Abstract
External feedback of performance is an important component of therapy, especially for children with impairments due to cerebral palsy because they lack intrinsic experience of “good movements” to compare effort and determine performance outcomes. A robotic therapy system was developed to provide feedback for specific upper extremity movements (gestures) which are therapeutically desirable. The purpose of this study was to compare changes in forearm supination/pronation or wrist extension/flexion motion following conventional therapy and gestural robotic feedback therapy intervention. Six subjects with cerebral palsy (ages 5–18, GMFCS level IV—three subjects, level III—one subject, and level I—two subjects) participated in a blinded crossover design study of conventional and robotic feedback therapy targeting either forearm supination or wrist extension. Functional upper extremity motion at baseline and following conventional and robotic feedback therapy interventions were obtained using a motion capture system by personnel blinded to the intervention order. All activities were approved by IRB. Use of the robotic feedback system did result in slightly increased movement in the targeted gesture without change in un-targeted motions. Data also suggest a decrease in both agonist and antagonist motion following conventional therapy intervention. Results suggest improved motion when robotic feedback therapy intervention precedes conventional therapy intervention. Robotic feedback therapy is no different than conventional therapy to improve supination or wrist extension function in upper extremity impairments of children with cerebral palsy when changes were considered as aggregate data. In this very small group of diverse patients, individual subject results suggested that intervention order could be responsible for obscuring differences due to intervention type. Outcomes from several individual subjects suggest that results could be different given a more homogeneous group of subjects which future studies should be considered to ultimately determine efficacy of the robotic feedback therapy. Future studies should also address efficacy in other neuromuscular patient populations.
Keywords: Cerebral palsy (CP), movement feedback, robotic feedback, upper extremity
1. Introduction
Cerebral palsy (CP), brain injury, and stroke are neurological disorders which affect body movements and muscle coordination [1]–[3]. With a prevalence of 260 per 100 000 children, CP ranks as the top disorder of childhood seen in comprehensive rehabilitation settings followed by traumatic brain injury (210 per 100 000) [4]. Nearly 660 000 of the 70 million children ages 17 and under in the United States have impairments to their neuromuscular system that impact their ability to engage fully in school or play with nondisabled peers. The developmental delays and severity of impairments due to CP, stroke, or brain injury are not only related to the amount and location of brain damage but also to the level of intervention (physical/occupational therapy). The residual impairments as the child grows cannot be predicted, but physical/occupational therapy does have an important role in enhancing functional capacity. Emphasis on family- and child-centered care often incorporates play into physical and/or occupational therapy [5]–[7]. McArdle [8] has indicated that when children play they are relaxed in the present setting, intrinsically motivated, and actively engaged, which are all behaviors conducive to learning.
Feedback is information attained during or after performing a task concerning the quality of the task performed. Feedback can facilitate sustained or complex play [9]. Motor learning experts have categorized feedback along several dimensions, including: 1) intrinsic (the skill itself provides feedback) versus extrinsic (external verbal input from a therapist), and 2) concurrent (feedback during the performance) versus terminal (feedback following the performance). Feedback regarding the movement outcome (especially from visual, kinesthetic, or other sources providing knowledge of the performance or the results) is essential to motor learning [10]. Children with movement impairments from CP have little reference to judge “good” movements from “poor” movements, and thus have little intrinsic feedback and must rely on external feedback to improve their skill. Children tend to need concurrent feedback, particularly when initially learning a task. Automatic concurrent feedback is also desirable over feedback from a therapist because it is objective and is provided faster to the subject than from another person [11], [12].
A new robotic therapy system was developed to meet the goals of timely feedback in a play type setting for children with movement impairments. The purpose of this study was to compare functional outcomes from therapy using the upper extremity robotic feedback system with conventional upper extremity therapy. Our null hypothesis was that there was no difference in upper extremity function of children with CP following interventions of conventional therapy and therapy using the CosmoBot robotic feedback system.
II. Methods
A repeated measures crossover design was used. Each subject participated in both conventional and robotic feedback therapy intervention targeted to improve either forearm supination or wrist extension. Each intervention was provided for 20 min two times per week for five weeks by a clinical therapist in either the child's home, school, or at an outpatient clinic. Measurements of motion were performed at baseline and following both the conventional and robotic feedback interventions by a research therapist in a motion analysis laboratory. Intervention order was randomized and the research therapist and personnel measuring functional motion were blinded to the intervention order.
Subjects were eligible if they were between the ages of 3 and 21 and had a diagnosis of CP with upper extremity involvement. Six volunteer subjects were selected by convenience from the pool of patients being seen in an urban outpatient therapy facility. All subjects provided written assent and parents provided written consent for participation via an IRB approved protocol. Subjects enrolled were between the ages of 5 and 18 with different forms of CP and functioning between GMFCS levels I and IV (Table I).
TABLE I.
Subject Demographics With Indication of Target Gesture for Therapy Interventions and Intervention Order
| Age | Type of CP | GMFCS Level | Target of Therapy | Conventional or Robotic Intervention First |
|---|---|---|---|---|
| 5 | Spastic Quadriplegia | IV | Forearm Supination | C |
| 9 | Spastic L Hemiplegia | I | Forearm Supination | R |
| 11 | Athetoid Quadriplegia | IV | Wrist Extension | R |
| 12 | Spastic R Hemiplegia | I | Forearm Supination | C |
| 12 | Spastic Quadriplegia | IV | Forearm Supination | C |
| 18 | Spastic Quadriplegia | III | Wrist Extension | C |
Therapy, both conventional and robotic feedback for each subject, was provided by a clinical therapist and was targeted to meet a clinical need specific for upper extremity function for that subject. All subjects worked to increase either forearm supination (four subjects) or wrist extension (two subjects). The more involved upper extremity was targeted as the study side. Intervention order was determined randomly with gestural feedback therapy during the first five week intervention period for subjects 3 and 4. The remainder of subjects received conventional therapy during the first intervention period.
The CosmoBot system [Fig. 1(a)] was designed to provide instantaneous automatic feedback in both a visual and auditory manner (watching the robot move and hearing the robot motor engage for movement) to provide immediate feedback to the child about the attempted movement. A triaxial accelerometer [Fig. 1(b)] provided the interface between the subject and the robot. Therapists established parameters of the target movement (gesture) required from the subject via a graphical user interface (GUI) on a laptop computer [Fig. 1(c) and (d)]. Parameters under the control of a therapist included direction and threshold magnitude of the target gesture and the time that a gesture must be sustained before the robot responded. The default robot response to a target gesture of satisfactory magnitude and duration was forward movement one length of the base. Turning the robot to the left or right was achieved by the therapist depressing either the left or right mouse button after which the next successful gesture turned the robot. Therapists could adjust threshold parameters at any time during treatment sessions as the subject improved in their ability to achieve the target gesture.
Fig. 1.
CosmoBot gestural feedback system (A) with mobile base used in this study. The robot moves in response to change in triaxial accelerometer (B) orientation. In this case, a reference orientation captured with the accelerometer in neutral rotation and pronation and supination are defined with respect to the neutral position. A graphical user interface (C and D) allows therapists to set thresholds of movement required by patient for robot movement. Here, the subject is required to supinate greater than 45° to successfully get feedback from the robot. (Note: In this example, the pronation setting is not being used.).
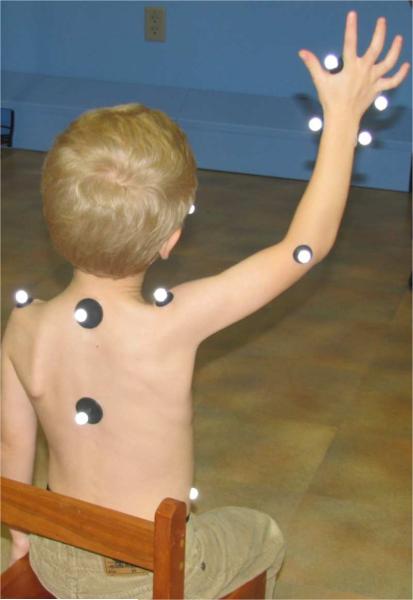
Subject motion of the study upper extremity was quantified at baseline and following each intervention period. Three-dimensional movement data was acquired at a frequency of 60 Hz using a 10 camera high-resolution optical motion capture system (Motion Analysis, Santa Rosa, CA, USA). Following the methods of Morrow et al. [13], 15 1.3-cm retro reflective markers were placed on subjects and used to construct a five segment upper extremity model (Fig. 2). Marker locations were identified and markers applied by the same research therapist. Kinematics were calculated in Visual3D (C-Motion, Germantown, MD, USA). At each measurement session, subjects performed the same six motion tasks (Table II), to the best of their ability, within the calibrated motion capture volume. Subjects were seated with both feet on the floor and the hips and knees flexed to 90° with lumbopelvic support if needed to maintain good sitting posture.
Fig. 2.
Subject with retro reflective markers used to simultaneously measure right shoulder, elbow, forearm, and wrist movements as the subject performs a functional reaching task.
TABLE II.
Motion Tasks Performed by Subjects During Each Measurement Session
| Task |
|---|
| Forearm pronation/supination with elbow flexed to 90° |
| Reaching to top of opposite shoulder |
| Reaching to nape of neck |
| Reaching for a book on a shelf on the same side of the body |
| Reaching for a book on a shelf on the opposite side |
| Elbow flexion and extension with forearm in self-selected position |
Three-dimensional kinematic data were calculated for each joint movement in each activity. Custom programming in MATLAB (The MathWorks, Inc., Natick, MA, USA) detected movement extrema for each of the target joint movements. The dependent variable was quantitative movement (either forearm supination/pronation or wrist extension/flexion) during motion tasks. The independent variable was the type of therapy intervention. Significance was tested using paired t-tests in JMP (SAS Institute, Inc. Cary, NC, USA) with statistical significance set at α = 0.05. Data were graphed using Excel (Microsoft Corporation, Redmond, WA, USA).
III. Results
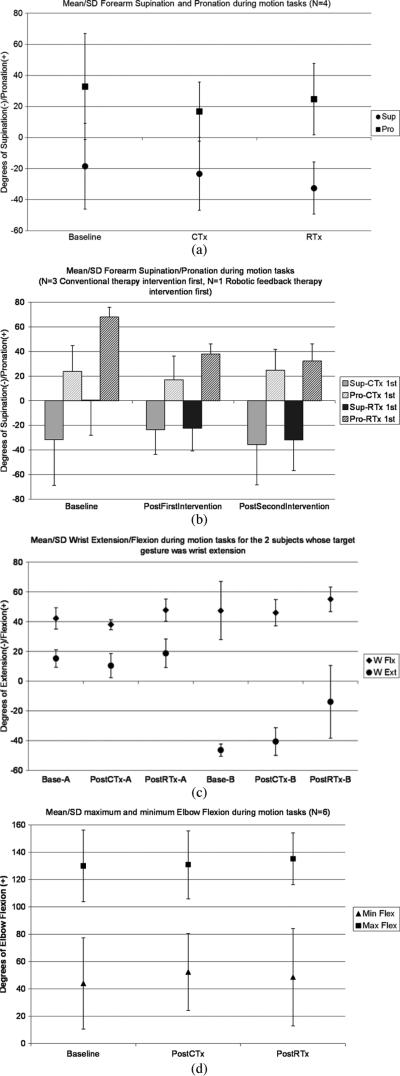
Supination during motion tasks was not different following either intervention [Fig. 3(a)] but robotic feedback therapy did show a trend toward larger increase in supination than conventional therapy (p = 0.06). The amount of pronation during motion tasks was significantly less following conventional therapy intervention (p = 0.05) than following robotic feedback intervention. There was no change in the supination/pronation range of motion (ROM) between the two therapy interventions. When conventional therapy was the first intervention (N = 3), supination decreased and recovered following the subsequent robotic feedback therapy, but failed to increase over baseline supination measurement [Fig. 3(b)]. When robotic feedback therapy was the first intervention (N = 1), supination increased and an additional increase in supination was noted following the conventional therapy intervention.
Fig. 3.
(A) Mean and standard deviation of forearm supination/pronation during motion tasks following conventional (CTx) versus robotic feedback (RTx) therapy interventions. Error bar indicates one standard deviation from the mean. * Indicates significant decrease in pronation (p = 0.05) following CTx as compared with RTx interventions. ** Indicates a trend toward increased supination (p = 0.06) following RTx as compared with CTx interventions. (B) Mean and standard deviation of forearm supination/pronation during motion tasks according to which therapy intervention was first. Error bar indicates one standard deviation from the mean. (C) Mean and standard deviation of wrist extension/flexion during motion tasks following CTx versus RTx interventions for the two subjects whose target gesture was wrist extension. Error bar indicates one standard deviation from the mean. (D) Mean and standard deviation of maximum and minimum elbow flexion during motion tasks following (CTx) versus (RTx) therapy interventions. Error bar indicates one standard deviation from the mean.
Of the two subjects whose target gesture was wrist extension, one subject had no discernible change in either flexion or extension motion during the motion tasks following either intervention [Subject A in Fig. 3(c)]. The second subject had no change following the conventional therapy intervention and a decrease in wrist extension following the robotic feedback therapy intervention.
Examination of the data revealed some interesting differences between the two interventions. First, the change in agonist and antagonist motion differed between the two interventions. Pronation decreased significantly (p = 0.05) more following the conventional therapy than robotic feedback therapy intervention when the goal was targeted to increase forearm supination. A presumption for physical and occupational therapy with the goal of increasing functional use of an agonist motion, in this case supination, is that no compromise will occur in the antagonist motion, in this case pronation. The greater loss of pronation motion following conventional therapy intervention than the robotic feedback therapy intervention suggests that the robotic feedback therapy was less detrimental to the antagonist motion of the targeted agonist motion. By contrast, supination motion increased slightly following the conventional therapy and demonstrated a trend toward greater improvement (p = 0.06) following robotic feedback therapy intervention. Despite no significant difference found in the overall supination/pronation ROM following the different interventions, considering the apparently greater gain in supination with the smaller loss of pronation suggests that the robotic feedback therapy is overall better for increasing functional motion without compromising the function of the antagonist motion.
Another difference between the interventions can be seen by inspecting the order of intervention among subjects whose goal was to increase forearm supination. There were three subjects who received the conventional therapy intervention during the first five week period and one subject who received the robotic feedback therapy intervention during the first five week period. It is clear that the subjects were not matched in their forearm supination and pronation motion measurements at baseline. The conventional therapy first intervention group demonstrated both supination and pronation motion during the tasks but the robotic feedback therapy first intervention subject had predominantly pronation. Following the initial intervention period, subjects receiving conventional therapy first demonstrated less pronation and supination during the motion tasks. The subject receiving robotic feedback therapy first demonstrated more supination (the target motion) and less pronation. This subject continued to demonstrate improvement in supination motion following the subsequent intervention of conventional therapy with no change in the amount of pronation motion. Although the subjects receiving conventional therapy intervention first did improve in both pronation and supination motion following the subsequent robotic feedback therapy, the improvement did not exceed their baseline measurements.
The pair of subjects whose targeted gesture was wrist extension also demonstrated several differences between the interventions. The first subject with wrist extension as the target gesture [Subject A in Fig. 3(c)] does not appear to have any change in wrist extension, flexion, or ROM following either intervention. In selecting the target gesture for this subject, therapists noted that the wrist remained in flexion during all of the motion tasks. A second consideration in selecting the target motion was the fact that the forearm was fixed in pronation due to soft tissue contractures. During the measurement sessions following interventions it was noted that, although passively the subject could be moved into an extended wrist position, voluntary wrist extension never exceeded approximately 5° of wrist flexion. In this flexed wrist position, primary wrist extension muscles are at a mechanical disadvantage for wrist extension motion as their moment arms are estimated to be minimal [14]. The intervention order for this subject was robotic feedback therapy before conventional therapy and the results do not suggest an order of intervention effect.
The therapeutic goal for the second subject for whom wrist extension was that targeted gesture, was to control the degree of wrist extension during activities. At baseline when performing the motion tasks, the wrist of this subject would move into maximum extension and the wrist would remain in that position which can be seen in the small standard deviation. When the wrist is in such extreme extension, functional hand use is decreased. Little change in the average wrist motion or standard deviation was found following conventional therapy intervention. Following the robotic feedback therapy there was less wrist extension on average with a greater standard deviation indicating that the goal of keeping the wrist in a less extreme degree of wrist extension to allow for increased hand function had been achieved.
This data also suggests that the order of intervention is a factor warranting further investigation. One of two subjects who received the robotic feedback therapy intervention prior to the conventional therapy had improvement following both intervention periods. By contrast, subjects having conventional therapy intervention first demonstrated little or no improvement in the target gesture and improvement following the robotic feedback therapy intervention, although it was not sufficient to exceed baseline measurements.
Movement measured in joints other than the targeted therapeutic joint motion did not change following either conventional or robotic feedback interventions [Fig. 3(d)]. The amount of elbow flexion and extension ROM did not change following either conventional or robotic therapy intervention periods.
IV. Discussion
This study demonstrated slight differences between conventional and robotic feedback therapy. Rationale for differences in response to gestural robotic feedback therapy could be due to several mechanisms. First, the robotic system provides feedback to subjects very quickly compared with the conventional therapy. The immediacy of the feedback from the robotic feedback therapy as compared with conventional therapy could be responsible for some of the gains in motion tasks. Feedback from the robot occurs <500 ms after the target gesture has exceeded the threshold, which is almost imperceptible to subjects. By contrast, the time required during conventional therapy for the therapist to visually process motion of the subject and provide feedback about the gesture, is so long that the subject might have attempted several strategies to perform the requested motion thus setting up confusion as to which strategy the subject should employ in the next attempt.
The implication that this sort of robotic feedback therapy is similar to interventions used to develop neuroplasticity is un-supported. The number of repetitions of the target gesture using robotic feedback in this study is limited to 3 h 20 min over a five week period. The literature reveals that neuroplastic changes are induced only after more than 100 repetitions per hour for multiple hours on a daily basis [15]–[17]. The neuromuscular rationale for improvement in motion following robotic feedback therapy will need to be developed in future studies.
Temporal factors with respect to intervention will also require additional research before a fixed protocol can be recommended. This study established 20 min treatment sessions following general education principles for anticipated ages of subjects covered in study inclusion criteria. Little evidence exists defining optimal time for therapy sessions with many institutions determining length based on external factors such as billing codes and reimbursement rates. Similarly, the number of therapy sessions per week and number of weeks necessary to elicit meaningful change in movement capability have yet to be defined. If we accept that greater improvement occurs when conventional therapy followed robotic feedback therapy interventions, another study would be necessary to determine the amount of time robotic feedback therapy should be undertaken prior to following with conventional therapy. Alternatively, combining the two therapies might provide greater gains with robotic feedback therapy for a targeted gesture followed by conventional or functional tasks, a strategy similar to working to increase movement by stretching prior to performing functional activities.
Three-dimensional quantification of movement using an optical motion capture system is a valuable tool for simultaneously measuring all joints in the upper extremities [18]. Consistent and repeatable measurements of upper extremity kinematics have been demonstrated in both subjects with and without CP [19], [20]. Movement tasks selected for inclusion in this study were selected from those in recent reports which would reflect both isolated joint motions [21] and simulated functional tasks [20]–[22]. Previous studies assessing change in motion of children have depended on parental recall of activities (motor activity logs) or subjective clinical tests to support change secondary to intervention. Quantification of motion during tasks used in this study is a less ambiguous methodology to determine change. Although supination motion for the group whose intervention targeted supination, when considered as the whole group, did not change significantly following interventions, the quantification of motion during tasks is sensitive enough to provide some evidence of changes in individual subjects.
Information about magnitude of joint ROM limits of pronation and supination in typically developing children is limited, but has been noted to be greater than that in children with CP [20], [22]. The magnitude of supination and pronation measured in this study during the motion tasks in subjects whose target gesture was supination, was generally greater than that reported by Reid et al. [20]. All four subjects in this study were able to achieve some supination at baseline (although the average supination across motion tasks for one subject at baseline showed no supination, the standard deviation demonstrates that supination was present in some of the motion tasks), and all demonstrated full pronation at some time during the study. Part of the difference between measurements is likely due to the fact that the majority of our subjects had quadriplegic CP involvement rather than hemiplegic CP as those in the Reid and Butler studies. Another source of difference between the two sets of subjects is that subjects in the Reid study were classified as having Manual Ability Classification System (MACS) levels between 1 and 3. Only one of our subjects was classified as GMFCS level 1 correlating to the MACS level 1 [23]. Most of our subjects were classified as GMFCS level IV, which would likely be ranked as level IV or V on the MACS.
There are several limitations that should be considered when interpreting the results of this study. It is not possible to clearly determine efficacy of the robotic feedback intervention given the limited number of subjects in this study. Subjects in this study also varied with respect to both diagnoses and functional capabilities at baseline making comparisons difficult. Greater numbers of subjects working toward a single target gesture are needed from a single diagnosis, e.g., spastic hemiplegia, with similar classification of function, e.g., GMFCS level III, and similar impairments at baseline to legitimately determine efficacy of robotic feedback therapy. Future studies should address targeted gestures at other joints and gestures for the lower extremities. In addition to continuing to assess robotic gestural feedback treatment for children with CP, expansion to other patient populations such as stroke and brain injury is warranted.
Acknowledgment
This work was completed with the collaboration of Valiant Technology Ltd, London (Roamer-Too base for the robot) and therapists at Stanley Jones and Associates, Inc., Rochester, MN, USA.
This work was supported by the National Institutes of Health under an SBIR Grant funded by the National Institute of Child Health and Development Grant #2R44HD042353-02A2.
Biography
Krista Coleman Wood received the B.S. degree in physical therapy from the University of Illinois, Peoria, IL, USA, the M.S. degree in physical therapy from the University of Minnesota, Minneapolis, MN, USA, the M.Sc. degree in bio-engineering from the University of Strathclyde, Glasgow, U.K., and the Ph.D. degree in biomechanics from the University of Minnesota, Minneapolis, MN, USA.
She is a Physical Therapist and Biomedical Engineer at the Motion Analysis Laboratory of Mayo Clinic, Rochester, MN, USA. Her practice is focused quantitative assessment of human performance to determine the source of movement disorders.
Corinna E. Lathan received the B.A. degree in biopsychology and mathematics from Swarthmore College, Swarthmore, PA, in 1988, and the S.M. degree in aeronautics and astronautics, and the Ph.D. degree in neuroscience from the Massachusetts Institute of Technology, Cambridge, MA, USA, in 1995 and 1994, respectively.
Since then she has been an Associate Professor of Biomedical Engineering at The Catholic University of America, Washington, DC, USA, and an Adjunct Associate Professor of Aerospace Engineering at the University of Maryland, College Park, MD, USA. In 1999, she co-founded and currently serves as Board Chair and CEO of AnthroTronix, Inc., Silver Spring, MD. Her research interests include assistive technologies and advanced multi-modal human–technology interfaces.
Kenton R. Kaufman received the B.S. degree in agricultural engineering (with highest honors, and a mathematics minor) and the M.S. degree in agricultural engineering from South Dakota State University, Brookings, SD, USA, in 1974 and 1976, respectively, and the Ph.D. degree in biomechanical engineering with a statistics minor in 1988 from North Dakota State University, Fargo, ND, USA, and Mayo Graduate School of Medicine, Rochester, MN, USA.
Currently, he is the W. Hall Wendel Jr. Musculoskeletal Research Professor, Professor of Bioengineering, Director of the Motion Analysis Laboratory, and Consultant in the Departments of Orthopedics, Physiology and Biomedical Engineering at Mayo Clinic, Rochester, MN, USA. He is also a registered professional engineer. His primary area of research is musculoskeletal rehabilitation science.
Contributor Information
Krista Coleman Wood, Motion Analysis Laboratory, Department of Orthopedic Surgery, Mayo Clinic, Rochester, MN 55905 USA.
Corinna E. Lathan, AnthroTronix, Inc., Silver Spring, MD 20910 USA.
Kenton R. Kaufman, Motion Analysis Laboratory, Department of Orthopedic Surgery, Mayo Clinic, Rochester, MN 55905 USA (kaufman.kenton@mayo.edu).
References
- 1.Campbell SK, et al. Physical Therapy for Children. 4th ed. Saunders; Philadelphia, PA: 2010. [Google Scholar]
- 2.Myklebust BM, Gottlieb GL. Development of the stretch reflex in the newborn: Reciprocal excitation and reflex irradiation. Child Develop. 1993;64:1036–1045. [PubMed] [Google Scholar]
- 3.Myklebust BM, et al. Reciprocal excitation of antagonistic muscles as a differentiating feature in spasticity. Ann. Neurol. 1982;12:367–374. doi: 10.1002/ana.410120409. [DOI] [PubMed] [Google Scholar]
- 4.DeLisa JA, et al. Physical Medicine and Rehabilitation: Principles and Practice. 4th ed. Lippincott Williams Wilkins; Philadelphia, PA: 2004. [Google Scholar]
- 5.Ketelaar M, et al. Parental participation in intervention programs for children with cerebral palsy: A review of research. Topics Early Childhood Special Edu. 1998;18:108–117. [Google Scholar]
- 6.Novak I, Cusick A. Home programmes in paediatric occupational therapy for children with cerebral palsy: Where to start? Aust. Occupat. Therapy J. 2006;53:251–264. [Google Scholar]
- 7.Valvano J, Rapport MJ. Activity-focused motor interventions for infants and young children with neurological conditions. Infants Young Children. 2006;19:292–307. [Google Scholar]
- 8.McArdle P. Children's play. Child: Care, Health Develop. 2001;27:509–514. doi: 10.1046/j.1365-2214.2001.00230.x. [DOI] [PubMed] [Google Scholar]
- 9.Swinnen SP, et al. Interlimb coordination: Learning and transfer under different feedback conditions. Hum. Movement Sci. 1997;16:749–785. [Google Scholar]
- 10.Schmidt RA. Motor schema theory after 27 years: Reflections and implications for a new theory. Res. Q. Exercise Sport. 2003;74:366–375. doi: 10.1080/02701367.2003.10609106. [DOI] [PubMed] [Google Scholar]
- 11.Fitts PM. Factors in comples skill training. In: Glaser R, editor. Training Research and Education. Univ. Pittsburgh Press; Pittsburgh, PA: 1962. [Google Scholar]
- 12.Gentile AM. A working model of skill acquisition with application to teching. Quest. 1972;17:3–28. [Google Scholar]
- 13.Morrow MMB, et al. Upper-limb joint kinetics expression during wheelchair propulsion. J. Rehabil. Res. Develop. 2009;46:939–944. doi: 10.1682/jrrd.2008.12.0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramsay JW, et al. Muscle moment arm and normalized moment contributions as reference data for musculoskeletal elbow and wrist joint models. J. Biomechan. 2009;42:463–473. doi: 10.1016/j.jbiomech.2008.11.035. [DOI] [PubMed] [Google Scholar]
- 15.Birkenmeier RL, et al. Translating animal doses of task-specific training to people with chronic stroke in 1-hour therapy sessions: A proof-of-concept study. Neurorehabil. Neural Repair. 2010;24:620–635. doi: 10.1177/1545968310361957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoare BJ, et al. Constraint-induced movement therapy in the treatment of the upper limb in children with hemiplegic cerebral palsy. Cochrane Database Systematic Rev. 2007 doi: 10.1002/14651858.CD004149.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Page SJ, et al. Efficacy of modified constraint-induced movement therapy in chronic stroke: A single-blinded randomized controlled trial. Arch. Phys. Med. Rehabil. 2004;85:14–18. doi: 10.1016/s0003-9993(03)00481-7. [DOI] [PubMed] [Google Scholar]
- 18.Jaspers E, et al. Review of quantitative measurements of upper limb movements in hemiplegic cerebral palsy. Gait Posture. 2009;30:395–404. doi: 10.1016/j.gaitpost.2009.07.110. [DOI] [PubMed] [Google Scholar]
- 19.Butler EE, et al. Three-dimensional kinematics of the upper limb during a reach and grasp cycle for children. Gait Posture. 2010;32:72–77. doi: 10.1016/j.gaitpost.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 20.Reid S, et al. Repeatability of upper limb kinematics for children with and without cerebral palsy. Gait Posture. 2010;32:10–17. doi: 10.1016/j.gaitpost.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 21.van Andel CJ, et al. Complete 3D kinematics of upper extremity functional tasks. Gait Posture. 2008 Jan;27:120–127. doi: 10.1016/j.gaitpost.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Butler EE, et al. Temporal-spatial parameters of the upper limb during a reach grasp cycle for children. Gait Posture. 2010;32:301–306. doi: 10.1016/j.gaitpost.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 23.Carnahan KD, et al. Association between gross motor function (GMFCS) and manual ability (MACS) in children with cerebral palsy. A population-based study of 359 children. BMC Musculoskeletal Disorders. 2007;8 doi: 10.1186/1471-2474-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]