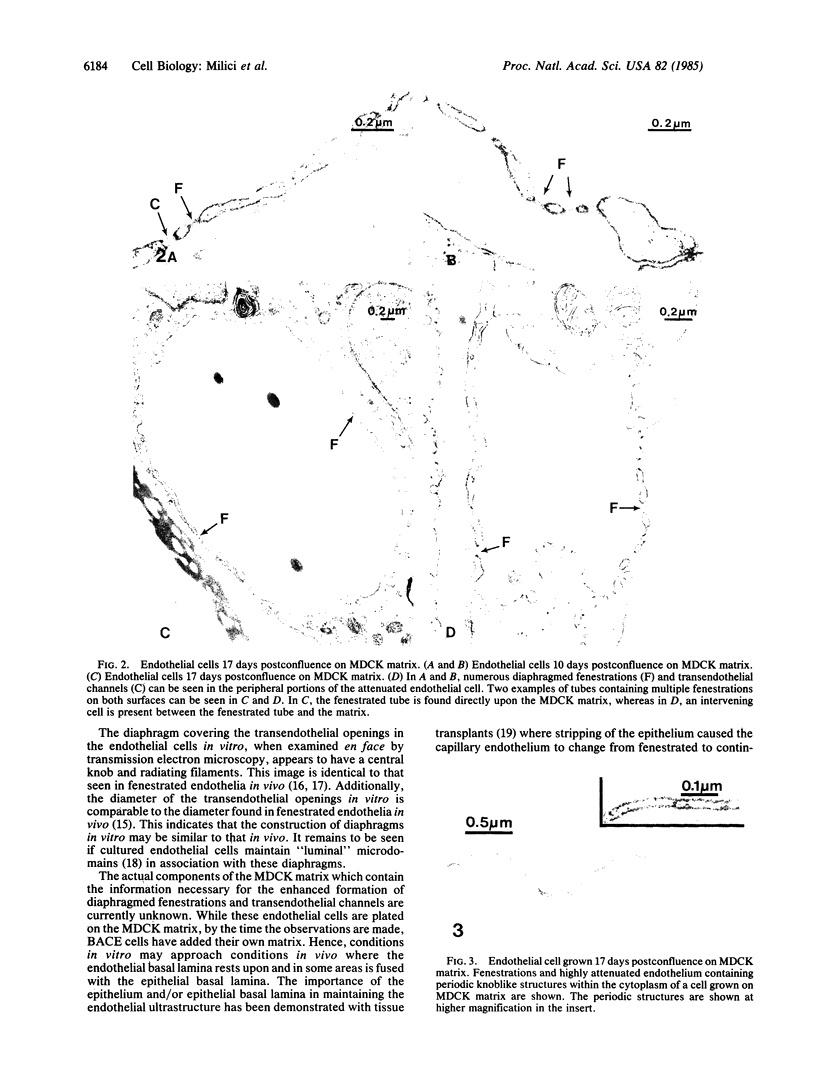
Microvascular endothelial cells isolated from fenestrated capillaries have been shown to form tubes in vitro, thereby demonstrating that they retain the ability to express some degree of their in vivo differentiated phenotype. However, some of their physiologically important structural features, such as transendothelial openings (i. e., diaphragmed fenestrations and transendothelial channels) are lost or are greatly reduced in number. In this study, cloned bovine adrenal cortex endothelial cells were cultured on plastic or on a basal lamina produced by Madin-Darby canine kidney (MDCK) cells for up to 17 days postconfluence. All cultures were then routinely fixed and processed for electron microscopic morphometry. For cells grown on plastic for 17 days postconfluence, the linear density of transendothelial openings in endothelial profiles less than 400 nm thick was found to be 0.007 openings per micron. On MDCK matrix, however, the linear density of transendothelial openings in endothelial profiles less than 400 nm thick was found to be 0.157 per micron. Occasionally some cells formed "tube-like" structures that also contained diaphragmed fenestrations and transendothelial channels on both sides of the tubes. These findings suggest that the substrate on which endothelial cells are grown can affect their differentiation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alby L., Auerbach R. Differential adhesion of tumor cells to capillary endothelial cells in vitro. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5739–5743. doi: 10.1073/pnas.81.18.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak L. S., Yocum R. R. 7-Nitrobenz-2-oxa-1,3-diazole (NBD)--phallacidin: synthesis of a fluorescent actin probe. Anal Biochem. 1981 Jan 1;110(1):31–38. doi: 10.1016/0003-2697(81)90107-x. [DOI] [PubMed] [Google Scholar]
- Braverman I. M., Yen A. Ultrastructure of the capillary loops in the dermal papillae of psoriasis. J Invest Dermatol. 1977 Jan;68(1):53–60. doi: 10.1111/1523-1747.ep12485169. [DOI] [PubMed] [Google Scholar]
- Campbell G. R., Uehara Y. Formation of fenestrated capillaries in mammalian vas deferens and ureter transplants. Z Zellforsch Mikrosk Anat. 1972;134(2):167–173. doi: 10.1007/BF00307150. [DOI] [PubMed] [Google Scholar]
- Elfvin L. G. The ultrastructure of the capillary fenestrae in the adrenal medulla of the rat. J Ultrastruct Res. 1965 Jun;12(5):687–704. doi: 10.1016/s0022-5320(65)80056-9. [DOI] [PubMed] [Google Scholar]
- Folkman J., Haudenschild C. C., Zetter B. R. Long-term culture of capillary endothelial cells. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5217–5221. doi: 10.1073/pnas.76.10.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J., Haudenschild C. Angiogenesis in vitro. Nature. 1980 Dec 11;288(5791):551–556. doi: 10.1038/288551a0. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Grund C., Schmid E., Mandelkow E. Paracrystalline arrays of membrane-to-membrane cross bridges associated with the inner surface of plasma membrane. J Cell Biol. 1978 May;77(2):323–328. doi: 10.1083/jcb.77.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Lüder M. R., Kartenbeck J., Zerban H., Keenan T. W. Involvement of vesicle coat material in casein secretion and surface regeneration. J Cell Biol. 1976 Apr;69(1):173–195. doi: 10.1083/jcb.69.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furie M. B., Cramer E. B., Naprstek B. L., Silverstein S. C. Cultured endothelial cell monolayers that restrict the transendothelial passage of macromolecules and electrical current. J Cell Biol. 1984 Mar;98(3):1033–1041. doi: 10.1083/jcb.98.3.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerritsen M. E., Cheli C. D. Arachidonic acid and prostaglandin endoperoxide metabolism in isolated rabbit and coronary microvessels and isolated and cultivated coronary microvessel endothelial cells. J Clin Invest. 1983 Nov;72(5):1658–1671. doi: 10.1172/JCI111125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern P. A., Knedler A., Eckel R. H. Isolation and culture of microvascular endothelium from human adipose tissue. J Clin Invest. 1983 Jun;71(6):1822–1829. doi: 10.1172/JCI110937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J., HECHTER O. An electron microscopic correlation of structure with function in the isolated perfused cow adrenal, preliminary observations. J Biophys Biochem Cytol. 1957 Jul 25;3(4):615–618. doi: 10.1083/jcb.3.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madri J. A., Williams S. K. Capillary endothelial cell cultures: phenotypic modulation by matrix components. J Cell Biol. 1983 Jul;97(1):153–165. doi: 10.1083/jcb.97.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul G. G. Structure and formation of pores in fenestrated capillaries. J Ultrastruct Res. 1971 Sep;36(5):768–782. doi: 10.1016/s0022-5320(71)90030-x. [DOI] [PubMed] [Google Scholar]
- Milici A. J., Bankston P. W. Fetal and neonatal rat intestinal capillaries: a TEM study of changes in the mural structure. Am J Anat. 1981 Apr;160(4):435–448. doi: 10.1002/aja.1001600407. [DOI] [PubMed] [Google Scholar]
- Milici A. J., L'Hernault N., Palade G. E. Surface densities of diaphragmed fenestrae and transendothelial channels in different murine capillary beds. Circ Res. 1985 May;56(5):709–717. doi: 10.1161/01.res.56.5.709. [DOI] [PubMed] [Google Scholar]
- Motta P., Muto M., Fujita T. Three dimensional organization of mammalian adrenal cortex. A scanning electron microscopic study. Cell Tissue Res. 1979 Jan 30;196(1):23–38. doi: 10.1007/BF00236346. [DOI] [PubMed] [Google Scholar]
- Simionescu N., Simionescu M., Palade G. E. Differentiated microdomains on the luminal surface of the capillary endothelium. I. Preferential distribution of anionic sites. J Cell Biol. 1981 Sep;90(3):605–613. doi: 10.1083/jcb.90.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. C., Matthews M. A. The isolation and culture of capillary endothelium from epididymal fat. Microvasc Res. 1975 Nov;10(3):286–297. doi: 10.1016/0026-2862(75)90033-3. [DOI] [PubMed] [Google Scholar]
- Wagner R. C., Williams S. K., Matthews M. A., Andrews S. B. Exclusion of albumin from vesicular ingestion by isolated microvessels. Microvasc Res. 1980 Jan;19(1):127–130. doi: 10.1016/0026-2862(80)90088-6. [DOI] [PubMed] [Google Scholar]
- Williams S. K., Gillis J. F., Matthews M. A., Wagner R. C., Bitensky M. W. Isolation and characterization of brain endothelial cells: morphology and enzyme activity. J Neurochem. 1980 Aug;35(2):374–381. doi: 10.1111/j.1471-4159.1980.tb06274.x. [DOI] [PubMed] [Google Scholar]