Abstract
Background
Excess cathelicidin and kallikrein 5 (KLK5) have been hypothesized to play a role in the pathophysiology of rosacea.
Objective
We sought to evaluate the effects of azelaic acid (AzA) on these elements of the innate immune system.
Methods
Gene expression and protease activity were measured in laboratory models and patients with rosacea during a 16-week multicenter, prospective, open-label study of 15% AzA gel.
Results
AzA directly inhibited KLK5 in cultured keratinocytes and gene expression of KLK5, Toll-like receptor-2, and cathelicidin in mouse skin. Patients with rosacea showed reduction in cathelicidin and KLK5 messenger RNA after treatment with AzA gel. Subjects without rosacea had lower serine protease activity (SPA) than patients with rosacea. Distinct subsets of patients with rosacea who had high and low baseline SPA were identified, and patients with high baseline exhibited a statistically significant reduction of SPA with 15% AzA gel treatment.
Limitations
Study size was insufficient to predict clinical efficacy based on the innate immune response to AzA.
Conclusions
These results show that cathelicidin and KLK5 decrease in association with AZA exposure. Our observations suggest a new mechanism of action for AzA and that SPA may be a useful biomarker for disease activity.
Keywords: antimicrobial peptides, azelaic acid, cathelicidin, kallikrein 5, LL-37, rosacea, serine protease
Rosacea is a chronic, inflammatory dermatosis that is characterized predominantly by persistent centrofacial erythema that flares episodically in intensity, and by telangiectasia. In addition, inflammatory papules and pustules, skin sensitivity, and associated symptomatology such as facial stinging and burning are also present. Several factors exacerbate rosacea symptoms; these include emotional stress, spicy food, hot beverages, alcohol consumption, high temperatures, sun exposure, and menopause. Given these diverse findings, a specific cause for rosacea has not been identified. Currently, a variety of therapeutic approaches based on empiric observations of improvement are used to treat patients given the diagnosis of this disorder.1–4
Several theories have recently emerged regarding the pathogenesis of rosacea, including neurovascular changes, stimulation by various microbes, and the concomitant abnormal function of innate immunity in the skin.5 One pathophysiologic factor that appears to unify these observations is the increased expression of the cathelicidin antimicrobial peptide gene (CAMP) in rosacea. Facial skin of patients with rosacea produces abnormally high levels of CAMP expressed in the peptide form of LL-37. In addition to its direct antimicrobial effects,6 LL-37 has the capacity to be both proinflammatory and angiogenic,7,8 properties not shared by other forms of active cathelicidin in healthy skin. Furthermore, data from mouse and human studies implicate the stratum corneum tryptic enzyme, kallikrein 5 (KLK5), as the enzyme responsible for posttranslational processing of cathelicidin and the resultant aberrant production of LL-37.9 In addition, increased expression of the pattern recognition receptor, TLR2, has been identified in rosacea, further supporting participation of augmented innate immune detection and response in the pathophysiology of this disorder.10 This model helps to explain the clinical heterogeneity of rosacea and permits direct testing to determine why some of our current therapies are effective in reducing at least some of the signs and symptoms of the disorder.
There are many treatment options currently available for rosacea, however it is not fully understood how each medication mediates its therapeutic effects. Clinical studies have shown that azelaic acid (AzA) 15% gel is a topical therapeutic option in patients with papulopustular rosacea.11 Many studies have investigated the pharmacologic mechanisms of AzA. These include inhibition of microbial survival, modulation of epidermal differentiation, and inhibition of reactive oxygen species.12 Although reactive oxygen species has been suggested to contribute to rosacea pathogenesis,13 we postulated that reactive oxygen species inhibition is not the sole mechanism of therapeutic effect.
In this study we investigated whether AzA could influence the expression and activity of KLK5 and CAMP by evaluating the response of normal human keratinocytes, and topical application of AzA gel to mice and during clinical treatment of patients with rosacea. We show evidence for the first time to our knowledge confirming that serine protease activity (SPA) is elevated in rosacea and show information supporting the hypothesis that the therapeutic action of AzA may include inhibition of the cathelicidin and KLK5 pathway.
METHODS
Patients
This study was designed as a multicenter study at University of California–San Diego; University of Alabama, Birmingham; and Valley Hospital Medical Center, Las Vegas, NV. After approval by the Human Research Protection Program at the University of California–San Diego (institutional review board reference number 100473), and upon receiving written informed consent before sampling, 60 adult individuals (age 18–80 years) with at least mild rosacea were enrolled and 55 of those completed the study. Each patient was instructed to apply AzA 15% gel to his or her involved skin twice daily for 16 weeks. Tape-strip samplings were taken at baseline and every 4 weeks (±7 days) until completion of the study.
AzA preparation (used in the in vitro study with human keratinocytes)
AzA powder was dissolved in dimethyl sulfoxide (DMSO) at 1 mol/L. A total of 1 mol/L AzA in DMSO was further diluted in DMSO and added to culture media at volume-to-volume to final. DMSO was added to control cultures at concentrations identical to that in AzA-treated samples.
In vitro cell stimulation and sample collection
Normal human epidermal keratinocytes were grown in EpiLife (Cascade/Invitrogen, Carlsbad, CA). Low-calcium basal media consisted of EpiLife (Cascade/Invitrogen) with 0.06-mmol/L calcuim cloride (CaCl2) to maintain keratinocytes in undifferentiated and monolayer conditions. High-calcium differentiation media consisted of EpiLife (Cascade/Invitrogen, Carlsbad, CA) with 1.0-mmol/L CaCl2 for 2 days plus 1.6-mmol/L CaCl2 for 2 days. After normal human epidermal keratinocytes reached confluence, the media were changed to either fresh high- or low-calcium basal media (as described above), and treated with AzA. Culture media were collected at 48 hours and cells restimulated with AzA in fresh media. Cultured media were collected at 96 hours and RNA extracted using TRIzol (Invitrogen, Carlsbad, CA). The purity and concentration of the final RNA product was analyzed by Nanodrop 100 spectrophotometry (Thermo Scientific, Waltham, MA).
Mouse skin stimulation with application of 15% AzA gel and sampling
On day 1, mouse (Balb/c, 9 weeks old, both female and male) backs were shaved and depilatory cream (Nair, Church and Dwight Co Inc, Princeton, NJ) applied for 3 minutes. On days 2 through 9, 200 μL of AzA gel 15% was applied topically to the skin on the right half of the back. The left half of the back was treated with control vehicle gel. This procedure was repeated once a day for 9 days.
Clinical assessments
Investigator Global Assessment (IGA) and Clinician Erythema Assessment (CEA) scales were both applied as in earlier studies.14
Tape-strip sampling and assessments
Four sites were selected from involved skin on the face (ie, cheeks, nose, glabella). Tape stripping was performed by firmly applying a (D-Squame D100, CuDerm, Dallas, TX) 1- × 1-cm2 tape for 10 seconds to each site, then repeating this 10 times with the same tape. This process was repeated with a total of 4 tapes at each site. Tape-strip samples were stored in a −80°C freezer until processing. Total RNA was isolated from tape-strips samples using TRIzol reagent (Invitrogen, Carlsbad, CA) per manufacturer’s protocol. RNA was then purified using the RNeasy Mini kit (Qiagen, Valencia, CA). After purifying steps, RNA was eluted with RNase-free water. The purity and concentration of the final RNA product was analyzed by Nanodrop 100 spectrophotometry (Thermo Scientific).
Protease activity fluorescence polarization assays
Two tapes from each visit were used for protease activity assay. Total protease activities were determined with the EnzChek protease assay kit (Invitrogen, Carlsbad, CA) according to the manufacturer’s instruction.
Data analysis
Experiments in keratinocytes and mice were performed at least 3 times, and the data are presented as mean ± SEM. Data from 6 patients were considered to be significant outliers by Grubbs test, also called the extreme studentized deviate method, and thus were not included in the final data set. Grubbs test detects 1 outlier at a time. This outlier is expunged from the data set and the test is iterated until no outliers are detected.15 To determine statistical significance between groups, comparisons were made using Friedman tests (analysis of variance) and linear regressions from GraphPad Prism (GraphPad Software Inc, La Jolla, CA), as appropriate. For all statistical tests, a P value of .05 was accepted for statistical significance.
RESULTS
AzA inhibits KLK5 protein expression in cultured human keratinocytes
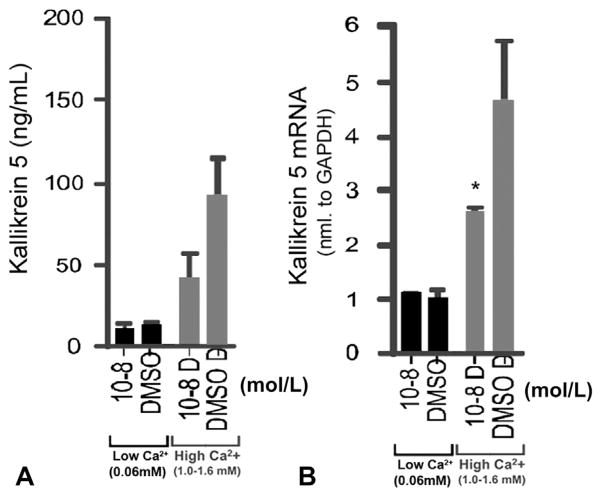
To evaluate the effects of AzA on the production of KLK5 in cultured keratinocytes, we compared KLK5 messenger RNA (mRNA) and protein expression in normal human epidermal keratinocytes treated for 24 hours with AzA at concentrations of 10−2 to 10−13. Protein expression of KLK5 was inhibited by AzA at concentrations of 10−8 mol/L, but only when keratinocytes were grown in high-calcium concentrations (Fig 1, A). There was also a corresponding decrease in KLK5 mRNA expression as measured by quantitative real-time polymerase chain reaction (Fig 1, B). Expression of mRNA for the human cathelicidin gene (CAMP) did not change after treatment with AzA. High concentration (10−2 mol/L) of AzA was toxic to cultured keratinocytes in both basal and high-calcium media (data not shown).
Fig 1.
Azelaic acid (AzA) inhibits kallikrein 5 (KLK5) expression in differentiated keratinocytes. A, KLK5 protein expression as measured by enzyme-linked immunosorbent assay in keratinocytes grown in basal (low-calcium) medium or differentiated in high-calcium culture conditions. B, KLK5 messenger RNA (mRNA) expression measured by quantitative real-time polymerase chain reaction in both basal and high-calcium growth conditions. AZA cells treated with AzA at 10−8 mol/L. Control cells treated with vehicle (dimethyl sulfoxide [DMSO]) only. *P <.05. Ca2+, Calcium.
15% AzA gel inhibits KLK5 mRNA expression in mouse skin
We next examined the effects of AzA by applying 200 μL of 15% AzA gel, or vehicle control, to depilated mouse back skin for a total of 9 days. Mouse skin treated with 15% AzA gel had a significant decrease in Klk5 mRNA compared with control (P = .0192). A trend toward a decrease in expression of Camp was also seen, although this decrease was not statistically significant (Fig 2). Furthermore, because recent observations have suggested abnormal TLR2 function may contribute to rosacea pathogenesis,10 the expression of Tlr2 mRNA was also measured. A small decrease in Tlr2 was observed after AzA but this did not reach significance in this study (P = .0683) (Fig 2).
Fig 2.
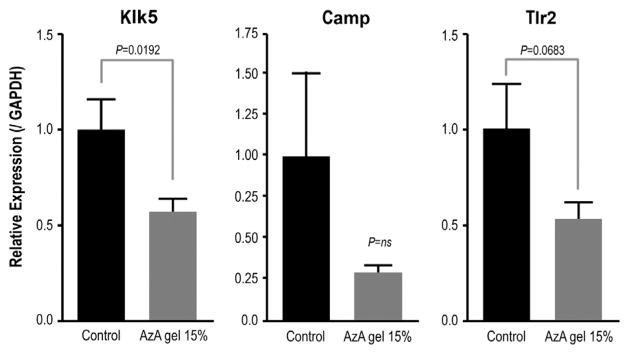
15% Azelaic acid (AzA) gel suppressed CAMP, kallikrein 5 (Klk5), and Toll-like receptor 2 (Tlr2) messenger RNA (mRNA) expression in mouse skin. mRNA expression measured by quantitative real-time polymerase chain reaction of mouse skin treated with 15% AzA gel. Klk5 (P = .0192), Camp (P = not significant [ns]), and Tlr2 (P = .0683) genes as compared with vehicle-treated skin. Relative gene expression is shown after normalization by expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Evaluation of cathelicidin and KLK5 in patients with rosacea treated with 15% AzA gel
To extend the studies of the molecular mechanisms of AzA action to human beings with rosacea, 49 patients with mild to moderate rosacea based on clinical assessment scores, with presence of both facial erythema and 3 or more inflammatory lesions (papules, pustules), were enrolled in a multicenter, open-label study testing response to the application of 15% AzA gel. Patients applied the gel twice daily and samples of the stratum corneum were collected by tape stripping of the central and lateral aspects of the face at baseline and every 4 weeks for 16 weeks (weeks 0, 4, 8, 12, and 16). Patient demographic data are shown in Table I.
Table I.
Study patient demographics
| Age, y | |
| Mean | 50.7 |
| Range | 28–79 |
| Gender | |
| Male | 8 (14.5%) |
| Female | 47 (85.5%) |
| Race | |
| White | 52 (96.4%) |
| Asian | 1 (0.2%) |
| Hispanic | 2 (0.4%) |
| Baseline clinical assessments | |
| IGA scores: 1, n = 9; 2, n = 43; 3, n = 11 | |
| CEA scores: 4–5, n = 4; 6–9, n = 35; 10, n = 16 | |
CEA, Clinician Erythema Assessment; IGA, Investigator Global Assessment.
KLK5 and CAMP mRNA decreased in rosacea skin after several weeks of application of 15% AzA gel, with CAMP mRNA decreasing by 4 weeks and KLK5 expression showing a more gradual decrease until week 12 (Fig 3).
Fig 3.
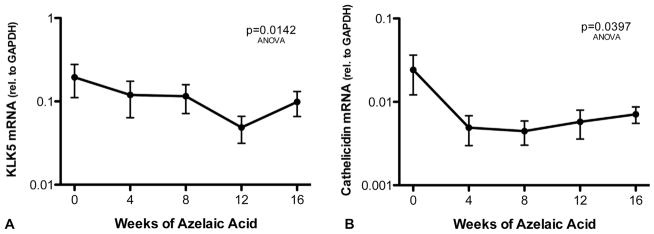
15% Azelaic acid (AzA) gel inhibits kallikrein 5 (KLK5) and cathelicidin messenger (CAMP) RNA (mRNA) in patients with rosacea. Rosacea-affected facial skin was treated twice daily with 15% AzA gel for 16 weeks (n = 49). KLK5 (A) and cathelicidin (B) mRNA were measured by quantitative real-time polymerase chain reaction from tape strips of facial skin taken at 4-week intervals. Relative KLK5 and cathelicidin mRNA abundance was normalized against GAPDH level. P values obtained by Friedman test analysis of variance (ANOVA) in GraphPad Prism (GraphPad Software Inc, La Jolla, CA).
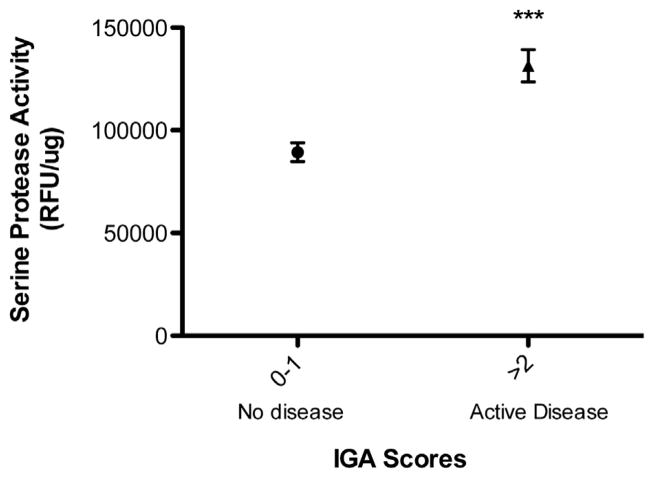
To assess whether SPA correlated with disease activity, we compared all SPA data with their corresponding CEA and IGA scores. Patients with low baseline SPA maintained significantly higher levels of SPA than patients with normal-appearing skin (59,758 vs 39,474 relative fluorescence units (RFU)/μg, P <.001) (Fig 4, A). CEA scores designated as “severe” were associated with patients who had significantly higher SPA measurements versus patients with mild to no disease activity (Fig 4, B). Furthermore, a subgroup of patients with IGA score greater than 1 had higher SPA levels than those with IGA score of 1 or less (P <.001) (Fig 5) although the number of patients with high IGA score was small (n = 9). The reduction in SPA activity seen after 16 weeks of AzA gel therapy did not correlate with improvement in CEA score (Supplemental Fig 1, A; see Appendix), but did show a weak correlation with clinical improvements in IGA score as measured by linear regression analysis that was not statistically significant (Supplemental Fig 1, B; see Appendix).
Fig 4.
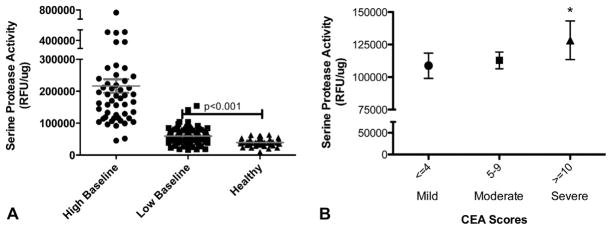
Association of elevated serine protease activity (SPA) with disease activity in rosacea. Tape-strip samples from patients with rosacea (n = 49) and facial skin of healthy patients (n = 10) were obtained, and SPA measured after normalization to total protein. A, Individual measurements of SPA are shown from separate sites on face. Data are clustered into patients with rosacea who had high mean baseline SPA value of >100,000 RFU/μg (high baseline), mean value <100,000 (low baseline), or healthy patients. Wide range of individual site SPA measurements is seen in high baseline group. Healthy patients have significantly lower SPA measurement than those with rosacea, *P <.001. B, Clinical assessment scores were obtained concurrently with tape-strip samples to evaluate correlation between SPA and disease activity. Patients with Clinician Erythema Assessment (CEA) scores designated as “severe” had significantly higher SPA (n = 21) than those with moderate or mild disease activity (n = 28). *P <.05. P values obtained by Student t test in GraphPad Prism (GraphPad Software Inc, La Jolla, CA).
Fig 5.
Higher Investigator Global Assessment (IGA) scores correspond to higher serine protease activity (SPA). SPA measurements were obtained as in Fig 4. Patients with high IGA (≥2) (n = 28) at baseline had significantly higher SPA value than patients with lower IGA (0–1) (n = 21).***P <.001.
KLK5 is a predominant serine protease in the epidermis. To determine if a functional correlation could be measured between KLK5 mRNA expression and SPA, a sensitive direct assay of human skin surface SPA was done.16 Two populations could be distinguished among patients with rosacea at baseline based on their starting SPA value (Fig 6). Both of these populations had higher SPA values than that measured from facial skin of patients without rosacea. Patients who started at low baseline did not show a decrease in SPA with 15% AzA gel treatment (r2 = 0.1421, P = .5317). However, those with a high baseline (>100,000 RFU/μg) showed that 16 weeks of AzA gel therapy positively correlated with a decrease in SPA (r2 = 0.7953, P = .0008) (Fig 6).
Fig 6.
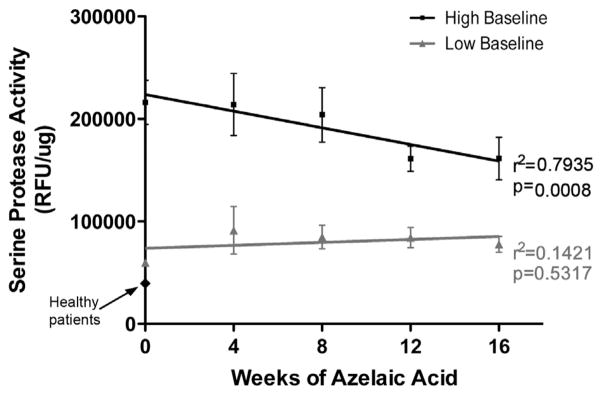
15% Azelaic acid (AzA) gel inhibits serine protease activity (SPA) in rosacea-affected facial skin. Rosacea-affected facial skin was treated twice daily with 15% AzA gel for 16 weeks. Tape-strip samples from facial skin were obtained, and SPA was measured as in Figs 4 and 5. Patient data were clustered based on mean baseline SPA into high (>100,000 RFU/μg) (n = 21) and low (n = 28) baseline subgroups as in Fig 4. 15% AzA gel significantly inhibited SPA in high baseline subgroup but not low baseline subgroup and revealed proportional decrease in SPA with time of therapy in high baseline subgroup (r2 = 0.7935). r2 and P values obtained by linear regression analysis in GraphPad Prism (GraphPad Software Inc, La Jolla, CA).
DISCUSSION
Although the cause of rosacea is a complex combination of environmental and genetic factors, considerable evidence suggests an aberrant innate immune detection and response system is important to its development. Previous studies have shown that facial skin from patients with rosacea has excess expression of genes that increase both the sensitivity and reactivity to a variety of environmental triggers including TLR2, KLK5, and CAMP.17 In this study we evaluated whether AzA, a commonly used topical therapy approved by the US Food and Drug Administration for rosacea, might alter the expression of cathelicidin and KLK5 in cultured human keratinocytes, in murine skin, and in human facial skin during use in patients with rosacea. We found that AzA could inhibit KLK5 and cathelicidin gene expression. Analysis of collections of stratum corneum also revealed inhibition of a surrogate marker of KLK5 activity (SPA) after treatment with AzA gel. These data show a previously unknown mechanism of action for AzA.
In previous studies of skin biopsy specimens from patients with rosacea it was observed that KLK5 and cathelicidin were overexpressed in rosacea compared with normal-appearing facial skin, and unlike normal-appearing skin, cathelicidin was processed to the LL-37 peptide.9 LL-37 has been shown in several experimental systems to be proinflammatory and also signals the formation of physical vascular changes (ie, vessel proliferation) that are consistent with findings observed in rosacea.18–20
To further test the hypothesis that the clinical manifestations of rosacea correlate with the abnormal production of cathelicidin and KLK5, the effects of AzA on these innate immune effector molecules was evaluated. The exposure of normal human keratinocytes to AzA produced decreased mRNA expression relative to the vehicle control. These observations supported extension of this research approach to an in vivo study with mouse skin and a human clinical trial, both with application of 15% AzA gel. Further study of the mechanism by which AzA inhibits KLK5 expression was justified because elements that influence innate immune function in the cathelicidin pathway include various factors such as the presence of microbial products, cytokines, vitamin D, and epigenetic factors.21–23 Interestingly, normal-appearing mouse skin showed significant inhibition of Klk5 mRNA after application of 15% AzA gel and trends toward a decrease in expression in Camp and Tlr2.
The goals of the clinical study to measure cathelicidin and KLK5 expression at several time points necessitated the development of a noninvasive collection technique to study these genes. Tape stripping has been used previously to study gene expression in the skin,24 and reflects both the expression and the stability of individual mRNAs. The tape-strip technique provided insufficient material to measure individual cathelicidin peptide abundance or specific KLK5 function. However, sufficient material was present to measure total SPA as a surrogate for KLK5 activity. SPA activity is necessary to activate the cathelicidin precursor protein (hCAP18) to active forms such as LL-37.25
The results reported here are the first to our knowledge to show that SPA is significantly elevated in rosacea, a finding consistent with prior immuno-staining and tissue zymography results from biopsy specimens.9 Although the total patient sample size was relatively small, and this study was not designed to include a large population of patients with severe IGA or CEA values, we observed trends that suggested clinical severity and response to treatment correlated with high SPA values. Ultimately, additional studies will be necessary to confirm the role of SPA measurements in the diagnosis of rosacea or as a predictor of therapeutic response. Because of the small number of patients with high IGA and SPA values, it is not possible to fully assess to what extent the baseline clinical severity may correlate with KLK5, SPA, and CAMP expression and/or activity.
In summary, we show that an association exists between treatment with 15% AzA gel and inhibition of SPA activity and cathelicidin and KLK5 expression. These data suggest that inhibition of cathelicidin may correlate directly with beneficial therapeutic activity in rosacea, and support prior laboratory evidence.16 Given that increased SPA underlies the activation of cathelicidin and, by proxy, the inflammation in rosacea, it is reasonable to consider targeting this system in a new class of treatment for rosacea. Further, our data provide evidence of a novel noninvasive sampling method for evaluating disease activity through the measurement of SPA. Ultimately, larger-scale studies are necessary to determine if similar techniques can be used as an objective marker for rosacea diagnosis and activity or for prognostic measures to predict therapy for individualization of treatment.
Supplementary Material
Acknowledgments
Supported in part by National Institutes of Health grant R01-AR052728 to Dr Gallo and an investigator-initiated grant from Bayer (Intendis) to Drs Gallo, Hata, Del Rosso, and Harper.
Abbreviations used
- AzA
azelaic acid
- CEA
Clinician Erythema Assessment
- DMSO
dimethyl sulfoxide
- IGA
Investigator Global Assessment
- KLK5
kallikrein 5
- mRNA
messenger RNA
- SPA
serine protease activity
Footnotes
Disclosure: Dr Del Rosso currently serves or has recently (within the last 2 years) served as a consultant, speaker, and/or researcher for Bayer Dermatology. Dr Coda, Dr Miller, Mr Audish, Mr Kotol, Dr Two, Dr Yamasaki, and Dr Shafiq have no conflicts of interest to declare.
References
- 1.Pelle MT, Crawford GH, James WD. Rosacea, II: therapy. J Am Acad Dermatol. 2004;51:499–512. doi: 10.1016/j.jaad.2004.03.033. quiz 3–4. [DOI] [PubMed] [Google Scholar]
- 2.Del Rosso JQ, Baldwin H, Webster G. American Acne and Rosacea Society rosacea medical management guidelines. J Drugs Dermatol. 2008;7:531–3. [PubMed] [Google Scholar]
- 3.Odom R, Dahl M, Dover J, Draelos Z, Drake L, Macsai M, et al. Standard management options for rosacea, part 2: options according to subtype. Cutis. 2009;84:97–104. [PubMed] [Google Scholar]
- 4.Kennedy Carney C, Cantrell W, Elewski BE. Rosacea: a review of current topical, systemic and light-based therapies. G Ital Dermatol Venereol. 2009;144:673–88. [PubMed] [Google Scholar]
- 5.Yamasaki K, Gallo RL. The molecular pathology of rosacea. J Dermatol Sci. 2009;55:77–81. doi: 10.1016/j.jdermsci.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larrick JW, Hirata M, Zhong J, Wright SC. Anti-microbial activity of human CAP18 peptides. Immunotechnology. 1995;1:65–72. doi: 10.1016/1380-2933(95)00006-2. [DOI] [PubMed] [Google Scholar]
- 7.Koczulla R, von Degenfeld G, Kupatt C, Krotz F, Zahler S, Gloe T, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111:1665–72. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorschner RA, Pestonjamasp VK, Tamakuwala S, Ohtake T, Rudisill J, Nizet V, et al. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A streptococcus. J Invest Dermatol. 2001;117:91–7. doi: 10.1046/j.1523-1747.2001.01340.x. [DOI] [PubMed] [Google Scholar]
- 9.Yamasaki K, Di Nardo A, Bardan A, Murakami M, Ohtake T, Coda A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975–80. doi: 10.1038/nm1616. [DOI] [PubMed] [Google Scholar]
- 10.Yamasaki K, Kanada K, Macleod DT, Borkowski AW, Morizane S, Nakatsuji T, et al. TLR2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. J Invest Dermatol. 2011;131:688–97. doi: 10.1038/jid.2010.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Zuuren EJ, Kramer SF, Carter BR, Graber MA, Fedorowicz Z. Effective and evidence-based management strategies for rosacea: summary of a Cochrane systematic review. Br J Dermatol. 2011;165:760–81. doi: 10.1111/j.1365-2133.2011.10473.x. [DOI] [PubMed] [Google Scholar]
- 12.Gollnick H, Layton A. Azelaic acid 15% gel in the treatment of rosacea. Expert Opin Pharmacother. 2008;9:2699–706. doi: 10.1517/14656566.9.15.2699. [DOI] [PubMed] [Google Scholar]
- 13.Bakar O, Demircay Z, Yuksel M, Haklar G, Sanisoglu Y. The effect of azithromycin on reactive oxygen species in rosacea. Clin Exp Dermatol. 2007;32:197–200. doi: 10.1111/j.1365-2230.2006.02322.x. [DOI] [PubMed] [Google Scholar]
- 14.Thiboutot D, Thieroff-Ekerdt R, Graupe K. Efficacy and safety of azelaic acid (15%) gel as a new treatment for papulopustular rosacea: results from two vehicle-controlled, randomized phase III studies. J Am Acad Dermatol. 2003;48:836–45. doi: 10.1067/mjd.2003.308. [DOI] [PubMed] [Google Scholar]
- 15.NIST/SEMATECH. [Accessed April 2012.];e-Handbook of statistical methods. Available at: http://www.itl.nist.gov/div898/handbook/
- 16.Kanada KN, Nakatsuji T, Gallo RL. Doxycycline indirectly inhibits proteolytic activation of tryptic kallikrein-related peptidases and activation of cathelicidin. J Invest Dermatol. 2012;132:1435–42. doi: 10.1038/jid.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamasaki K, Gallo RL. Rosacea as a disease of cathelicidins and skin innate immunity. J Investig Dermatol Symp Proc. 2011;15:12–5. doi: 10.1038/jidsymp.2011.4. [DOI] [PubMed] [Google Scholar]
- 18.Schauber J, Dorschner RA, Coda AB, Buchau AS, Liu PT, Kiken D, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117:803–11. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hata TR, Gallo RL. Antimicrobial peptides, skin infections, and atopic dermatitis. Semin Cutan Med Surg. 2008;27:144–50. doi: 10.1016/j.sder.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sorensen OE, Thapa DR, Roupe KM, Valore EV, Sjobring U, Roberts AA, et al. Injury-induced innate immune response in human skin mediated by transactivation of the epidermal growth factor receptor. J Clin Invest. 2006;116:1878–85. doi: 10.1172/JCI28422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schauber J, Gallo RL. Antimicrobial peptides and the skin immune defense system. J Allergy Clin Immunol. 2009;124:R13–8. doi: 10.1016/j.jaci.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Frohm M, Agerberth B, Ahangari G, Stahle-Backdahl M, Liden S, Wigzell H, et al. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem. 1997;272:15258–63. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- 23.Schauber J, Dorschner RA, Yamasaki K, Brouha B, Gallo RL. Control of the innate epithelial antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli. Immunology. 2006;118:509–19. doi: 10.1111/j.1365-2567.2006.02399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang CY, Maibach HI. Why minimally invasive skin sampling techniques? A bright scientific future. Cutan Ocul Toxicol. 2011;30:1–6. doi: 10.3109/15569527.2010.517230. [DOI] [PubMed] [Google Scholar]
- 25.Sorensen OE, Follin P, Johnsen AH, Calafat J, Tjabringa GS, Hiemstra PS, et al. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood. 2001;97:3951–9. doi: 10.1182/blood.v97.12.3951. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.