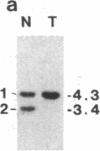
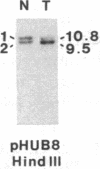
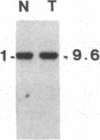
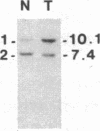
Abstract
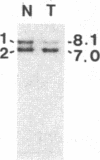
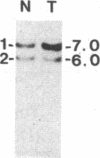
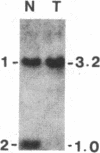
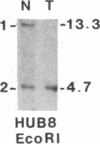
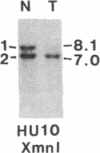
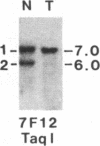
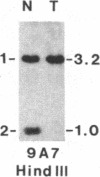
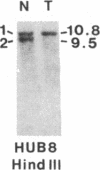
Survivors of the heritable form of retinoblastoma subsequently develop second primary osteosarcomas at substantially greater frequency than either the general population or survivors of nonheritable retinoblastoma. Here we present molecular genetic evidence that the development of these two disparate tumor types involves specific somatic loss of constitutional heterozygosity for the region of human chromosome 13 that includes the RB1 locus. Similar events occur during the genesis of nonheritable osteosarcoma but not in several other embryonal tumors or sarcomas. These findings suggest that a conceptual approach toward defining the number of genes whose recessive mutant forms predispose to cancer is the molecular genetic analysis of clinically associated tumor types. They also suggest that the molecular basis of mixed cancer families may be the differential expression of a single pleiotropic recessive mutation by tissue specific mitotic segregation abnormalities.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson D. H., Ellsworth R. M., Kitchin F. D., Tung G. Second nonocular tumors in retinoblastoma survivors. Are they radiation-induced? Ophthalmology. 1984 Nov;91(11):1351–1355. doi: 10.1016/s0161-6420(84)34127-6. [DOI] [PubMed] [Google Scholar]
- Abramson D. H., Ellsworth R. M., Zimmerman L. E. Nonocular cancer in retinoblastoma survivors. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1976 May-Jun;81(3 Pt 1):454–457. [PubMed] [Google Scholar]
- Aherne G. Retinoblastoma associated with other primary malignant tumours. Trans Ophthalmol Soc U K. 1974;94(4):938–944. [PubMed] [Google Scholar]
- Balaban G., Gilbert F., Nichols W., Meadows A. T., Shields J. Abnormalities of chromosome #13 in retinoblastomas from individuals with normal constitutional karyotypes. Cancer Genet Cytogenet. 1982 Jul;6(3):213–221. doi: 10.1016/0165-4608(82)90058-9. [DOI] [PubMed] [Google Scholar]
- Barker D., Holm T., White R. A locus on chromosome 11p with multiple restriction site polymorphisms. Am J Hum Genet. 1984 Nov;36(6):1159–1171. [PMC free article] [PubMed] [Google Scholar]
- Barker D., Schafer M., White R. Restriction sites containing CpG show a higher frequency of polymorphism in human DNA. Cell. 1984 Jan;36(1):131–138. doi: 10.1016/0092-8674(84)90081-3. [DOI] [PubMed] [Google Scholar]
- Benedict W. F., Murphree A. L., Banerjee A., Spina C. A., Sparkes M. C., Sparkes R. S. Patient with 13 chromosome deletion: evidence that the retinoblastoma gene is a recessive cancer gene. Science. 1983 Feb 25;219(4587):973–975. doi: 10.1126/science.6336308. [DOI] [PubMed] [Google Scholar]
- Berg J. W., Schottenfeld D. Multiple primary cancers at Memorial Hospital 1949-1962. Cancer. 1977 Oct;40(4 Suppl):1801–1805. doi: 10.1002/1097-0142(197710)40:4+<1801::aid-cncr2820400805>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- Cavenee W. K., Hansen M. F., Nordenskjold M., Kock E., Maumenee I., Squire J. A., Phillips R. A., Gallie B. L. Genetic origin of mutations predisposing to retinoblastoma. Science. 1985 Apr 26;228(4698):501–503. doi: 10.1126/science.3983638. [DOI] [PubMed] [Google Scholar]
- Cavenee W., Leach R., Mohandas T., Pearson P., White R. Isolation and regional localization of DNA segments revealing polymorphic loci from human chromosome 13. Am J Hum Genet. 1984 Jan;36(1):10–24. [PMC free article] [PubMed] [Google Scholar]
- Dracopoli N. C., Houghton A. N., Old L. J. Loss of polymorphic restriction fragments in malignant melanoma: implications for tumor heterogeneity. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1470–1474. doi: 10.1073/pnas.82.5.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryja T. P., Cavenee W., White R., Rapaport J. M., Petersen R., Albert D. M., Bruns G. A. Homozygosity of chromosome 13 in retinoblastoma. N Engl J Med. 1984 Mar 1;310(9):550–553. doi: 10.1056/NEJM198403013100902. [DOI] [PubMed] [Google Scholar]
- Dryja T. P., Rapaport J. M., Weichselbaum R., Bruns G. A. Chromosome 13 restriction fragment length polymorphisms. Hum Genet. 1984;65(4):320–324. doi: 10.1007/BF00291555. [DOI] [PubMed] [Google Scholar]
- François J. Retinoblastoma and osteogenic sarcoma. Ophthalmologica. 1977;175(4):185–191. doi: 10.1159/000308656. [DOI] [PubMed] [Google Scholar]
- François J., de Sutter E., Coppieters R., de Bie S. Late extraocular tumours in retinoblastoma survivors. Ophthalmologica. 1980;181(2):93–99. doi: 10.1159/000309033. [DOI] [PubMed] [Google Scholar]
- Godbout R., Dryja T. P., Squire J., Gallie B. L., Phillips R. A. Somatic inactivation of genes on chromosome 13 is a common event in retinoblastoma. Nature. 1983 Aug 4;304(5925):451–453. doi: 10.1038/304451a0. [DOI] [PubMed] [Google Scholar]
- Goldfarb M., Shimizu K., Perucho M., Wigler M. Isolation and preliminary characterization of a human transforming gene from T24 bladder carcinoma cells. Nature. 1982 Apr 1;296(5856):404–409. doi: 10.1038/296404a0. [DOI] [PubMed] [Google Scholar]
- Gordon H. Family studies in retinoblastoma. Birth Defects Orig Artic Ser. 1974;10(10):185–190. [PubMed] [Google Scholar]
- Jensen R. D., Miller R. W. Retinoblastoma: epidemiologic characteristics. N Engl J Med. 1971 Aug 5;285(6):307–311. doi: 10.1056/NEJM197108052850602. [DOI] [PubMed] [Google Scholar]
- Kitchin F. D., Ellsworth R. M. Pleiotropic effects of the gene for retinoblastoma. J Med Genet. 1974 Sep;11(3):244–246. doi: 10.1136/jmg.11.3.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson A. G., Jr, Hethcote H. W., Brown B. W. Mutation and childhood cancer: a probabilistic model for the incidence of retinoblastoma. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5116–5120. doi: 10.1073/pnas.72.12.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson A. G., Jr, Meadows A. T., Nichols W. W., Hill R. Chromosomal deletion and retinoblastoma. N Engl J Med. 1976 Nov 11;295(20):1120–1123. doi: 10.1056/NEJM197611112952007. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971 Apr;68(4):820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson A. G., Jr, Strong L. C., Anderson D. E. Heredity and cancer in man. Prog Med Genet. 1973;9:113–158. [PubMed] [Google Scholar]
- Koufos A., Hansen M. F., Lampkin B. C., Workman M. L., Copeland N. G., Jenkins N. A., Cavenee W. K. Loss of alleles at loci on human chromosome 11 during genesis of Wilms' tumour. Nature. 1984 May 10;309(5964):170–172. doi: 10.1038/309170a0. [DOI] [PubMed] [Google Scholar]
- Newell G. R., Krementz E. T. Multiple malignant neoplasms in the Charity Hospital of Louisiana Tumor Registry. Cancer. 1977 Oct;40(4 Suppl):1812–1820. doi: 10.1002/1097-0142(197710)40:4+<1812::aid-cncr2820400807>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Schappert-Kimmijser J., Hemmes G. D., Nijland R. The heredity of retinoblastoma. Ophthalmologica. 1966;151(2):197–213. doi: 10.1159/000304891. [DOI] [PubMed] [Google Scholar]
- Schimke R. N., Lowman J. T., Cowan A. B. Retinoblastoma and osteogenic sarcoma in siblings. Cancer. 1974 Dec;34(6):2077–2079. doi: 10.1002/1097-0142(197412)34:6<2077::aid-cncr2820340630>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Schoenberg B. S., Myers M. H. Statistical methods for studying multiple primary malignant neoplasms. Cancer. 1977 Oct;40(4 Suppl):1892–1898. doi: 10.1002/1097-0142(197710)40:4+<1892::aid-cncr2820400820>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Skolnick M. H., Willard H. F., Menlove L. A. Report of the Committee on Human Gene Mapping by Recombinant DNA Techniques. Cytogenet Cell Genet. 1984;37(1-4):210–273. doi: 10.1159/000132011. [DOI] [PubMed] [Google Scholar]
- Sorsby A. Bilateral retinoblastoma: a dominantly inherited affection. Br Med J. 1972 Jun 3;2(5813):580–583. doi: 10.1136/bmj.2.5813.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkes R. S., Murphree A. L., Lingua R. W., Sparkes M. C., Field L. L., Funderburk S. J., Benedict W. F. Gene for hereditary retinoblastoma assigned to human chromosome 13 by linkage to esterase D. Science. 1983 Feb 25;219(4587):971–973. doi: 10.1126/science.6823558. [DOI] [PubMed] [Google Scholar]
- Spratt J. S., Jr Multiple primary cancers: review of clinical studies from two Missouri hospitals. Cancer. 1977 Oct;40(4 Suppl):1806–1811. doi: 10.1002/1097-0142(197710)40:4+<1806::aid-cncr2820400806>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Yunis J. J., Ramsay N. Retinoblastoma and subband deletion of chromosome 13. Am J Dis Child. 1978 Feb;132(2):161–163. doi: 10.1001/archpedi.1978.02120270059012. [DOI] [PubMed] [Google Scholar]