Abstract
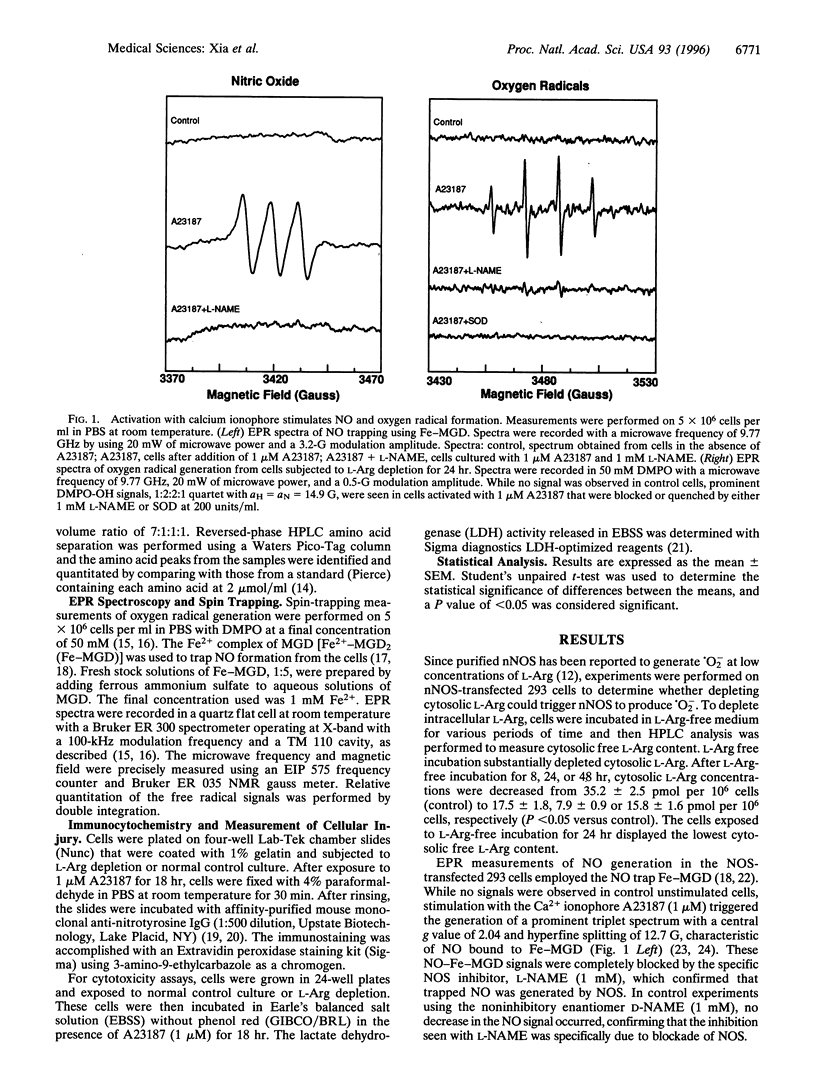
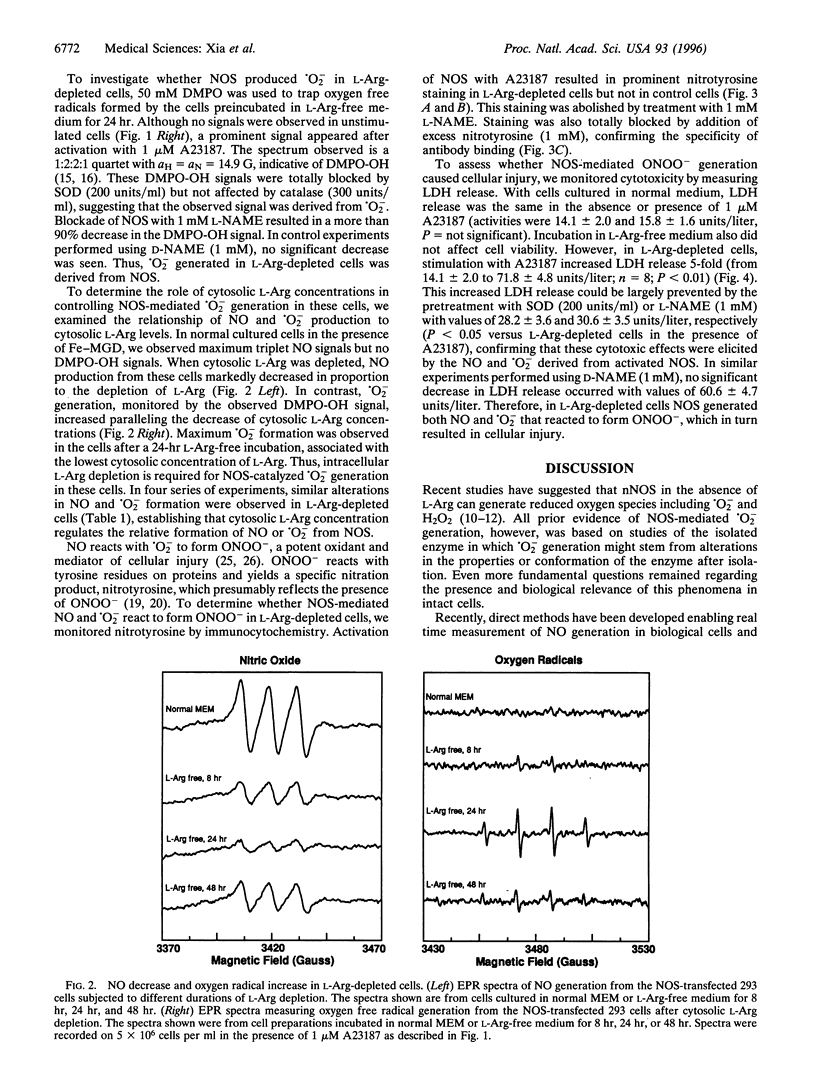
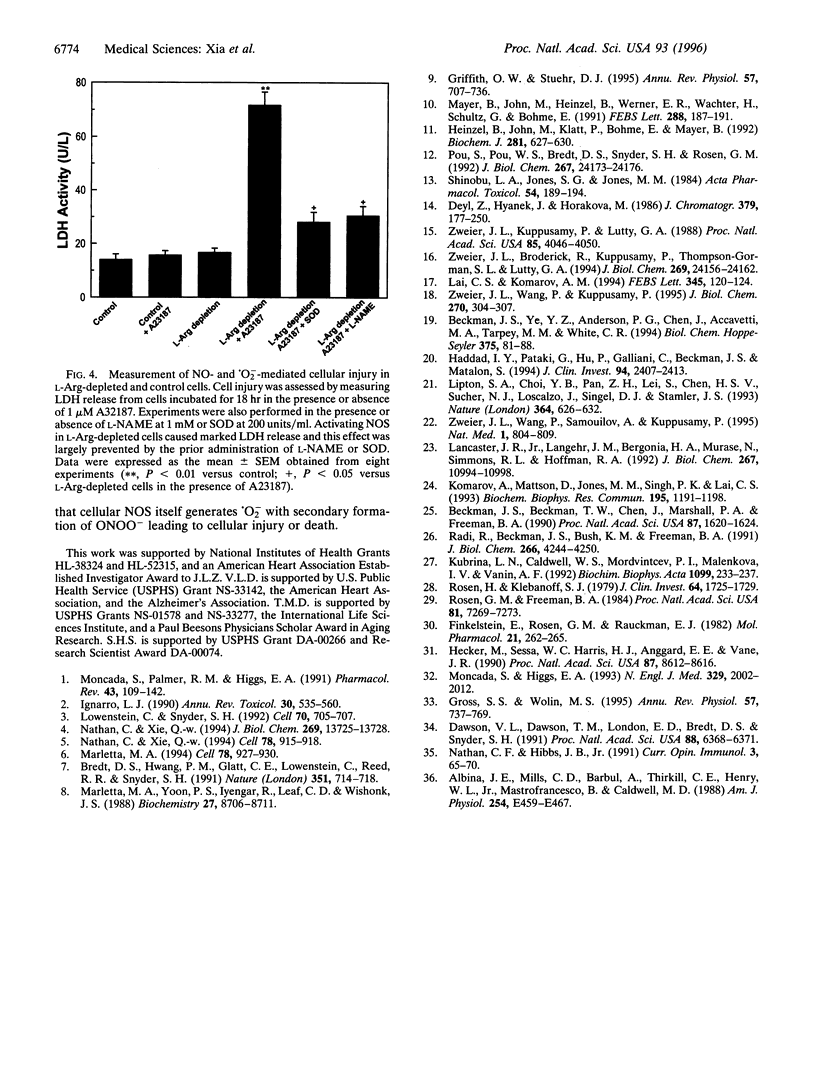
Besides synthesizing nitric oxide (NO), purified neuronal NO synthase (nNOS) can produce superoxide (.O2-) at lower L-Arg concentrations. By using electron paramagnetic resonance spin-trapping techniques, we monitored NO and .O2- formation in nNOS-transfected human kidney 293 cells. In control transfected cells, the Ca2+ ionophore A23187 triggered NO generation but no .O2- was seen. With cells in L-Arg-free medium, we observed .O2- formation that increased as the cytosolic L-Arg levels decreased, while NO generation declined. .O2- formation was virtually abolished by the specific NOS blocker, N-nitro-L-arginine methyl ester (L-NAME). Nitrotyrosine, a specific nitration product of peroxynitrite, accumulated in L-Arg-depleted cells but not in control cells. Activation by A23187 was cytotoxic to L-Arg-depleted, but not to control cells, with marked lactate dehydrogenase release. The cytotoxicity was largely prevented by either superoxide dismutase or L-NAME. Thus, with reduced L-Arg availability NOS elicits cytotoxicity by generating .O2- and NO that interact to form the potent oxidant peroxynitrite. Regulating arginine levels may provide a therapeutic approach to disorders involving .O2-/NO-mediated cellular injury.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albina J. E., Mills C. D., Barbul A., Thirkill C. E., Henry W. L., Jr, Mastrofrancesco B., Caldwell M. D. Arginine metabolism in wounds. Am J Physiol. 1988 Apr;254(4 Pt 1):E459–E467. doi: 10.1152/ajpendo.1988.254.4.E459. [DOI] [PubMed] [Google Scholar]
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann J. S., Ye Y. Z., Anderson P. G., Chen J., Accavitti M. A., Tarpey M. M., White C. R. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol Chem Hoppe Seyler. 1994 Feb;375(2):81–88. doi: 10.1515/bchm3.1994.375.2.81. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Glatt C. E., Lowenstein C., Reed R. R., Snyder S. H. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991 Jun 27;351(6329):714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- Dawson V. L., Dawson T. M., London E. D., Bredt D. S., Snyder S. H. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyl Z., Hyanek J., Horakova M. Profiling of amino acids in body fluids and tissues by means of liquid chromatography. J Chromatogr. 1986 Jun 20;379:177–250. doi: 10.1016/s0378-4347(00)80685-4. [DOI] [PubMed] [Google Scholar]
- Finkelstein E., Rosen G. M., Rauckman E. J. Production of hydroxyl radical by decomposition of superoxide spin-trapped adducts. Mol Pharmacol. 1982 Mar;21(2):262–265. [PubMed] [Google Scholar]
- Griffith O. W., Stuehr D. J. Nitric oxide synthases: properties and catalytic mechanism. Annu Rev Physiol. 1995;57:707–736. doi: 10.1146/annurev.ph.57.030195.003423. [DOI] [PubMed] [Google Scholar]
- Gross S. S., Wolin M. S. Nitric oxide: pathophysiological mechanisms. Annu Rev Physiol. 1995;57:737–769. doi: 10.1146/annurev.ph.57.030195.003513. [DOI] [PubMed] [Google Scholar]
- Haddad I. Y., Pataki G., Hu P., Galliani C., Beckman J. S., Matalon S. Quantitation of nitrotyrosine levels in lung sections of patients and animals with acute lung injury. J Clin Invest. 1994 Dec;94(6):2407–2413. doi: 10.1172/JCI117607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker M., Sessa W. C., Harris H. J., Anggård E. E., Vane J. R. The metabolism of L-arginine and its significance for the biosynthesis of endothelium-derived relaxing factor: cultured endothelial cells recycle L-citrulline to L-arginine. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8612–8616. doi: 10.1073/pnas.87.21.8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel B., John M., Klatt P., Böhme E., Mayer B. Ca2+/calmodulin-dependent formation of hydrogen peroxide by brain nitric oxide synthase. Biochem J. 1992 Feb 1;281(Pt 3):627–630. doi: 10.1042/bj2810627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J. Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu Rev Pharmacol Toxicol. 1990;30:535–560. doi: 10.1146/annurev.pa.30.040190.002535. [DOI] [PubMed] [Google Scholar]
- Komarov A., Mattson D., Jones M. M., Singh P. K., Lai C. S. In vivo spin trapping of nitric oxide in mice. Biochem Biophys Res Commun. 1993 Sep 30;195(3):1191–1198. doi: 10.1006/bbrc.1993.2170. [DOI] [PubMed] [Google Scholar]
- Kubrina L. N., Caldwell W. S., Mordvintcev P. I., Malenkova I. V., Vanin A. F. EPR evidence for nitric oxide production from guanidino nitrogens of L-arginine in animal tissues in vivo. Biochim Biophys Acta. 1992 Mar 13;1099(3):233–237. doi: 10.1016/0005-2728(92)90032-w. [DOI] [PubMed] [Google Scholar]
- Lai C. S., Komarov A. M. Spin trapping of nitric oxide produced in vivo in septic-shock mice. FEBS Lett. 1994 May 30;345(2-3):120–124. doi: 10.1016/0014-5793(94)00422-6. [DOI] [PubMed] [Google Scholar]
- Lancaster J. R., Jr, Langrehr J. M., Bergonia H. A., Murase N., Simmons R. L., Hoffman R. A. EPR detection of heme and nonheme iron-containing protein nitrosylation by nitric oxide during rejection of rat heart allograft. J Biol Chem. 1992 Jun 5;267(16):10994–10998. [PubMed] [Google Scholar]
- Lipton S. A., Choi Y. B., Pan Z. H., Lei S. Z., Chen H. S., Sucher N. J., Loscalzo J., Singel D. J., Stamler J. S. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. 1993 Aug 12;364(6438):626–632. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- Lowenstein C. J., Snyder S. H. Nitric oxide, a novel biologic messenger. Cell. 1992 Sep 4;70(5):705–707. doi: 10.1016/0092-8674(92)90301-r. [DOI] [PubMed] [Google Scholar]
- Marletta M. A. Nitric oxide synthase: aspects concerning structure and catalysis. Cell. 1994 Sep 23;78(6):927–930. doi: 10.1016/0092-8674(94)90268-2. [DOI] [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- Mayer B., John M., Heinzel B., Werner E. R., Wachter H., Schultz G., Böhme E. Brain nitric oxide synthase is a biopterin- and flavin-containing multi-functional oxido-reductase. FEBS Lett. 1991 Aug 19;288(1-2):187–191. doi: 10.1016/0014-5793(91)81031-3. [DOI] [PubMed] [Google Scholar]
- Moncada S., Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993 Dec 30;329(27):2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Nathan C. F., Hibbs J. B., Jr Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991 Feb;3(1):65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- Nathan C., Xie Q. W. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994 Sep 23;78(6):915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Nathan C., Xie Q. W. Regulation of biosynthesis of nitric oxide. J Biol Chem. 1994 May 13;269(19):13725–13728. [PubMed] [Google Scholar]
- Pou S., Pou W. S., Bredt D. S., Snyder S. H., Rosen G. M. Generation of superoxide by purified brain nitric oxide synthase. J Biol Chem. 1992 Dec 5;267(34):24173–24176. [PubMed] [Google Scholar]
- Radi R., Beckman J. S., Bush K. M., Freeman B. A. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991 Mar 5;266(7):4244–4250. [PubMed] [Google Scholar]
- Rosen G. M., Freeman B. A. Detection of superoxide generated by endothelial cells. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7269–7273. doi: 10.1073/pnas.81.23.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Hydroxyl radical generation by polymorphonuclear leukocytes measured by electron spin resonance spectroscopy. J Clin Invest. 1979 Dec;64(6):1725–1729. doi: 10.1172/JCI109637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinobu L. A., Jones S. G., Jones M. M. Sodium N-methyl-D-glucamine dithiocarbamate and cadmium intoxication. Acta Pharmacol Toxicol (Copenh) 1984 Mar;54(3):189–194. doi: 10.1111/j.1600-0773.1984.tb01916.x. [DOI] [PubMed] [Google Scholar]
- Zweier J. L., Broderick R., Kuppusamy P., Thompson-Gorman S., Lutty G. A. Determination of the mechanism of free radical generation in human aortic endothelial cells exposed to anoxia and reoxygenation. J Biol Chem. 1994 Sep 30;269(39):24156–24162. [PubMed] [Google Scholar]
- Zweier J. L., Kuppusamy P., Lutty G. A. Measurement of endothelial cell free radical generation: evidence for a central mechanism of free radical injury in postischemic tissues. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4046–4050. doi: 10.1073/pnas.85.11.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweier J. L., Wang P., Kuppusamy P. Direct measurement of nitric oxide generation in the ischemic heart using electron paramagnetic resonance spectroscopy. J Biol Chem. 1995 Jan 6;270(1):304–307. doi: 10.1074/jbc.270.1.304. [DOI] [PubMed] [Google Scholar]
- Zweier J. L., Wang P., Samouilov A., Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat Med. 1995 Aug;1(8):804–809. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]




