Abstract
Galectin-3 (Gal-3) is a multifunctional protein involved in cancer through regulation of cell adhesion, cell growth, apoptosis, and metastasis, while p21 (Cip1/WAF1) is a negative regulator of the cell cycle, involved in apoptosis, transcription, DNA repair and metastasis. The results presented here demonstrate for the first time that the level of Gal-3 protein is associated with the level of p21 protein expression in human prostate cancer cells and the effects of Gal-3 on cell growth and apoptosis were reversed by modulating p21 expression level. Furthermore, Gal-3 regulates p21 expression at the post-translational level by stabilizing p21 protein via the carbohydrate-recognition domain (CRD). This is the first report suggesting a molecular function not yet described for Gal-3 as the regulator of p21 protein stability. This study provides a unique insight into the relationship of these two molecules during prostate cancer progression, and may provide a novel therapeutic target.
Keywords: galectin-3, p21, stability, CRD
Introduction
Gal-3, a carbohydrate-binding protein, consists of three structural domains: an NH2-terminal domain, repeated collagen-like sequence, and COOH-terminal containing a single CRD (1). Gal-3 exerts multiple functions through CRD binding to the carbohydrate portion of glycoproteins and glycolipids. Literature suggests that the alteration of Gal-3 expression is associated with the malignant transformation and progression of many kinds of human tumors (2–4). In prostate cancer, Gal-3 expression may be decreased compared with normal prostate and prostatic intraepithelial neoplasia (5). Using differential immunohistochemistry, we found that, although the expression level of intact Gal-3 decreased, its cleavage by matrix metalloproteinases (MMPs) is associated with the progression of prostate cancer (6). It has been demonstrated that Gal-3 plays important roles in the development and progression of various tumors through regulating cell growth, apoptosis, and metastasis (7, 8).
p21, also known as cyclin-dependent kinase (CDK) inhibitor 1 or CDK-interacting protein 1, is encoded by the CDKN1A gene located on chromosome 6 (9). It was originally described as a potent negative regulator of cell cycle progression by inhibiting the activity of cyclin/CDK complex (10). In addition, p21 has now been recognized to be involved in apoptosis (11), transcription (12), and DNA repair (13).
Previously, Lin et al., (7) reported that genistein induced p21 expression in Gal-3 transfected BT549 cells (human breast cancer cell line), but not in the control Gal-3 null BT549 cells, moreover, genistein induced apoptosis in control BT549 cells without directly affecting cell cycle arrest, whereas, Gal-3 transfected BT549 cells responded to genistein by cell cycle arrest without apoptosis. p21 protein expression was up-regulated by the Gal-3 specific inhibitor modified citrus pectin (MCP/GCS-100) along with G1 arrest and apoptosis in myeloma cells (14). Based on the above, we examined whether p21 protein expression is regulated by Gal-3 to exert related functions. In the present study, we have demonstrated that in human prostate cancer cells the expression level of Gal-3 protein is associated with that of p21 protein. p21 partially mediates the effects of Gal-3 on cell growth and apoptosis, while Gal-3 stabilizes p21 protein via its CRD. Thus, this study reports an undescribed function of Gal-3 and may assist in better understanding of the molecular mechanisms of Gal-3 actions in relation to p21, and may provide a new insight into the relationship between Gal-3 and p21 during human prostate cancer progression.
Results
Gal-3 regulates the expression of p21 in human prostate cancer cells
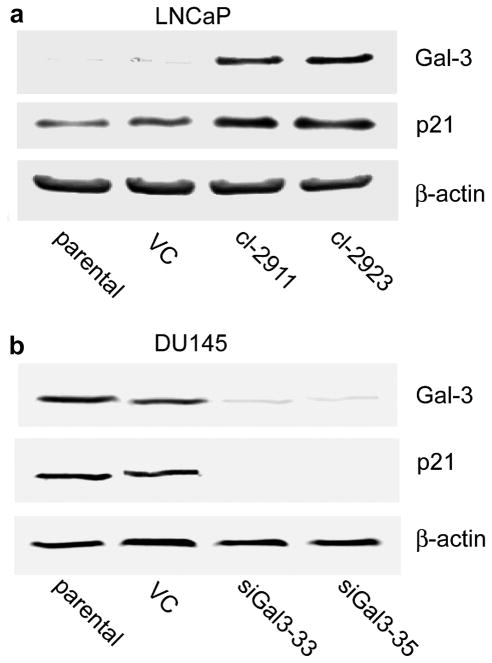
To study the possible effect of Gal-3 on p21 protein expression, two prostate cancer cell lines LNCaP (Gal-3 null) and DU145 (Gal-3 expressing) were used. Gal-3 over-expressing LNCaP and Gal-3 knockdown DU145 cell clones were established as described in Materials and Methods. As shown in Figure 1, compared to control cells, Gal-3 over-expressing LNCaP cells exhibited higher expression levels of p21 protein, while Gal-3 knockdown DU145 cells displayed markedly decreased expression of p21 protein, indicating that in human prostate cancer cells the expression of p21 can be regulated/associated with Gal-3 protein expression.
Figure 1.
The regulation of p21 expression by Gal-3 in human prostate cancer cells. The expression levels of Gal-3 and p21 were analyzed by Western blot analysis. (a) Gal-3 over-expression in LNCaP cells up-regulated the endogenous level of p21 protein. (b) Gal-3 knockdown in DU145 cells down-regulated the endogenous level of p21. β-actin was used as the loading control. Data are representative of three independent experiments.
Gal-3 functions are partially mediated by p21
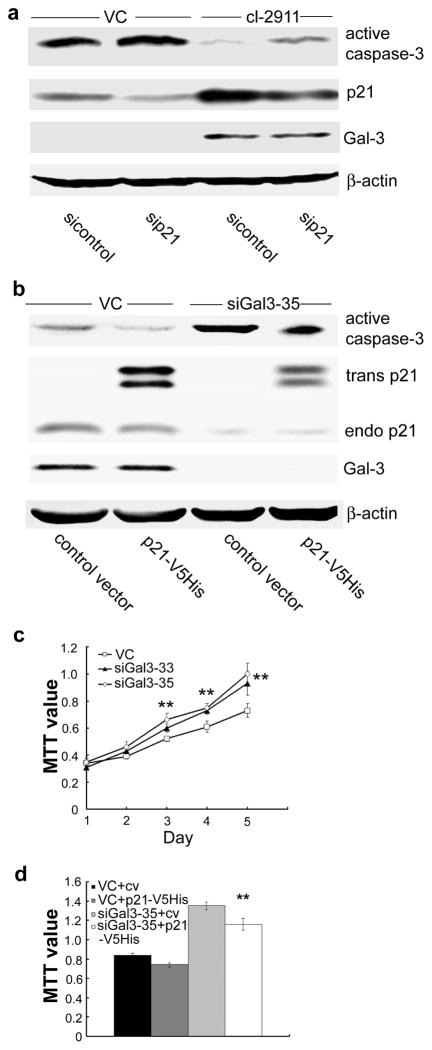
Since Gal-3 regulates the expression level of p21 protein, we presumed that elevated levels of p21 protein might in turn mediate Gal-3 associated functions. In LNCaP cells, Gal-3 protein over-expression resulted in a decrease in caspase-3 activation induced by cisplatin (indicating reduced apoptosis) (15). We show here that the inhibition of apoptosis could be reversed by p21 knockdown (Figure 2a). In DU145 cells, Gal-3 knockdown resulted in an increase of caspase-3 activation induced by cisplatin, the increased caspase-3 activation was attenuated by p21 over-expression (Figure 2b). Of note, neither p21 over-expression nor knockdown has altered Gal-3 expression. The results suggest that p21 mediates at least in part the anti-apoptotic function of Gal-3 in prostate cancer cells. In addition, we observed the effect of Gal-3 knockdown on the growth of DU145 cells. As shown in Figure 2c, Gal-3 knockdown DU145 cells grew faster than control cells. The accelerated growth of DU145 cells mediated by Gal-3 knockdown was slowed down by p21 over-expression (Figure 2d), which suggests that p21 also mediates the regulatory effect of Gal-3 on cell growth.
Figure 2.
p21 partially mediates the functions of Gal-3. The expression levels of active caspase-3, p21, Gal-3, and β-actin were analyzed by Western blot analysis. (a) Gal-3 over-expression-mediated inhibition of apoptosis was reversed by p21 knockdown. LNCaP VC and cl-2911 were transfected with p21 siRNA or control siRNA, 48 h later, cells were treated with cisplatin (50 μM) for 12 h. (b) Gal-3 knockdown-induced apoptosis was attenuated by p21 over-expression. DU145 VC and siGal3-35 were transfected with pcDNA 6.0-p21-V5His or control vector, 48 h later, cells were treated with cisplatin (50 μM) for 12 h. β-actin was used as the loading control. (c) Gal-3 knockdown led to increased cell growth of DU145. Cell growth was analyzed by MTT assay. **, P<0.01 vs. control vector. (d) Gal-3 knockdown-induced increase in cell growth of DU145 was slowed down by p21 over-expression. Cells were seeded into 96-well plate, the next day, transfected with pcDNA 6.0-p21-V5His or control vector, two days later, cell growth was analyzed by MTT assay. **, P<0.01 vs. siGal3-35 transfected with control vector. Data are representative of three independent experiments.
Regulation of p21 expression by Gal-3 at the post-translational level
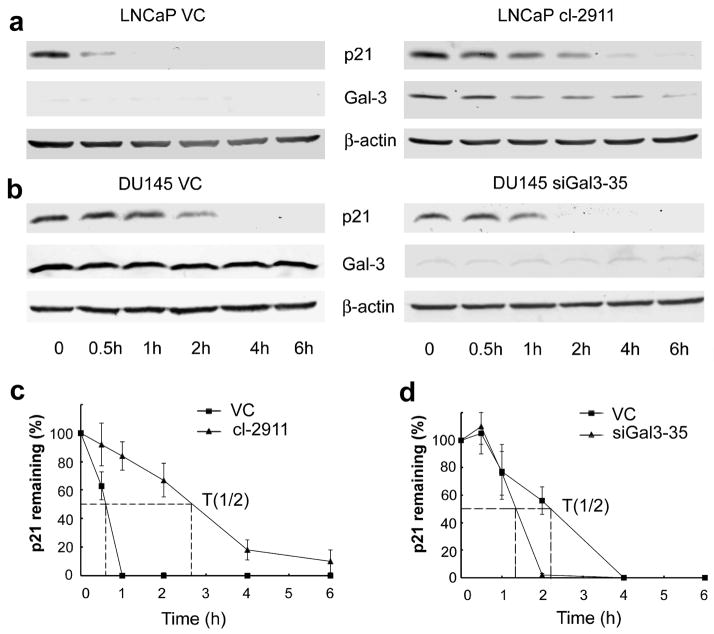
Next, we investigated how Gal-3 regulates the expression of p21 protein. p21 mRNA levels were evaluated by semi-quantitative PCR and quantitative PCR. The results did not show an obvious effect of Gal-3 on p21 mRNA levels in both LNCaP and DU145 cells (Data not shown), indicating that the regulation of p21 protein expression by Gal-3 does not occur at the transcriptional level. Next, we considered the possibility that the regulation might occur at the post-translational level. The stability of p21 was examined by Western Blot analysis of p21 in cells treated with protein translation inhibitor cycloheximide for appropriate times. Compared to control cells, Gal-3 over-expressing LNCaP cells displayed increased stability of p21 (Figure 3a), whereas, Gal-3 knockdown in DU145 cells led to decreased p21 stability (Figure 3b). The half-life of p21 was increased from less than 1 h in LNCaP control cells to more than 2 h in Gal-3 over-expressing LNCaP cells (Figure 3c). Gal-3 knockdown in DU145 cells decreased the half-life of p21 from more than 2 h in control cells to about 1.3 h (Figure 3d).
Figure 3.
Gal-3 enhances the stability of p21. (a) Gal-3 over-expression in LNCaP cells increased the stability of p21. (b) Gal-3 knockdown in DU145 cells decreased the stability of p21. Cells were treated with cycloheximide (50 μg/ml) for indicated times, collected and subjected to Western blot analysis. β-actin was used as the loading control. Data are representative of three independent experiments. The graph of half-life of p21 in LNCaP (c) and DU145 (d) cells. p21 expression was quantified by densitometric analysis and normalized to β-actin. The relative abundance of p21 was represented as the percentage remaining relative to time zero.
The stabilization of p21 by Gal-3 is mediated by CRD
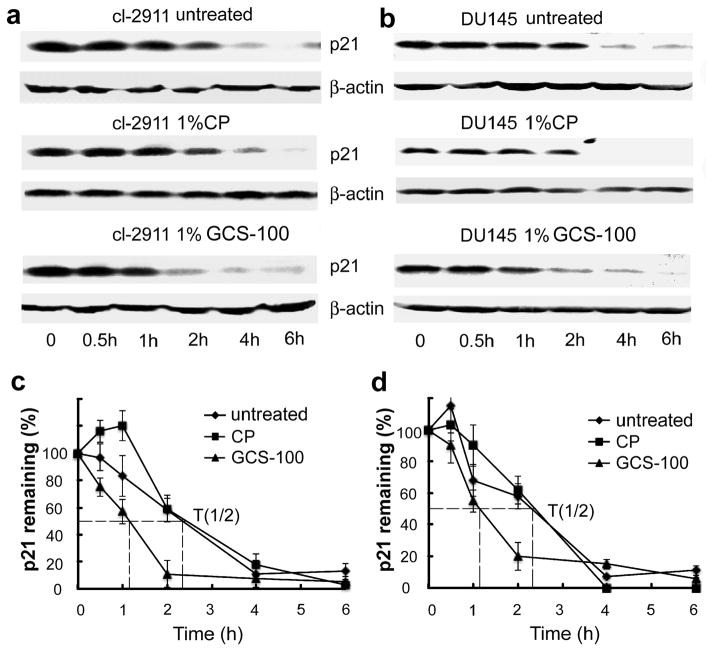
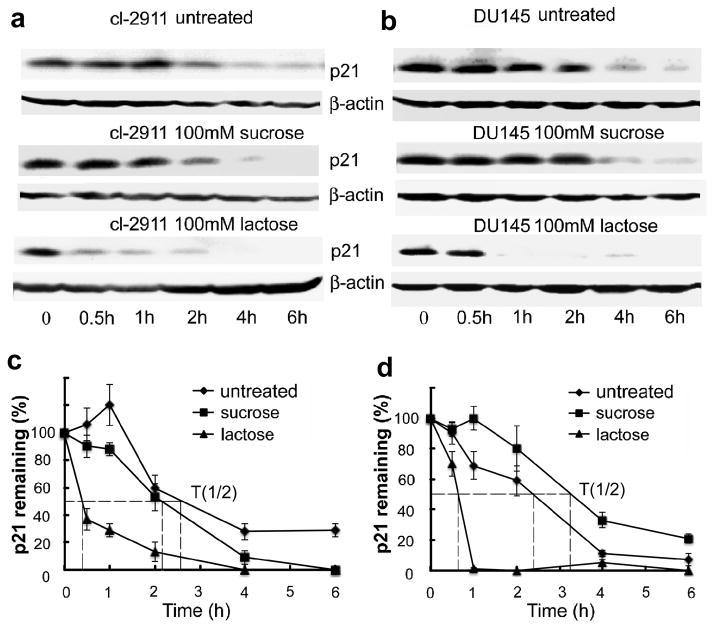
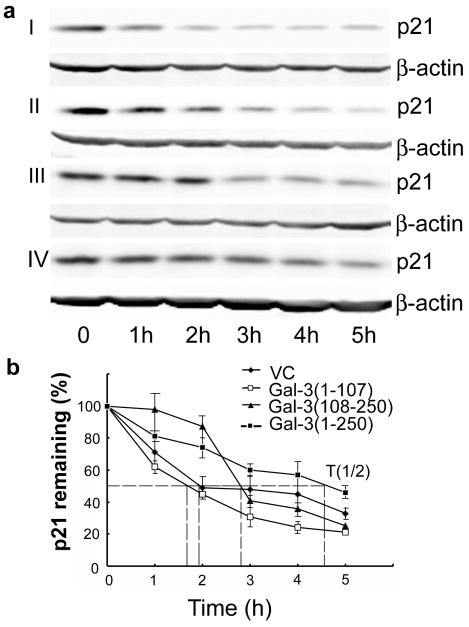
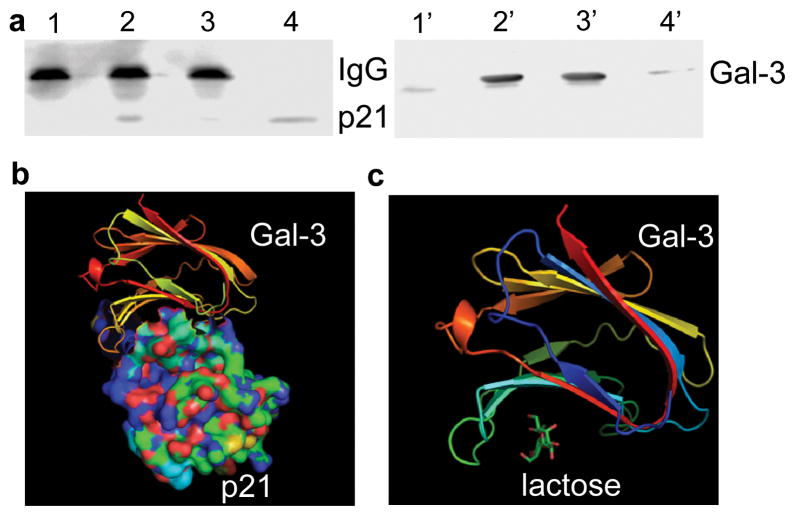
To investigate if p21 stability is mediated by CRD, GCS-100 and lactose were used to block Gal-3 functions by competitively binding to its CRD. As shown in Figure 4a and b, compared with untreated and CP treated cells, GCS-100 treated cells showed decreased stability of p21. To exclude the possibility of this effect resulting from GCS-100 binding to other lectins besides Gal-3, we treated Gal-3 null LNCaP parental cells with GCS-100, it did not decrease the stability of p21 in Gal-3 null LNCaP cells (data not shown), suggesting that GCS-100 binds to the CRD of Gal-3 to inhibit the effect of Gal-3 on p21 stability. The addition of GCS-100 decreased the half-life of p21 in LNCaP cl-2911 (Figure 4c) and DU145 cells (Figure 4d) from about 2.3 h to only about 1 h. Similarly, the addition of lactose also decreased the stability of p21 in both LNCaP (Figure 5a) and DU145 (Figure 5b) cells. The half-life of p21 in LNCaP cl-2911 cells was decreased by lactose to less than half an hour (Figure 5c), and that in DU145 cells was decreased by lactose to about 45 min (Figure 5d). Our results indicate that Gal-3 enhances the stability of p21 via the CRD domain. To further confirm our findings, we transfected C4-2B cells (Gal-3 null) with plasmids containing three different fragments of Gal-3 (1-107aa non-CRD portion, 108-250aa CRD portion, 1-250aa full length). Compared to C4-2B cells transfected with scramble vector, cells transfected with both Gal-3 (108-250) and full length displayed increased stability of p21, however, Gal-3 (1-107) over-expression had no obvious effect on p21 stability (Figure 6a). The half-life of p21 is about 2 h in control and Gal-3 (1-107) transfected cells, about 3 h in Gal-3 (108-250) transfected cells, and about 4.5 h in Gal-3 (1-250) transfected cells (Figure 6b). Co-IP assays have indicated recognition and binding of p21 to Gal-3 which was attenuated by a specific sugar inhibitor i.e. lactose (Figure 7a), implying that the binding site resides within the CRD motif of Gal-3. In silico docking analysis confirms the potential interaction of Gal-3 CRD with p21 protein (Figure 7b) or lactose (Figure 7c).
Figure 4.
The stabilization of p21 by Gal-3 is impaired by GCS-100. LNCaP Cl-2911 (a) and DU145 parental cells (b) were treated with 1% GCS-100 or 1%CP for 24 h, and treated with cycloheximide (50 μg/ml) for indicated times. Cells were harvested and subjected to Western blot analysis. Data are representative of three independent experiments. The graph of half-life of p21 in LNCaP cl-2911 (c) and DU145 cells (d) after GCS-100 treatment. p21 expression was quantified by densitometric analysis and normalized to β-actin. The relative abundance of p21 was represented as the percentage remaining relative to time zero.
Figure 5.
The stabilization of p21 by Gal-3 is impaired by lactose. LNCaP Cl-2911 (a) and DU145 parental cells (b) were treated with 100mM lactose or 100mM sucrose or remained untreated, 24 h later, treated with cycloheximide (50 μg/ml) for indicated times. Cells were harvested and subjected to Western blot analysis. β-actin was used as the loading control. Data are representative of three independent experiments. The graph of half-life of p21 in LNCaP cl-2911 (c) and DU145 (d) cells after lactose treatment. p21 expression was quantified by densitometric analysis and normalized to β-actin. The relative abundance of p21 was represented as the percentage remaining relative to time zero.
Figure 6.
The effects of different Gal-3 fragments on p21 stability. Gal-3 null C4-2B cells were transfected with plasmids containing three different Gal-3 fragments sequence (1-107aa, 108-250aa, and 1-250aa). (a) Gal-3 (108-250) and Gal-3 (1-250) stabilized p21 protein in C4-2B cells. Cells were treated with cycloheximide (50 μg/ml) for indicated times, collected and subjected to Western blot analysis. Compared to control transfectants (I), p21 stability was increased in Gal-3 (108-250) (III) and Gal-3 (1-250) (IV) transfected clones, but not in Gal-3 (1-107) (II) transfected clone. β-actin was used as the loading control. Data are representative of three independent experiments. (b) The graph of half-life of p21 in Gal-3 fragments transfected clones. p21 expression was quantified by densitometric analysis and normalized to β-actin. The relative abundance of p21 was represented as the percentage remaining relative to time zero.
Figure 7.
The interaction of p21 protein with the CRD of Gal-3. (a) Detection of the interaction of p21 with the CRD of Gal-3 by Co-IP. LNCaP cl-2911 cells were treated with 100 mM lactose or remained untreated for 24 h, collected and analyzed by Co-IP as described in Materials and Methods. 1-4, immunoblot for p21; 1′-4′, immunoblot for Gal-3; 1 and 1′, untreated cell lysate IP with normal rat IgG; 2 and 2′, untreated cell lysate IP with rat anti-Gal-3; 3 and 3′, lactose treated cell lysate IP with rat anti-Gal-3; 4 and 4′, untreated total cell lysate. Prediction of the interaction of CRD of Gal-3 with p21 (b) and lactose (c). The references about the structure of Gal-3 CRD, lactose, and p21 were indicated in Meterials and Methods. In silico docking was performed using PatchDock and FireDock online server http://bioinfo3d.cs.tau.ac.il/PatchDock/.
Discussion
The roles of Gal-3 in human cancer are well documented (16). According to previous reports, Gal-3 seems to have dual roles as both a tumor suppressor and an oncogene in tumorigenesis and progression depending on the types of tumors. Elevated expression level of Gal-3 is detected in malignant thyroid tumor (17) and colon cancer (18), whereas, compared to normal tissue, expression of intact Gal-3 decreases in breast (19), ovarian (20), and prostate cancer (5). The functions of Gal-3 involved in tumor progression include the regulation of cell growth, apoptosis, and metastasis. In a prostate cancer study, Gal-3 transfected LNCaP cells were found to proliferate at a slower rate than Gal-3 null control cells. Four of six Gal-3 transfected clones formed tumors in nude mice at a slower rate than control LNCaP cells (21). Gal-3 inhibition increases cisplatin-induced apoptosis of PC3 cells, and this effect is mediated at least in part by calpain activation (22).
A different protein i.e., p21 also exhibits both tumor-suppressive and oncogenic activities (23). In fact, the negative regulatory role of p21 in cell proliferation is counterbalanced by preventing drug-induced apoptosis in tumor cells (24, 25), and by promoting a metastatic potential (26). The anti- or pro-tumor activities of p21 may vary due to a tumor’s origin. The loss of p21 expression along with increased p53 is associated with poor prognosis of breast, gastric, and ovarian cancers (27–29). In prostate cancer, p21 over-expression associates with the worst clinical outcome before and after androgen deprivation therapy (30). It was reported that in response to genistein (7) or the loss of cell anchorage (31), Gal-3 transfected human breast cancer cells displayed cell cycle arrest and inhibited apoptosis, which involves up-regulation of p21. Our results showed that Gal-3 protein over-expression in LNCaP cells (p53 wild type) up-regulated the expression of p21, Gal-3 protein silencing in DU145 cells (p53 mutant) down-regulated p21 protein expression (Figure 1), which suggests that Gal-3 is the regulator of p21 expression in human prostate cancer cells. Interestingly, our previous study in another human prostate cancer cell line PC3 (p53 null) showed that Gal-3 knockdown in PC3 cells induced the expression of p21. The possible reason for this discrepancy is that Gal-3 might regulate p21 expression through different mechanisms in p53 null and p53 expressing prostate cancer cells.
Initially, p21 was described as a cell cycle regulator. Later, the implication of p21 in apoptosis was revealed. Previous reports indicate the contradictive effects of p21 on apoptosis: anti- and pro-apoptotic activities (24, 32), which might be influenced by several factors including the nature of the apoptotic stimuli (33), subcellular localization (34), and the extent of DNA damage (35). It was reported that human prostate cancer cell line LNCaP is resistant to docetaxel-induced apoptosis because docetaxel treatment results in the up-regulation of p53 and p21 proteins mediated by p38 phosphorylation, and that knockdown of p21 sensitizes LNCaP cells to docetaxel treatment (36). In the present study, we found that up-regulated p21 by Gal-3 partially mediated the anti-apoptotic function of Gal-3. In addition, we found that the effect of Gal-3 on cell growth was also mediated by p21. Our results suggest that p21 may act as the target of Gal-3 to execute its functions at least in part.
The expression of p21 can be regulated at both transcriptional and post-translational levels. The transcriptional regulation of p21 protein expression has been extensively studied. The transcription of CDKN1A gene can be activated through both p53-dependent and p53-independent mechanisms (37, 38). In the present study, we did not find obvious changes of p21 transcription after modulating Gal-3 expression. So, we assumed that Gal-3 might regulate the expression of p21 at post-translational level. The proteasome degradation of p21 can occur in both ubiquitin-dependent and –independent mechanisms (39, 40). The ubiquitin-dependent mechanisms have been described to occur via three E3 ubiquitin ligases, namely SCFSkp2, APC/CCdc20, and CRLCdt2 (39, 41, 42). p21 also can be degraded independently of ubiquitin by interaction of its C terminus with C8α subunit of 20S proteasome (43). Various factors and signaling molecules were reported to affect the stability of p21. For example, tumor growth factor (TGF)-β and bone morphogenetic protein 2 (BMP2) suppress the growth of human colon cancer cells partly owing to increased p21 stability, although the mechanism is poorly understood (44, 45). Our study showed that Gal-3 over-expression enhances the stability of p21 protein in human prostate cancer cells, whereas, Gal-3 knockdown leads to decreased stability of p21 (Figure 3), suggesting the regulation of p21 stability by Gal-3.
To investigate which fragment of Gal-3 mediates its effect on p21 stability, we used two Gal-3 inhibitors: GCS-100 and lactose to block CRD activity of Gal-3, then re-evaluated the effect of Gal-3 on p21 stability. Our results showed that compared to control, both Gal-3 inhibitors lead to a decrease in the stability of p21 protein expression associated with reduced half-life of p21 protein (Figure 4 and 5), suggesting that CRD is responsible for the effect of Gal-3 on p21 stability. To further confirm this, we transfected LNCaP-derivative C4-2B cells (Gal-3 null) with three different fragments of Gal-3: non-CRD portion (1-107aa), CRD portion (108-250aa), and the full length (1-250aa). The results showed that both full length Gal-3 and CRD of Gal-3 enhanced p21 stability, non-CRD portion had no obvious effect (Figure 6). Compared to the strong effect of full length, the effect of CRD is moderate, suggesting that even though the regulation of p21 stability by Gal-3 is mediated by CRD, the exhibition of most effect may also require the facilitation of non-CRD portion. Based on Co-IP assays and in silico docking analysis, the results indicated the binding of p21 protein is with the CRD of Gal-3 (Figure 7). Based on these data, we propose the following model for the mechanism by which Gal-3 regulates p21 in p53 expressing prostate cancer cells (Figure 8). The interaction between Gal-3 and p21 through CRD stabilizes p21 protein, which contributes to Gal-3 regulated functions such as cell growth, apoptosis, and so on. However, the mechanism for the regulation of p21 by Gal-3 in p53 null prostate cancer cells remains unclear and might be different. This study will help us to better understand the mechanisms of actions of Gal-3. Consideration of the relationship between Gal-3 and p21 will provide a novel therapeutic strategy.
Figure 8.
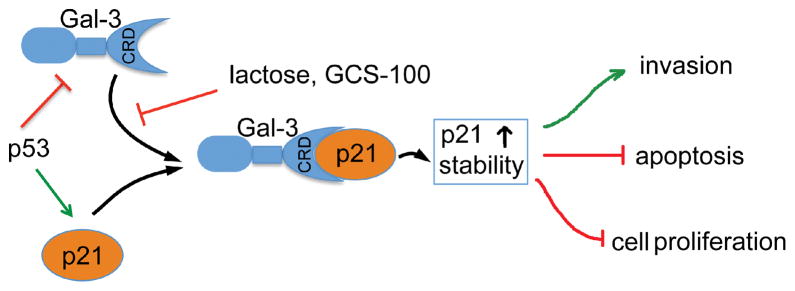
Proposed model for the regulation of p21 by Gal-3 in p53 expressing prostate cancer cells. Gal-3 binds to p21 through CRD, which can be blocked by sugars i.e. lactose or GCS-100. The interaction between Gal-3 and p21 promotes p21 stability, and the regulated p21 partially mediates Gal-3 functions associated with cell proliferation, apoptosis, and invasion. Acting as a transcription factor, p53 positively regulates p21 and negatively regulates Gal-3.
Materials and Methods
Antibodies
Monoclonal rat anti-Gal-3 antibody was isolated from the supernatant of hybridoma (catalog number: TIB-166, American Type Culture Collection, Manassas, VA, USA); Customized polyclonal rabbit anti-Gal-3 antibody was created by Invitrogen (Grand Island, NY, USA); mouse anti-p21 was purchased from BD Biosciences (San Jose, CA, USA); cisplatin, thiazolyl blue tetrazolium bromide (MTT), cycloheximide, mouse anti-β-actin was purchased from Sigma Chemicals (St. Louis, MO, USA); rabbit anti-caspase-3 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Citrus pectin (CP) was purchased from Sigma Chemicals and modified to MCP as previously described (22).
Cell culture
Human prostate cancer cells LNCaP (ATCC#CRL-1740) and DU145 (ATCC#HTB-81) were purchased from American Type Culture Collection. LNCaP-derivative C4-2B cells were purchased from Urocor Labs (Oklahoma City, OK, USA). These cell lines have been tested and authenticated by the supplier. Gal-3 over-expressing LNCaP clones, cl-2911 and cl-2923, and non-target control vector clone VC were kindly provided by Dr. Reuben Lotan (University of Texas MD Anderson cancer center, Houston, Texas, USA). Parental LNCaP, DU145, and C4-2B cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum. To maintain clones, 200 μg/ml G418 (Invitrogen) for LNCaP and DU145 transfectants, or 5 μg/ml blasticidin (Invitrogen) for C4-2B transfectants were added to the culture media.
Plasmids and transfection
shRNA expression plasmid targeting Gal-3 and control plasmid were previously described (6). Gal-3 shRNA oligonucleotides sequence is as follows: 5′-GATCCCGGGAAGAAAGACAGTCGGTTTCAAGAGAACCGACTGTCTTTCTTCC CTTTTTTGGAAA-3′. To construct pcDNA 6.0-p21-V5His, human p21 complete cDNA was amplified by polymerase chain reaction (PCR) using primers: 5′-GCTTGGATCCACCATGTCAGAACCGGCTGGG-3′ and 5′-CGTTCTCGAGGCGGGCTTCCTCTTGGAGAAGAT-3′. After sequencing, the PCR products were purified and cloned into pcDNA 6.0-V5His plasmid and verified by plasmid sequencing. Plasmids containing human Gal-3 fragments cDNA (1-107aa, 108-250aa, and 1-250aa) and scramble control were prepared as previously described (46). DU145 and C4-2B cells were transfected with appropriate plasmids using FuGENE HD transfection reagent (Roche Applied Science, Nutley, NJ, USA) according to the manufacturer’s instructions. Stable clones of DU145 cells were selected by G418, two Gal-3 knockdown clones, siGal3-33 and siGal3-35 were used for the following experiments. Stable clones of C4-2B cells were selected by blasticidin, clones over-expressing three Gal-3 fragments Gal-3 (1-107), Gal-3 (108-250), and Gal-3 (1-250) were used for the following experiments.
siRNA transfection
Custom siRNA duplex targeting human p21 (sense sequence as UGGCGGGCUGCAUCCAGGAUU) and non-target control siRNA (D-001810) were ordered from Thermo Scientific Dharmacon (Lafayette, CO, USA). siRNA transfection was performed using TransIT-siQUEST transfection reagent (Mirus, Madison, WI, USA) according to the manufacturer’s instructions.
Western blot analysis
Cells were lysed in RIPA buffer containing protease inhibitors (Roche Applied Science). Western blot was performed as previously described (17). The quantification of band intensity was performed using Gel-Pro Analyzer (Media Cybernetics, Bethesda, MD, USA).
Cell growth assay
Cell growth was examined by MTT assay. Briefly, cells were seeded at the density of 8000 cells/well into 96-well plate. At the time of assay, 0.5 mg/ml MTT in basic medium was added to each well and incubated for 4 h. After removing MTT, dimethyl sulfoxide (DMSO) was added and mixed vigorously. Absorbance was measured at 490 nm using a Vmax microplate reader (Molecular Devices, Sunnyvale, CA, USA).
In silico docking
Human p21 structure was predicted using I-TASSER online server (47). We used PatchDock web server http://bioinfo3d.cs.tau.ac.il/PatchDock/ to perform rigid-body docking of CRD domain of Gal-3 (1A3K) with lactose (48) or predicted structure of p21, FireDock method for flexible refinement of the accepted complexes (49, 50).
Co-immunoprecipitation (IP) assay
Cells were lysed in buffer (50 mM Tris-HCl pH 7.5, 100 mM NaCl, 0.5% NP-40) containing protease inhibitors (Roche Applied Science). Cell lysates (3 mg) were incubated with 12 μg of monoclonal rat anti-Gal-3 antibody or normal rat IgG and 50 μl of protein G Sepharose (GE Healthcare, Piscataway, NJ, USA) for 2 h at 4 °C. The beads were washed twice with 10 ml of lysis buffer, twice with 10 ml of lysis buffer containing 0.5 M LiCl, and twice with 10 ml of phosphate-buffered saline. Beads were boiled in 1 X sample buffer, subsequent supernatant was subjected to SDS-PAGE and immunoblotted for mouse anti-p21 and rabbit anti-Gal-3.
Statistical analysis
Data are expressed as mean±S.D. of three independent experiments and analyzed by one-way ANOVA test using SPSS 14.0 software (SPSS Incorporated, Chicago, IL, USA). P<0.05 was considered statistically significant.
Acknowledgments
Financial support: We thank Dr. Reuben Lotan for providing Gal-3 transfected LNCaP clones. This study was supported by NIH, National Institutes of Health grant R37CA46120-19 (A Raz) and the American Cancer Society grant 11-053-01-IRG (V Balan).
Financial support: American Cancer Society grant 11-053-01-IRG (to Vitaly Balan), and National Institutes of Health grant R37CA46120-19 (to Avraham Raz)
Footnotes
Conflicts of interest
The authors declare no conflict of interest.
References
- 1.Barondes SH, Cooper DN, Gitt MA, Leffler H. Galectins. Structure and function of a large family of animal lectins. J Biol Chem. 1994;269:20807–20810. [PubMed] [Google Scholar]
- 2.Feilchenfeldt J, Totsch M, Sheu SY, Robert J, Spiliopoulos A, Frilling A, et al. Expression of galectin-3 in normal and malignant thyroid tissue by quantitative PCR and immunohistochemistry. Mod Pathol. 2003;16:1117–1123. doi: 10.1097/01.MP.0000096047.99202.31. [DOI] [PubMed] [Google Scholar]
- 3.Puglisi F, Minisini AM, Barbone F, Intersimone D, Aprile G, Puppin C, et al. Galectin-3 expression in non-small cell lung carcinoma. Cancer Lett. 2004;212:233–239. doi: 10.1016/j.canlet.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Idikio H. Galectin-3 expression in human breast carcinoma: correlation with cancer histologic grade. Int J Oncol. 1998;12:1287–1290. doi: 10.3892/ijo.12.6.1287. [DOI] [PubMed] [Google Scholar]
- 5.Merseburger AS, Kramer MW, Hennenlotter J, Simon P, Knapp J, Hartmann JT, et al. Involvement of decreased Galectin-3 expression in the pathogenesis and progression of prostate cancer. Prostate. 2008;68:72–77. doi: 10.1002/pros.20688. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Nangia-Makker P, Tait L, Balan V, Hogan V, Pienta KJ, et al. Regulation of prostate cancer progression by galectin-3. Am J Pathol. 2009;174:1515–1523. doi: 10.2353/ajpath.2009.080816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin HM, Moon BK, Yu F, Kim HR. Galectin-3 mediates genistein-induced G(2)/M arrest and inhibits apoptosis. Carcinogenesis. 2000;21:1941–1945. doi: 10.1093/carcin/21.11.1941. [DOI] [PubMed] [Google Scholar]
- 8.Yang LP, Jiang S, Liu JQ, Miao XY, Yang ZL. Association of Immunostaining of Galectin-3 and Sambucus nigra Agglutinin with Invasion, Metastasis and Poor Progression of Gallbladder Adenocarcinoma. Hepatogastroenterology. 2012:59. doi: 10.5754/hge12129. [DOI] [PubMed] [Google Scholar]
- 9.Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 10.Brugarolas J, Moberg K, Boyd SD, Taya Y, Jacks T, Lees JA. Inhibition of cyclin-dependent kinase 2 by p21 is necessary for retinoblastoma protein-mediated G1 arrest after gamma-irradiation. Proc Natl Acad Sci U S A. 1999;96:1002–1007. doi: 10.1073/pnas.96.3.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garner E, Raj K. Protective mechanisms of p53-p21-pRb proteins against DNA damage-induced cell death. Cell Cycle. 2008;7:277–282. doi: 10.4161/cc.7.3.5328. [DOI] [PubMed] [Google Scholar]
- 12.Coqueret O. New roles for p21 and p27 cell-cycle inhibitors: a function for each cell compartment? Trends Cell Biol. 2003;13:65–70. doi: 10.1016/s0962-8924(02)00043-0. [DOI] [PubMed] [Google Scholar]
- 13.Podust VN, Podust LM, Goubin F, Ducommun B, Hubscher U. Mechanism of inhibition of proliferating cell nuclear antigen-dependent DNA synthesis by the cyclin-dependent kinase inhibitor p21. Biochemistry. 1995;34:8869–8875. doi: 10.1021/bi00027a039. [DOI] [PubMed] [Google Scholar]
- 14.Streetly MJ, Maharaj L, Joel S, Schey SA, Gribben JG, Cotter FE. GCS-100, a novel galectin-3 antagonist, modulates MCL-1, NOXA, and cell cycle to induce myeloma cell death. Blood. 2010;115:3939–3948. doi: 10.1182/blood-2009-10-251660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 16.Califice S, Castronovo V, Van Den Brule F. Galectin-3 and cancer (Review) Int J Oncol. 2004;25:983–992. [PubMed] [Google Scholar]
- 17.Bartolazzi A, D’Alessandria C, Parisella MG, Signore A, Del Prete F, Lavra L, et al. Thyroid cancer imaging in vivo by targeting the anti-apoptotic molecule galectin-3. PLoS One. 2008;3:e3768. doi: 10.1371/journal.pone.0003768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iacovazzi PA, Notarnicola M, Caruso MG, Guerra V, Frisullo S, Altomare DF. Serum levels of galectin-3 and its ligand 90k/mac-2bp in colorectal cancer patients. Immunopharmacol Immunotoxicol. 2010;32:160–164. doi: 10.1080/08923970902936880. [DOI] [PubMed] [Google Scholar]
- 19.Nangia-Makker P, Sarvis R, Visscher DW, Bailey-Penrod J, Raz A, Sarkar FH. Galectin-3 and L1 retrotransposons in human breast carcinomas. Breast Cancer Res Treat. 1998;49:171–183. doi: 10.1023/a:1005913810250. [DOI] [PubMed] [Google Scholar]
- 20.Brustmann H. Epidermal growth factor receptor expression in serous ovarian carcinoma: an immunohistochemical study with galectin-3 and cyclin D1 and outcome. Int J Gynecol Pathol. 2008;27:380–389. doi: 10.1097/PGP.0b013e31815d060d. [DOI] [PubMed] [Google Scholar]
- 21.Ellerhorst JA, Stephens LC, Nguyen T, Xu XC. Effects of galectin-3 expression on growth and tumorigenicity of the prostate cancer cell line LNCaP. Prostate. 2002;50:64–70. doi: 10.1002/pros.10033. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Nangia-Makker P, Balan V, Hogan V, Raz A. Calpain activation through galectin-3 inhibition sensitizes prostate cancer cells to cisplatin treatment. Cell Death Dis. 2010;1:e101. doi: 10.1038/cddis.2010.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer. 2009;9:400–414. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki A, Tsutomi Y, Miura M, Akahane K. Caspase 3 inactivation to suppress Fas-mediated apoptosis: identification of binding domain with p21 and ILP and inactivation machinery by p21. Oncogene. 1999;18:1239–1244. doi: 10.1038/sj.onc.1202409. [DOI] [PubMed] [Google Scholar]
- 25.Le HV, Minn AJ, Massague J. Cyclin-dependent kinase inhibitors uncouple cell cycle progression from mitochondrial apoptotic functions in DNA-damaged cancer cells. J Biol Chem. 2005;280:32018–32025. doi: 10.1074/jbc.M504689200. [DOI] [PubMed] [Google Scholar]
- 26.Stivala LA, Cazzalini O, Prosperi E. The cyclin-dependent kinase inhibitor p21CDKN1A as a target of anti-cancer drugs. Curr Cancer Drug Targets. 2012;12:85–96. doi: 10.2174/156800912799095126. [DOI] [PubMed] [Google Scholar]
- 27.Caffo O, Doglioni C, Veronese S, Bonzanini M, Marchetti A, Buttitta F, et al. Prognostic value of p21(WAF1) and p53 expression in breast carcinoma: an immunohistochemical study in 261 patients with long-term follow-up. Clin Cancer Res. 1996;2:1591–1599. [PubMed] [Google Scholar]
- 28.Anttila MA, Kosma VM, Hongxiu J, Puolakka J, Juhola M, Saarikoski S, et al. p21/WAF1 expression as related to p53, cell proliferation and prognosis in epithelial ovarian cancer. Br J Cancer. 1999;79:1870–1878. doi: 10.1038/sj.bjc.6690298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogawa M, Onoda N, Maeda K, Kato Y, Nakata B, Kang SM, et al. A combination analysis of p53 and p21 in gastric carcinoma as a strong indicator for prognosis. Int J Mol Med. 2001;7:479–483. doi: 10.3892/ijmm.7.5.479. [DOI] [PubMed] [Google Scholar]
- 30.Baretton GB, Klenk U, Diebold J, Schmeller N, Lohrs U. Proliferation- and apoptosis-associated factors in advanced prostatic carcinomas before and after androgen deprivation therapy: prognostic significance of p21/WAF1/CIP1 expression. Br J Cancer. 1999;80:546–555. doi: 10.1038/sj.bjc.6690390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim HR, Lin HM, Biliran H, Raz A. Cell cycle arrest and inhibition of anoikis by galectin-3 in human breast epithelial cells. Cancer Res. 1999;59:4148–4154. [PubMed] [Google Scholar]
- 32.Waldman T, Lengauer C, Kinzler KW, Vogelstein B. Uncoupling of S phase and mitosis induced by anticancer agents in cells lacking p21. Nature. 1996;381:713–716. doi: 10.1038/381713a0. [DOI] [PubMed] [Google Scholar]
- 33.Kraljevic Pavelic S, Cacev T, Kralj M. A dual role of p21waf1/cip1 gene in apoptosis of HEp-2 treated with cisplatin or methotrexate. Cancer Gene Ther. 2008;15:576–590. doi: 10.1038/cgt.2008.28. [DOI] [PubMed] [Google Scholar]
- 34.Blagosklonny MV. Are p27 and p21 cytoplasmic oncoproteins? Cell Cycle. 2002;1:391–393. doi: 10.4161/cc.1.6.262. [DOI] [PubMed] [Google Scholar]
- 35.Lee EW, Lee MS, Camus S, Ghim J, Yang MR, Oh W, et al. Differential regulation of p53 and p21 by MKRN1 E3 ligase controls cell cycle arrest and apoptosis. Embo J. 2009;28:2100–2113. doi: 10.1038/emboj.2009.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gan L, Wang J, Xu H, Yang X. Resistance to docetaxel-induced apoptosis in prostate cancer cells by p38/p53/p21 signaling. Prostate. 2011;71:1158–1166. doi: 10.1002/pros.21331. [DOI] [PubMed] [Google Scholar]
- 37.el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 38.Parker SB, Eichele G, Zhang P, Rawls A, Sands AT, Bradley A, et al. p53-independent expression of p21Cip1 in muscle and other terminally differentiating cells. Science. 1995;267:1024–1027. doi: 10.1126/science.7863329. [DOI] [PubMed] [Google Scholar]
- 39.Bornstein G, Bloom J, Sitry-Shevah D, Nakayama K, Pagano M, Hershko A. Role of the SCFSkp2 ubiquitin ligase in the degradation of p21Cip1 in S phase. J Biol Chem. 2003;278:25752–25757. doi: 10.1074/jbc.M301774200. [DOI] [PubMed] [Google Scholar]
- 40.Sheaff RJ, Singer JD, Swanger J, Smitherman M, Roberts JM, Clurman BE. Proteasomal turnover of p21Cip1 does not require p21Cip1 ubiquitination. Mol Cell. 2000;5:403–410. doi: 10.1016/s1097-2765(00)80435-9. [DOI] [PubMed] [Google Scholar]
- 41.Amador V, Ge S, Santamaria PG, Guardavaccaro D, Pagano M. APC/C(Cdc20) controls the ubiquitin-mediated degradation of p21 in prometaphase. Mol Cell. 2007;27:462–473. doi: 10.1016/j.molcel.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim Y, Starostina NG, Kipreos ET. The CRL4Cdt2 ubiquitin ligase targets the degradation of p21Cip1 to control replication licensing. Genes Dev. 2008;22:2507–2519. doi: 10.1101/gad.1703708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Touitou R, Richardson J, Bose S, Nakanishi M, Rivett J, Allday MJ. A degradation signal located in the C-terminus of p21WAF1/CIP1 is a binding site for the C8 alpha-subunit of the 20S proteasome. Embo J. 2001;20:2367–2375. doi: 10.1093/emboj/20.10.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gong J, Ammanamanchi S, Ko TC, Brattain MG. Transforming growth factor beta 1 increases the stability of p21/WAF1/CIP1 protein and inhibits CDK2 kinase activity in human colon carcinoma FET cells. Cancer Res. 2003;63:3340–3346. [PubMed] [Google Scholar]
- 45.Beck SE, Jung BH, Del Rosario E, Gomez J, Carethers JM. BMP-induced growth suppression in colon cancer cells is mediated by p21WAF1 stabilization and modulated by RAS/ERK. Cell Signal. 2007;19:1465–1472. doi: 10.1016/j.cellsig.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Balan V, Nangia-Makker P, Kho DH, Wang Y, Raz A. Tyrosine-phosphorylated galectin-3 protein is resistant to prostate-specific antigen (PSA) cleavage. J Biol Chem. 2012;287:5192–5198. doi: 10.1074/jbc.C111.331686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roy A, Kucukural A, Zhang Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc. 2010;5:725–738. doi: 10.1038/nprot.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seetharaman J, Kanigsberg A, Slaaby R, Leffler H, Barondes SH, Rini JM. X-ray crystal structure of the human galectin-3 carbohydrate recognition domain at 2. 1-A resolution. J Biol Chem. 1998;273:13047–13052. doi: 10.1074/jbc.273.21.13047. [DOI] [PubMed] [Google Scholar]
- 49.Schneidman-Duhovny D, Inbar Y, Nussinov R, Wolfson HJ. PatchDock and SymmDock: servers for rigid and symmetric docking. Nucleic Acids Res. 2005;33:W363–367. doi: 10.1093/nar/gki481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mashiach E, Schneidman-Duhovny D, Andrusier N, Nussinov R, Wolfson HJ. FireDock: a web server for fast interaction refinement in molecular docking. Nucleic Acids Res. 2008;36:W229–232. doi: 10.1093/nar/gkn186. [DOI] [PMC free article] [PubMed] [Google Scholar]