Abstract
Although many rapid and high throughput molecular methods have been developed in the recent years for the multiplex detection of foodborne pathogens, the simultaneous recovery and enrichment of sublethally injured cells is still a problem that needs to be considered. Combined with previous established multiplex real-time PCR assay, the capability of simultaneous recovery and enrichment of sublethally injured Salmonella, E. coli O157:H7 and L. monocytogenes cells was evaluated in a multiplex selective enrichment broth SEL. The injured cells were obtained by heat shock. After evaluation of different procedures, 1 h of recovery period prior to 20 h of enrichment was proved to be necessary for the detection of less than 10 CFU/5 mL broth of injured L. monocytogenes. When the detection method was applied to artificially contaminated ground beef, all the three injured pathogens could be simultaneously detected without discrimination by real-time PCR combined with SEL broth, the detection limit was < 5 CFU/10 g ground beef. Comparatively, when BPW was employed as the enrichment broth in the same detection procedure, injured L. monocytogenes could not be detected if the initially spiked level was below 102 CFU/10 g ground beef. Considering the capability of co-enrichment and high detection effectiveness, the real-time PCR assay combined with SEL broth herein appears to be a promising tool for high-throughput screening of a large number of processed food samples, which require either single or multiple pathogen detection. More important, the sublethally injured foodborne pathogen cells were also detectable.
Keywords: sublethal injury, SEL broth, multiplex detection, foodborne pathogen
Introduction
Microbial foodborne pathogens are widespread and cause millions of cases of human illness every year around the world, with nearly a quarter of the population at higher risk for illness today (Oliver et al., 2005), resulting in major public health issues and substantial economic burden. Among the known pathogens, Salmonella spp., Escherichia coli O157:H7 and Listeria monocytogenes are important because of the severity of disease or the number of illness cases they cause (CDC, 2006). For the truth that many foods are common carriers of Salmonella spp., E. coli O157:H7 and L. monocytogenes, traditional pathogen detection has burdened the industry and regulatory agencies, for the testing of foods that have a high risk of contamination with these pathogens (Nugen and Baeumner, 2008). Current research trends emphasize the development of multipathogen detection platforms in a single-assay format (Shi et al., 2010). The multipathogen detection approach is attractive and economically favorable since it can reduce labour requirement for handling a large number of samples, as well as reducing the overall cost of testing per pathogen.
Though the sensitivity of many multiplex detection methods have improved significantly, an enrichment step is still needed. This step is required not only to increase the target-pathogen concentration in a sample but also to resuscitate physiologically stressed or injured cells (Wu, 2008). Additionally, selective enrichment is also necessary to suppress the natural background microorganisms, to improve detection efficiency and to avoid false results. However, previously widely used broths for simultaneous enrichment of multiple pathogens before detection should belong to the nonselective media, such as buffered peptone water (BPW) (Alarcon et al., 2004; Wang and Suo, 2011), No. 17 medium (Kawasaki et al., 2010), universal pre-enrichment broth (UPB) (Bhagwat, 2003), et al. This kind of broth could recover and enrich target pathogens along with background flora, which was prone to cause false negative detection results, especially induced by the complex flora in tested food sample (Lindner et al., 2011). Although selective enrichment broth promises to avoid the interference of background flora in food samples, the drawbacks of previous selective enrichment broths are aimed to enrich one specular pathogen in one detection system, which was unsuitable for multiplex detection of one more pathogens. Moreover, the selective agents can be inhibitory or can delay the recovery and growth of healthy or stressed target pathogens (Jacobsen, 1999) and thus affecting the detection of pathogens by causing false negative results (Wu, 2008).
Therefore, a desired enrichment broth for multi-pathogen detection should have the capabilities of both recovery of sublethally injured cells and selective enrichment of target cells from complex background flora in food (Yu et al., 2010). SEL is a recently developed multiplex enrichment broth for simultaneously and selectively enrich Salmonella, E. coli O157:H7 and L. monocytogenes (Kim and Bhunia, 2008). So far, based on SEL broth, a multiplex real-time PCR (Suo et al., 2010b) and a microarray assays (Suo et al., 2010a) have been developed. Although SEL broth has been proven to be able to resuscitate acid- and cold-stressed cells preliminarily (Kim and Bhunia, 2008), its capability in simultaneous recovery and enrichment of stressed cells is still not confirmed thoroughly by low number and pure stressed cells, which might represent the real condition of multipathogens contamination in food.
Materials and Methods
Bacterial strains and culture conditions
The bacterial reference strains, including S. Typhimurium ATCC14028, E. coli O157:H7 ATCC43889 and L. monocytogenes CMCC54002 were obtained from the American Type Culture Collection (ATCC), or China Microbiological Culture Collection Center (CMCC). All strains were aerobically grown at 37 °C in Brain Heart Infusion medium (Becton Dickinson Co., Sparks, MD) shaking at 150 rpm. After overnight incubation from single colony, bacterial cells were collected for analysis.
Preparation of sublethally injured bacterial cells
One colony of each pathogen was inoculated in 5 mL of nutrient broth and cultivated to later exponential phase (cv. 108 cfu/mL, the growth curves of all tested pathogens were pre-determined). 0.5 mL of enriched broth was heat shocked in a preheated glass tube, and the survival curves were plotted according to the viable cells counts on differential plate. S. Typhimurium ATCC14028 and E. coli O157:H7 ATCC43889 were heated at 55 °C, and L. monocytogenes CMCC54002 was heated at 60 °C. Differential plate counting was performed in tryptic soy agar plus 0.6% yeast extract (TSAYE, from BD) for enumeration of total viable cells, and in TSAYE with NaCl (TSAYE-NA, 4% NaCl for Salmonella and E. coli O157:H7, and 6% NaCl for L. monocytogenes) for enumeration of uninjured cells only. Based on our pre-experiments, concentrations used to detect sublethal injury correspond to the highest NaCl concentrations that did not affect the growth of untreated cells (Suo et al., 2012). After 6 min of heat shock, each broth was serially 10-fold diluted in 0.1% sterile peptone solution and used as sublethally injured cells. All sublethally injured cells were fresh prepared and used for the next enrichment experiment within 1 h.
Recovery of sublethally injured cells in SEL broth
To optimize the condition for recovery of sublethally injured cells, for each pathogen, 100 μL of serially diluted cell broth with known cell concentration were added to different 5 mL of Buffered Listeria Enrichment Broth Base (BLEB) (Becton Dickinson) and incubated at 37 °C with shaking at 150 rpm. After 0, 1 and 2 h of incubations for each group, selective antibiotics were added to achieve final concentrations of 0.01 g/L for Acriflavine (ICN Biomedical Inc., Aurora, OH), 0.05 g/L for Cycloheximide, 0.05 g/L for Fosfomycin, and 0.002 g/L for Nalidixic acid (Sigma, St. Louis, MO) as described for SEL (Kim and Bhunia, 2008). Following by another 20 h of incubation, the cell number was counted by spreading on TSAYE and TSAYE-NA plates. All experiments were performed at least three times.
Genomic DNA preparation and multiplex real-time PCR detection
One mL of freshly grown bacterial culture was subjected to DNA extraction and purification using the DNeasy Blood & Tissue kit (Qiagen, Valencia, CA) following the manufacturer’s recommendations. Before DNA extraction, the cell pellets were digested in 180 μL of 20 mg/mL lysozyme (containing 20 mM Tis-Cl, pH = 8.0, 2 mM sodium EDTA and 1.2% Triton® X-100) at 37 °C for 30 min. The primer and probe sets, multiplex real-time PCR procedure for the simultaneous detection and quantification of Salmonella spp., E. coli O157:H7 and L. monocytogenes were referred to Suo et al. (2010b), and performed on a 7500 Real-Time PCR system (Applied Biosystems, Foster City, CA).
Application to artificially contaminated ground beef by sublethally injured cells
Ground beef samples were artificially contaminated by seven different combinations of initial sublethally injured cell numbers to examine the simultaneous recovery and enrichment capability of each pathogen in SEL. Before spiking, the ground beef was confirmed by traditionally culture-based methods to be lacking the three target pathogens. 100 μL of serial diluted later-exponential cell broth ranged from 101 cfu/mL to 104 cfu/mL were inoculated to 10 g of ground beef samples. The initial cell numbers before dilution were 3.3 × 108 cfu/mL for S. Typhimurium ATCC14028, 4.1 × 108 cfu/mL for E. coli O157:H7 ATCC43889 and 3.7 × 108 cfu/mL for L. monocytogenes CMCC54002. The final cell counts of the four combinations of injured pathogens in beef samples were: A, 103:103:103; B, 102:102:102; C, 101:101:101; D, 100:100:100; E, 103:100:101; F, 100:101:103; and G, 101:103:100, respectively. All samples were put into a filter stomacher bag (Nasco Whirl-Pak, Fort Atkinson, WI) before adding 90 mL of BLEB broth. After 1 h of incubation at 37 °C, selective antibiotics were added to obtain SEL broth as described above. After enrichment under the same conditions for 20 h, 1 mL aliquot was collected from each sample for DNA extraction and quantitative real-time PCR determination. As comparisons, the same artificially contaminated beef samples were also recovered and enriched by a nonselective universal enrichment buffered peptone water (BPW) for 20 h. These experiments were repeated three times with two replicates per trial.
Results and Discussion
Heat injury of Salmonella, E. coli O157:H7 and L. monocytogenes
Before the thermal injury experiment, newly cultivated cells of S. Typhimurium ATCC14028, E. coli O157:H7 ATCC43889 and L. monocytogenes CMCC54002 on later exponential phase were sprayed on TSAYE agars plus increasing concentration of NaCl ranged from 0% to 10%, respectively, which was to determine the most tolerance of these pathogens. According to the results, the difference of cell counts was not significant between the sprayed results on TSAYE and TSAYE-NA, until the concentration of NaCl was increased to 6% for L. monocytogenes, and 4% for Salmonella and E. coli O157:H7 (data no presented). Thus, the concentrations were considered as the most tolerant to NaCl and were used to count noninjured number of these pathogens, respectively.
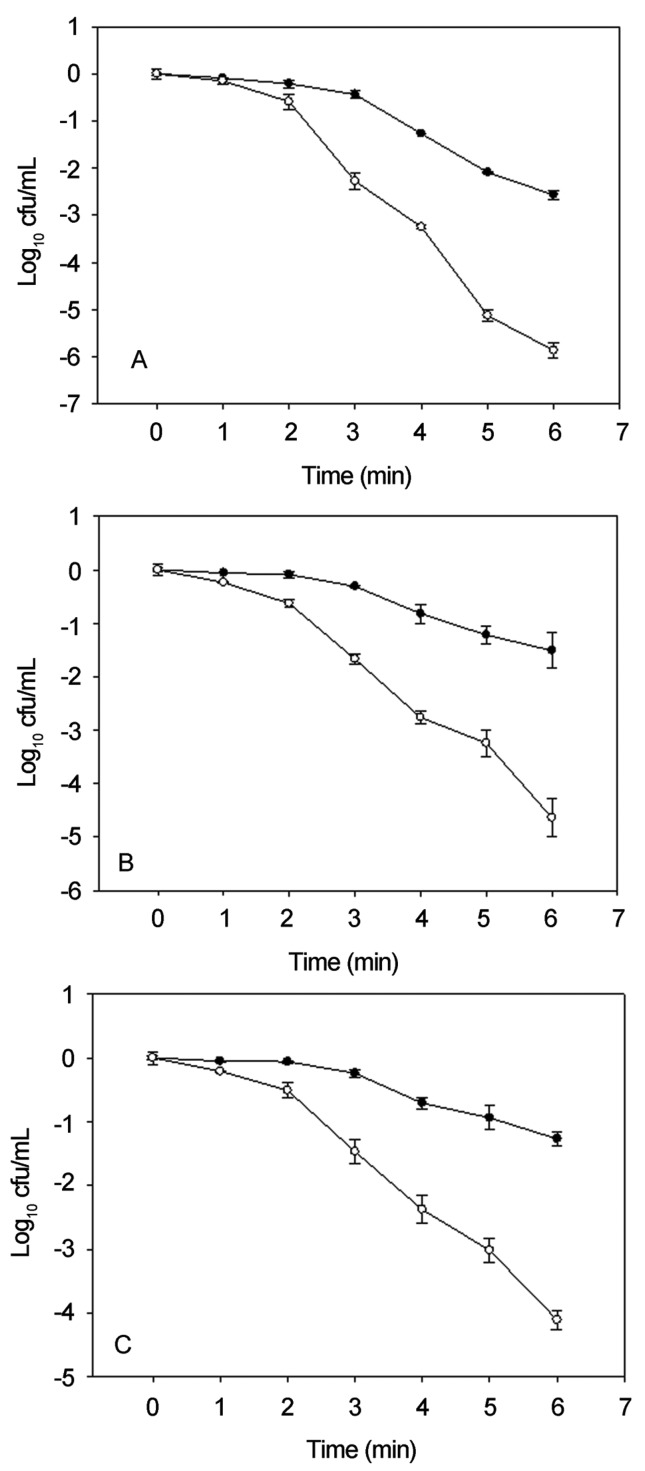
Before sublethally injured cells obtainment, Salmonella, E. coli O157:H7 and L. monocytogenes were mild heated under the same condition (55 °C), the L. monocytogenes cells showed a greater thermal stress resistance. This was expressed by both lower rate of lethality and less than 1 Log10 cfu/mL difference in the number between survivors on TSAYE and selective TSAYE-NA (data not presented), which was coincident with previous comparative results of the same three pathogens (Juneja, 2003). In this case, a higher temperature of 60 °C was imposed on L. monocytogenes to obtain the thermal injured cells. As results shown in Figure 1, after 6 min of heat treatment, at least 3 Log10 cfu/mL difference of viable cells were counted on differential plates, indicated more than 99.9 percent of survival cells were sublethally injured (Wesche et al., 2009).
Figure 1.
Thermal sublethal injury of Salmonella (A), E. coli O157:H7 (B) and L. monocytogenes (C) cells in nutrient broth. Cells were heated under 60 °C for L. monocytogenes and 55 °C for Salmonella and E. coli O157:H7. ●, number of total viable cells counted on TSAYE; °, number of uninjured cells counted on TSAYE-NA.
Evaluation of the recovery of sublethally injured cells in SEL
To simplify the operation steps and minimize the possibility of cross-contamination during enrichment of multiplex pathogens, a new enrichment procedure was designed and evaluated for the capability of recovery and enrichment of sublethally injured Salmonella, E. coli O157:H7 and L. monocytogenes. The enrichment procedure was imitated the selective enrichment step in FDA recommended BAM method for detection of L. monocytogenes (BAM, 2011). With the purpose of shortening the enrichment period as much as possible, 0, 1, 2 h of periods were compared by the recovery effect of sublethally injured cells in nonselective BLEB broth. As shown in Table 1, the 100 μL of initially spiked cell number in 5 mL BLEB broth could only be detected viable cells on nonselective TSAYE agars but could not on selective TSAYE-NA agars, which indicated that the spiked cells were all sublethally injured (Aljarallah and Adams, 2007; Jofre et al., 2010; Saldana et al., 2010).
Table 1.
Result of recovery and enrichment of thermal injured cells in SEL broth.
| Strains | Spiked cells (cfu/5 mL BLEB) | Recovery time (h) | Cells after 20 h enrichment (cfu/mL) | ||
|---|---|---|---|---|---|
|
|
|
||||
| TSAYE | TSAYE-NA | TSAYE | TSAYE-NA | ||
| S. Typhimurium | 1.80×102 | 0 * | 0 | 3.70×109 | 2.86×109 |
| 1.80×101 | 0 | 0 | 2.15×109 | 1.98×109 | |
| 1.80×100 | 0 | 0 | 1.44×109 | 1.39×109 | |
| 0 | 0 | 0 | 0 | 0 | |
|
| |||||
| 1.80×102 | 0 | 1 | 5.95×109 | 5.70×109 | |
| 1.80×101 | 0 | 1 | 5.03×109 | 4.49×109 | |
| 1.80×100 | 0 | 1 | 4.00×109 | 3.97×109 | |
| 0 | 0 | 1 | 0 | 0 | |
|
| |||||
| 1.80×102 | 0 | 2 | 6.72×109 | 6.29×109 | |
| 1.80×101 | 0 | 2 | 6.19×109 | 5.90×109 | |
| 1.80×100 | 0 | 2 | 5.28×109 | 5.47×109 | |
| 0 | 0 | 2 | 0 | 0 | |
|
| |||||
| E. coli O157:H7 | 2.00×102 | 0 | 0 | 3.27×109 | 1.58×109 |
| 2.00×101 | 0 | 0 | 4.16×109 | 2.10×109 | |
| 2.00×100 | 0 | 0 | 1.65×109 | 1.08×109 | |
| 0 | 0 | 0 | 0 | 0 | |
|
| |||||
| 2.00×102 | 0 | 1 | 4.40×109 | 3.77×109 | |
| 2.00×101 | 0 | 1 | 3.99×109 | 3.29×109 | |
| 2.00×100 | 0 | 1 | 2.22×109 | 2.01×109 | |
| 0 | 0 | 1 | 0 | 0 | |
|
| |||||
| 2.00×102 | 0 | 2 | 4.78×109 | 3.96×109 | |
| 2.00×101 | 0 | 2 | 4.21×109 | 4.07×109 | |
| 2.00×100 | 0 | 2 | 2.38×109 | 2.15×109 | |
| 0 | 0 | 2 | 0 | 0 | |
|
| |||||
| L. monocytogenes | 8.80×102 | 0 | 0 | 2.12×109 | 1.56×109 |
| 8.80×101 | 0 | 0 | 1.22×109 | 1.29×109 | |
| 8.80×100 | 0 | 0 | 0 | 0 | |
| 0 | 0 | 0 | 0 | 0 | |
|
| |||||
| 8.80×102 | 0 | 1 | 2.63×109 | 2.33×109 | |
| 8.80×101 | 0 | 1 | 1.85×109 | 2.65×109 | |
| 8.80×100 | 0 | 1 | 1.95×108 | 2.48×108 | |
| 0 | 0 | 1 | 0 | 0 | |
|
| |||||
| 8.80×102 | 0 | 2 | 3.86×109 | 3.86×109 | |
| 8.80×101 | 0 | 2 | 1.67×109 | 1.08×109 | |
| 8.80×100 | 0 | 2 | 4.20×108 | 3.11×108 | |
| 0 | 0 | 2 | 0 | 0 | |
0 cfu/mL represented non viable cells could be detected in 100 μL of broth.
The recovery and enrichment results of sublethally injured pathogens in SEL were shown in Table 1, S. Typhimurium and E. coli O157:H7 both could be reached to the level of 109 cfu/mL after 20 h of selective enrichment, even when only 2 cfu of cells were inoculated initially in 5 mL of BLEB broth, and no matter whether had passed 1, 2 h of recovery periods or not. However, L. monocytogenes showed a lower growth rate and less recovery capability in SEL plus antibiotics. Only the level of 108 cfu/mL viable cells could be detected on TSAYE and TSAYE-NA agars when < 10 cfu of sublethally injured cells were initially inoculated. Moreover, under this inoculation level, no viable cell was shown on plates after 20 h of selective enrichment without pre-recovery period. This inhibition might be attributed to the susceptibility of membrane injured L. monocytogenes cells to the antibiotics in SEL, thus these cells would be inactivated if without enough recovery period was imposed (Jacobsen, 1999). For this reason, 1 h of nonselective recovery period before 20 h of selective enrichment step was chosen for following multiplex real-time PCR detection.
Evaluation of SEL in artificially contaminated food
BPW was a commonly used enrichment broth that has been widely applied to recover and enrich sublethally injured cells in USDA/FSIS recommended methods for many foodborne pathogens detections (USDA/FSIS Microbiology Laboratory Guidebook), as well as it is a generally recommended nonselective media, in which the three bacteria are able to grow (Alarcon et al., 2004). Since the complex background flora in food could also be enriched along with detection target pathogens in these kinds of nonselective both, which were tended to cause false negative/positive detection results, selective enrichment has been considered as a necessary step before molecular detection procedure (Kim and Bhunia, 2008).
In present research, seven different combinations of sublethally injured Salmonella, E. coli O157:H7 and L. monocytogenes in spiked beef were used to compare the capability of SEL and BPW in simultaneous recovery and enrichment of injured cells. As results shown in Table 2 calculated from the cycle threshold (Ct) value by amplification standard curve of quantitative real-time PCR, when BPW was used for the simultaneous enrichment of the three food-borne pathogens, for Salmonella and E. coli O157:H7, lower cell numbers ranged from 0 to 2 Log10 genome copy/mL were determined in BPW than that in SEL. The growth of L. monocytogenes was much more suppressed by 5~6 Log10 genome copy/mL when other two pathogens existed in BPW broth. Evenly, when the initially spiked level was below 102 cfu/10 g ground beef, no positive genome copy of L. monocytogenes could be detected by multiplex real-time PCR.
Table 2.
Comparisons of simultaneous recovery and enrichment of sublethally injured Salmonella, E. coli O157:H7 and L. monocytogenes in SEL and BPW broth using multiplex real-time PCR method in spiked ground beef.
| Pathogens mixture of Salmonella, E. coli O157:H7 and L. monocytogenes (cfu/10 g ground beef) | Recovery and enrichment in SEL | Recovery and enrichment in BPW | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| invA | rfbE | hlyA | invA | rfbE | hlyA | |
| 103:103:103 | 1.83 × 108 | 7.91 × 108 | 6.69× 107 | 9.91 × 107 | 9.26 × 107 | 2.30 × 101 |
| 102:102:102 | 1.39 × 108 | 9.10 × 108 | 3.21 × 107 | 8.28 × 106 | 6.25 × 107 | 1.73 × 100 |
| 101:101:101 | 8.89 × 107 | 9.23 × 108 | 5.66 × 106 | 8.12 × 106 | 8.29 × 106 | - |
| 100:100:100 | 6.64 × 107 | 9.27 × 108 | 4.23 × 105 | 6.17 × 106 | 5.61 × 106 | - |
| 103:100:101 | 6.51 × 108 | 2.47 × 107 | 4.07 × 105 | 6.61 × 108 | 4.58 × 106 | - |
| 100:101:103 | 2.09 × 107 | 3.62× 108 | 5.29 × 106 | 4.21 × 106 | 6.56 × 106 | 4.51 × 101 |
| 101:103:100 | 6.43 × 107 | 2.29 × 109 | 1.48 × 105 | 7.28 × 107 | 5.33 × 108 | - |
Comparatively, when the same combinations of three foodborne pathogens were inoculated in SEL broth, all the three target pathogens could be simultaneously detected at a detection limit of < 5 cfu/10 g ground beef, after 1 h of recovery and 20 h of selective enrichment periods, no matter in each combination of pathogens. The detection limit was comparable to the previously reported real-time PCR assays for the detection of inoculated healthy cells (Kawasaki et al., 2010; Suo et al., 2010b). Althoug Log10 1~4 genome copy/mL lower was observed on L. monocytogenes compared to Salmonella and E. coli O157:H7, indicated the growth of L. monocytogenes was still partially competitively inhibited by other bacteria, this inhibition was comparable to optimized No. 17 broth without dextrose and was considered as not influence on the obtainment of positive detection results by real-time PCR assays (Omiccioli et al., 2009).
In terms of detection speed of multiple pathogens, the entire process of the multiplex assay from sample enrichment to data analysis can be completed within 24 h. The positive detection results could be obtained when either healthy or sublethally injured cells existed in food samples with complex background microflora. The effectiveness of the detection was significantly improved from traditional culturing methods, which require 5–7 days to analyze a single species (Jasson et al., 2010).
Conclusion
In conclusion, sublethally injured cells of Salmonella, E. coli O157 and L. monocytogenes were obtained by heat shock, and their capability of simultaneous recovery and enrichment in SEL was evaluated in present research. After evaluation of different enrichment procedure, 1 h of recovery before 20 h of enrichment period is necessary for the detection of less than 10 cfu of all the sublethally injured target cells. Considering the capability of anti-competitive growth inhibition and high effectiveness, the real-time PCR assay combined with SEL selective enrichment broth herein appears to be a promising tool for high-throughput screening of a large number of thermal processed food samples, which require either single or multiple pathogen detection but sublethally injured cells probably existed.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (grant no. U1204331), the Science and Technology Department of Henan Province (grant no. 122102310310), the Education Department of Henan Province (grant no. 13A550486) and the Start-up Funding, Dr. of Henan Agricultural University (grant no. 30300168).
References
- Alarcon B, Garcia-Canas V, Cifuentes A, Gonzalez R, Aznar R. Simultaneous and sensitive detection of three foodborne pathogens by multiplex PCR, capillary gel electrophoresis, and laser-induced fluorescence. J Agric Food Chem. 2004;52:7180–7186. doi: 10.1021/jf049038b. [DOI] [PubMed] [Google Scholar]
- Aljarallah KM, Adams MR. Mechanisms of heat inactivation in Salmonella serotype Typhimurium as affected by low water activity at different temperatures. J Appl Microbiol. 2007;102:153–160. doi: 10.1111/j.1365-2672.2006.03054.x. [DOI] [PubMed] [Google Scholar]
- BAM. Detection and enumeration of Listeria monocytogenes. [April 2011];FDA Bacteriological Analytical Manual (online) 2011 Available from http://www.fda.gov/food/scienceresearch/laboratorymethods/bacteriologicalanalyticalmanualbam/ucm071400.htm.
- Bhagwat AA. Simultaneous detection of Escherichia coli O157:H7, Listeria monocytogenes and Salmonella strains by real-time PCR. Int J Food Microbiol. 2003;84:217–224. doi: 10.1016/s0168-1605(02)00481-6. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food - 10 states, United States, 2005. Morb Mortal Wkly Rep. 2006;55:392–395. [PubMed] [Google Scholar]
- Jacobsen CN. The influence of commonly used selective agents on the growth of Listeria monocytogenes. Int J Food Microbiol. 1999;50:221–226. [Google Scholar]
- Jasson V, Jacxsens L, Luning P, Rajkovic A, Uyttendaele M. Alternative microbial methods: An overview and selection criteria. Food Microbiol. 2010;27:710–730. doi: 10.1016/j.fm.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Jofre A, Aymerich T, Bover-Cid S, Garriga M. Inactivation and recovery of Listeria monocytogenes, Salmonella enterica and Staphylococcus aureus after high hydrostatic pressure treatments up to 900 MPa. Int Microbiol. 2010;13:105–112. doi: 10.2436/20.1501.01.115. [DOI] [PubMed] [Google Scholar]
- Juneja VK. A comparative heat inactivation study of indigenous microflora in beef with that of Listeria monocytogenes, Salmonella serotypes and Escherichia coli O157:H7. Lett Appl Microbiol. 2003;37:292–298. doi: 10.1046/j.1472-765x.2003.01393.x. [DOI] [PubMed] [Google Scholar]
- Kawasaki S, Fratamico PM, Horikoshi N, Okada Y, Takeshita K, Sameshima T, Kawamoto S. Multiplex real-time polymerase chain reaction assay for simultaneous detection and quantification of Salmonella species, Listeria monocytogenes, and Escherichia coli O157:H7 in ground pork samples. Foodborne Pathog Dis. 2010;7:1–6. doi: 10.1089/fpd.2009.0465. [DOI] [PubMed] [Google Scholar]
- Kim H, Bhunia AK. SEL, a selective enrichment broth for simultaneous growth of Salmonella enterica, Escherichia coli O157:H7, and Listeria monocytogenes. Appl Environ Microbiol. 2008;74:4853–4866. doi: 10.1128/AEM.02756-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner JDD, Santarelli M, Yamaguishi CT, Soccol CR, Neviani E. Recovery and identification of bovine colostrum microflora using traditional and molecular approaches. Food Technol Biotechnol. 2011;49:364–368. [Google Scholar]
- Nugen SR, Baeumner AJ. Trends and opportunities in food pathogen detection. Anal Bioanal Chem. 2008;391:451–454. doi: 10.1007/s00216-008-1886-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver SP, Jayarao BM, Almeida RA. Foodborne pathogens in milk and the dairy farm environment: food safety and public health implications. Foodborne Pathog Dis. 2005;2:115–129. doi: 10.1089/fpd.2005.2.115. [DOI] [PubMed] [Google Scholar]
- Omiccioli E, Amagliani G, Brandi G, Magnani M. A new platform for Real-Time PCR detection of Salmonella spp., Listeria monocytogenes and Escherichia coli O157 in milk. Food Microbiol. 2009;26:615–622. doi: 10.1016/j.fm.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Saldana G, Puertolas E, Condon S, Alvarez I, Raso J. Modeling inactivation kinetics and occurrence of sublethal injury of a pulsed electric field-resistant strain of Escherichia coli and Salmonella Typhimurium in media of different pH. Innov Food Sci Emerg Technol. 2010;11:290–298. [Google Scholar]
- Shi XM, Long F, Suo B. Molecular methods for the detection and characterization of foodborne pathogens. Pure Appl Chem. 2010;82:69–79. [Google Scholar]
- Suo B, He Y, Paoli G, Gehring A, Tu SI, Shi X. Development of an oligonucleotide-based microarray to detect multiple foodborne pathogens. Mol Cell Probes. 2010a;24:77–86. doi: 10.1016/j.mcp.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Suo B, He Y, Tu SI, Shi X. A multiplex real-time polymerase chain reaction for simultaneous detection of Salmonella spp., Escherichia coli O157, and Listeria monocytogenes in meat products. Foodborne Pathog Dis. 2010b;7:619–628. doi: 10.1089/fpd.2009.0430. [DOI] [PubMed] [Google Scholar]
- Suo B, Shi C, Shi X. Inactivation and occurrence of sublethal injury of Salmonella Typhimurium under mild heat stress in broth. J Verbr Lebensm. 2012;7:125–131. [Google Scholar]
- Wang Y, Suo B. A new 7-plex PCR assay for simultaneous detection of shiga toxin-producing Escherichia coli O157 and Salmonella Enteritidis in meat products. J Verbr Lebensm. 2011;6:441–447. [Google Scholar]
- Wesche AM, Gurtler JB, Marks BP, Ryser ET. Stress, sublethal injury, resuscitation, and virulence of bacterial foodborne pathogens. J Food Prot. 2009;72:1121–1138. doi: 10.4315/0362-028x-72.5.1121. [DOI] [PubMed] [Google Scholar]
- Wu VC. A review of microbial injury and recovery methods in food. Food Microbiol. 2008;25:735–744. doi: 10.1016/j.fm.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Yu YG, Wu H, Liu YY, Li SL, Yang XQ, Xiao XL. A multipathogen selective enrichment broth for simultaneous growth of Salmonella enterica serovar Enteritidis, Staphylococcus aureus, and Listeria monocytogenes. Can J Microbiol. 2010;56:585–597. doi: 10.1139/w10-040. [DOI] [PubMed] [Google Scholar]