Abstract
The U.S. elderly population (≥85 years old) is estimated to increase from 5 to 20 million people between the years 2000 to 2050. Among the medical disorders facing the elderly, anorectal problems are not only highly prevalent, but cause significant morbidity and mortality, and have deleterious effects on health care burden and quality of life. These include disorders such as fecal incontinence, fecal impaction with overflow fecal incontinence, chronic constipation, dyssynergic defecation, hemorrhoids, anal fissure, and pelvic floor disorders. Here, we discuss the latest advances in age-related changes in anal sphincter morphology and function, changes in cellular and molecular biology, alterations in neurotransmitters and reflexes, and their impact on functional changes of the anorectum in the elderly. These biophysiologic changes have implications for the pathophysiology of anorectal disorders. A clear understanding and working knowledge of the functional anatomy and pathophysiology will enable appropriate diagnosis and treatment of these disorders.
Introduction
Anorectal disorders such as fecal incontinence (FI), chronic constipation, dyssynergic defecation, fecal impaction, and overflow FI are highly prevalent in the elderly. They significantly affect quality of life as well as pose a large health care burden. Estimates from the U.S. Bureau of the Census indicate that the size of the U.S. population 85 years of age and older will increase from approximately 5 to 20 million between 2000 to 2050.[1] Although a benign condition, constipation can result in chronic illness with potentially serious complications (FI, impaction, and bowel perforation).[2] FI affects between 6–19% of elderly individuals aged 65 years and older living in the community and unlike younger patients, men and women are equally affected.[3] It is associated with significant social stigma and psychological distress, leads to dependency, poor health, a high caregiver burden, and is a leading reason for nursing home placement in the elderly.[4] Although there is improved understanding of the mechanisms of some of these disorders outlined above, there is significant lack of knowledge regarding normal and abnormal changes of anorectal function and the biologic changes with aging. Here, we will discuss relevant structural and functional changes of the anorectum in the elderly and discuss their implications for the pathogenesis of common anorectal disorders.
Functional Anatomy and Physiology
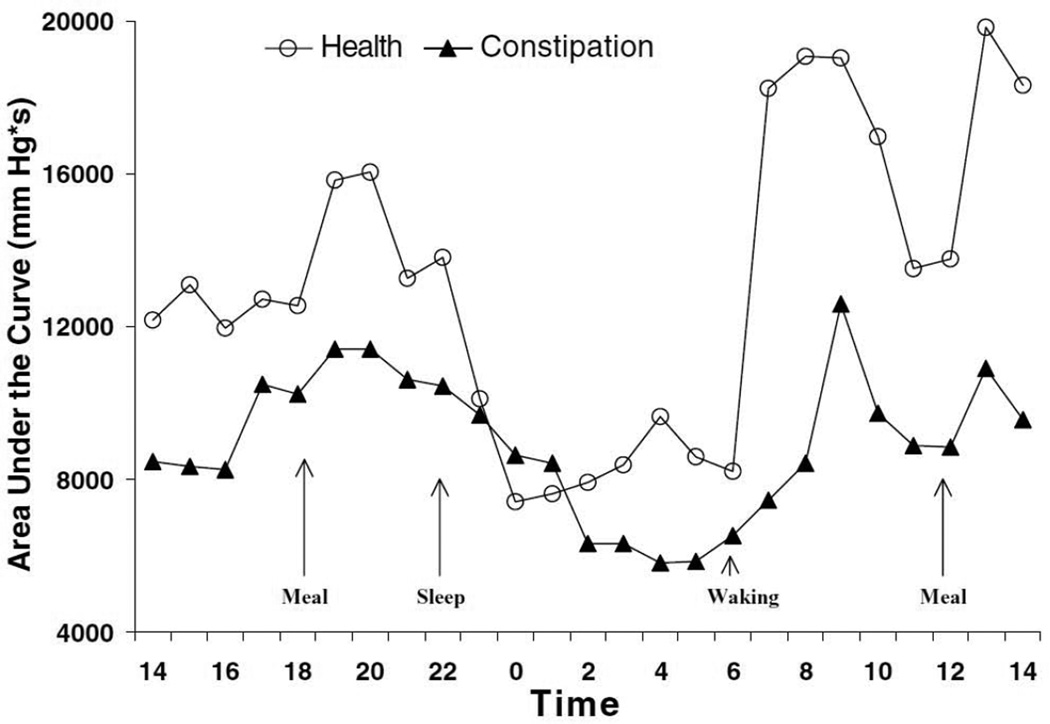
The colon has a well-established circadian rhythm, with a significant increase in motility after meals and after waking. During waking hours, the transverse/descending colon exhibits more activity, attributed to its role of mixing, storage, and salvaging digestive residue, while nocturnal activity is predominated by periodic rectal motor activity, which presumably acts as an intrinsic nocturnal brake that helps to maintain continence during sleep.[5, 6] (See Figure 1) Between 3 to10 times a day, intermittent high amplitude (>100 mm Hg) prolonged duration propagating contractions (HAPC’s) sweep through the colon, delivering fecal material into the rectum. The numbers of HAPC’s are significantly decreased or absent in patients with slow transit constipation, but whether their characteristics are different in the elderly is not known.[6]
Figure 1. A 24-hour profile of the mean area under the curve of colonic pressure waves in healthy and constipated patients.
There is significant increase in colonic motility after meals and after waking. From Rao SS, et al. Ambulatory 24-hour colonic manometry in slow-transit constipation. American journal of gastroenterology, 2004; with permission.
The rectum acts as a reservoir for stool and as a pump for emptying stool, and has three involutions known as the valves of Houston. The lateral angulations of the sigmoid colon and valves of Houston provide a mechanical barrier that helps retard progression of stool, with the weight of the stool enhancing this barrier effect.[7] Stool volume and consistency are important because patients with a weakened continence mechanism may be continent for firm stool but incontinent for liquid feces. The adaptive compliance of the rectum along with rectal capacity are important for its reservoir function.[8]
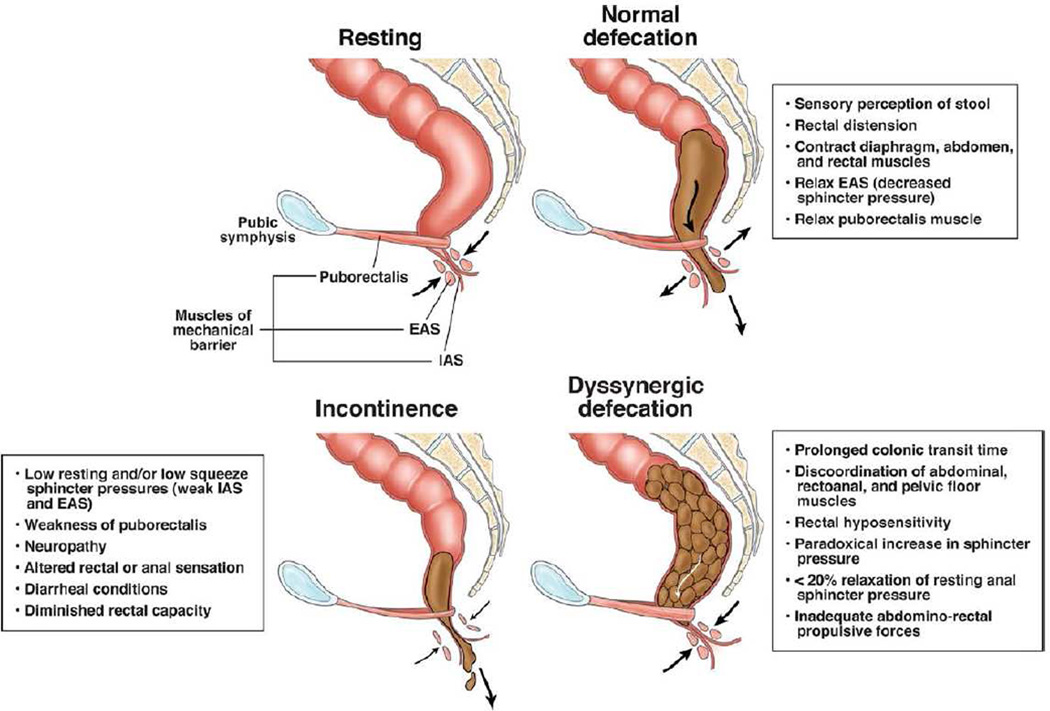
The anus is a muscular tube 2 to 4 cm in length, and the upper anal canal is lined with mostly columnar epithelium, the same tissue that lines the rectum. About 1 cm above the dentate line, there is a change from columnar to squamous epithelium in an area 1 to 1.5 cm, called the transitional zone. The anal sphincter consists of the internal anal sphincter, a 0.3 to 0.5 cm thick expansion of the circular smooth muscle layer of the rectum, which is under involuntary control, and the external anal sphincter, a 0.6 to 1 cm thick expansion of the striated levator ani muscles, which is under voluntary control. The anus forms an angle with the axis of the rectum, approximately 90 degrees at rest. With voluntary squeeze, it becomes more acute, around 70 degrees, and during defecation it becomes more obtuse, at 110 to 130 degrees.[9] (See Figure 2)
Figure 2. Normal Anatomy and Physiology of the Pelvic Floor in the Sagittal Plane at Rest, During Defecation, and the Key Pathophysiologic Changes in Subjects with Fecal Incontinence and Dyssynergic Defecation.
This profile shows the arrangement of the 3 anal muscles during rest, normal defecation, and the impairment that occurs with fecal incontinence and dyssynergic defecation. From Rao SS, et al. Advances in diagnostic assessment of fecal incontinence and dyssynergic defecation. Clin Gastroenterol Hepatol, 2010; with permission.
The anus is normally closed by the tonic activity of the internal anal sphincter, which keeps the canal in the collapsed position to maintain continence.[10] When the rectal ampulla fills and induces distention, the sensory stimulus for evacuation releases the state of tonic contraction, and begins the process of defecation. If volume increases rapidly over a short period of time, the accommodation response fails and leads to urgent emptying.[8]
The pelvic floor consists of the pubococcygeus, iliococcygeus, and puborectalis, a group of muscles that forms the levator ani. The pubococcygeus and iliococcygeus participate in continence by applying lateral pressure to narrow the levator hiatus. Recent work has shown that the puborectalis plays an integral role in maintaining continence and is either scarred or damaged in women with FI.[11] The external anal sphincter is composed of multiple interrelated skeletal muscle loops that lie in close approximation to the levator ani and the muscles of the pelvic floor, and is under voluntary control. This sphincter system contains three muscular loops that surround the anal canal. The external anal sphincter is innervated by the inferior hemorrhoidal branch of the pudendal nerve. Continuous tonic activity of the external anal sphincter and pelvic floor muscles has been recorded at rest and during sleep, which helps maintain fecal continence.[12]
The internal anal sphincter has both sympathetic and parasympathetic innervation. The parasympathetic nerves are inhibitory to the internal anal sphincter, while the sympathetic outflow mediates contraction and relaxation.[13, 14] Activity of the sphincters is the most important factor in maintaining anal continence. The internal anal sphincter is estimated to account for 52 to 85% of the pressure recorded in the high-pressure zone of the anal canal.[15] The hemorrhoidal cushions also play a significant physiologic role in protecting the anus, augmenting closure of the anal canal in response to increased abdominal pressure by engorging with increased inferior vena cava pressure, and contributes 15 to 20% of resting anal canal pressure—another important mechanism preserving fecal continence.[16]
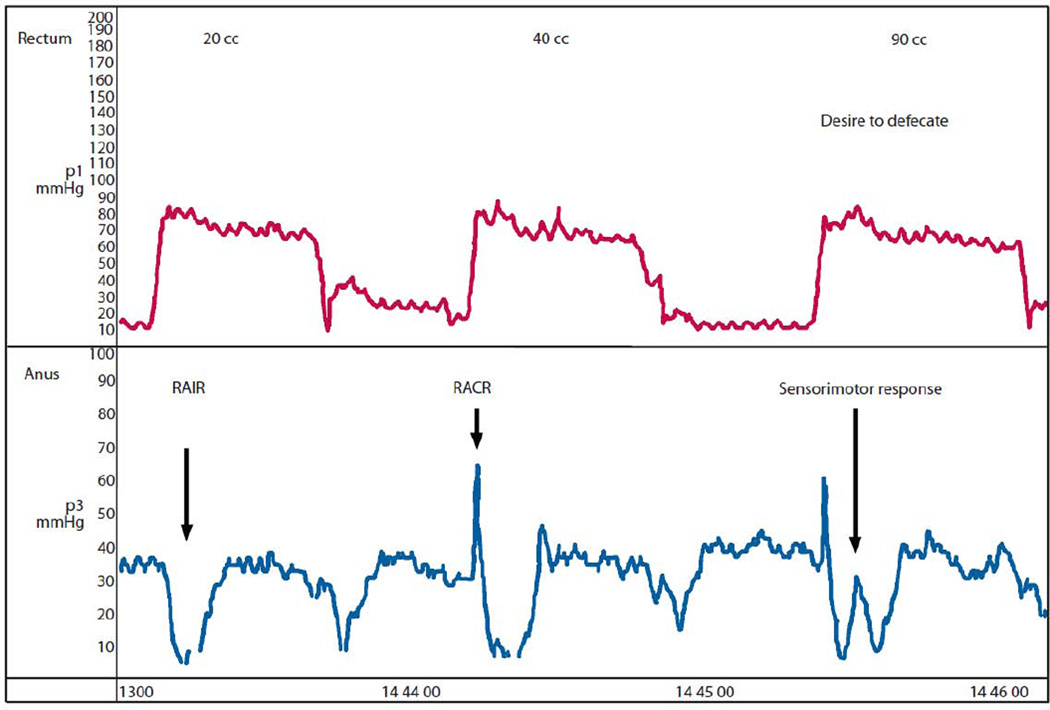
Three anal reflexes have been described and include the Recto-anal Inhibitory Reflex (RAIR), the Recto-anal Contractile Reflex (RACR), and the Sensorimotor Response (SMR). (See Figure 3) These reflexes are probably mediated by the pudendal nerve and subserved by the sacral spinal cord segments S1-S4. The RAIR is characterized by differential anal relaxation along the anterior–posterior axis, longitudinally along the length of anal canal, and is dependent on the rectal distention volume. Multidimensional analyses demonstrate that there is specific asymmetry in the RAIR. Recent work shows that it is maximally seen at the internal anal sphincter pressure zone, and is characterized manometrically by the loss of anal canal pressure during rectal balloon distension (relaxation pressure), and the lowest anal canal pressure point of the reflex (residual pressure).[17] The SMR is a transient anal contraction that is primarily induced by the activation and contraction of the puborectalis muscle in response to sensation of a desire to defecate.[18] It usually overlies the initial relaxation phase of the RAIR, and normally coincides with the onset of a sensation to defecate.[19] The RACR is a primordial reflex that prevents accidental release of rectal contents and is mediated by the pelvic splanchnic and pudendal nerves.[20]
Figure 3. Manometric Representation of the Recto-anal Inhibitory Reflex (RAIR), Recto-anal Contractile Reflex (RACR), and the Sensorimotor Response.
From Remes-Troche, J.M., et al., Rectoanal reflexes and sensorimotor response in rectal hyposensitivity. Dis Colon Rectum, 2010; with permission.
The sympathetic fibers to the rectum are derived from L1-L3 spinal cord segments. Parasympathetic fibers, which transmit the pathway for the sensation of rectal distention via the nervi erigentes, are derived from the S2-S4 spinal cord segments.[21] Rectal sensory perception is mediated by mechanoreceptors in the pelvic floor and wall itself.[22] Rectal hyposensitivity in patients with normal rectal compliance may reflect impaired afferent nerve function, whereas in patients with increased compliance, it may be due to abnormal rectal wall properties.[22]
Biophysiologic and Molecular effects of Aging on Neuromuscular Function
The key mechanisms underlying age-related cellular dysfunction include oxidative damage affecting the nucleotide pool and biochemical pathways associated with cell structure and function, and epigenetic alterations in gene expression that affect the plasticity of senescent cells. Endogenous reactive oxygen species are a mechanism of age-associated loss of myenteric neurons. The insufficient ability of cells to degrade damaged cytosolic macromolecules and organelles through autophagy results in accumulation of dysfunctional mitochondria in aged individuals that may result in cell death independent of the classic apoptosis pathway. The manipulation of factors that enhance autophagy function, such as SIRT1 and mTOR, has demonstrated significant increases in survival in several organisms, and emphasizes the role of autophagy in regulation of lifespan.[23, 24] In rodent models of aging, a significant reduction in the number of neurons occurs in the enteric nervous system, and is more prominent distally (60% in the colon, and 40% in the small intestine).[25] Interestingly, in the klotho murine model of premature aging, less contractile proteins are expressed with generalized intestinal neuromuscular hypoplasia, in the presence of accelerated colonic and whole-gut transit, suggesting that decreased fecal output is due to reduced food intake rather than intestinal dysmotility.[26]
In the colons of human patients, age-related neuronal loss is also associated with an increased proportion of abnormal appearing myenteric ganglia with cavities, which may contribute to disturbed colonic motility with aging.[27] Study of inhibitory innervation of human descending colon obtained at surgery has shown an age-related decrease in inhibitory junction potentials, suggesting a decrease in inhibitory nerves, neurotransmitter, density of bindings sites, and alternatively, a possible change in the interaction of inhibitory neurotransmitters with the smooth muscle membrane.[28] Rectal sensory thresholds have been reported to be higher in aged healthy human volunteers, despite absence of changes in colorectal smooth muscle compliance and tone, and age is therefore suggested to be a potential confounding factor when studying rectal sensitivity.[29] It is possible that this observation may point to potential alterations in the normal accommodation reflexes involved in defecation.
The enteric nervous system (ENS) is complex, and enteric neurons are heterogeneous in their morphology, projections, and physiological roles.[30] (See Table 1) Wang et al have studied the changes in innervation of the mouse internal anal sphincter with aging.[31] They found a significant reduction in the density of neuronal nitric oxide synthetase (nNOS) and substance P (SP) immunoreactivity in the nerve fibers of aging murine internal anal sphincter circular muscle (3 versus 25 months), with significant decreases in the density of nNOS, vasoactive intestinal peptide (VIP), and SP in anal mucosal nerve fibers. These changes point to pathophysiological alterations of anorectal motility and the defecation reflex with the aging process.
Table 1.
Summary of the Major Types of Myenteric and Submucosal Neurons in the Mammalian Gastrointestinal Tract*
| Main functional groups (% of total, guinea-pig*) | Projections to target cells (morphological classification) | Major neurotransmitter/s modulator/s (additional neurochemical markers) |
|---|---|---|
| Myenteric plexus | ||
| Intrinsic sensory neurons (26%) | Mucosal epithelium | Tachykinins/Acetylcholine (calbindin) |
| Motor neurons that stimulate smooth muscle relaxation (18%) | Anal, to smooth muscle | Nitric oxide/vasoactive intestinal peptide/pituitary adenylate cyclase-activating polypeptide/ATP |
| Motor neurons that stimulate smooth muscle contraction (37%) | Oral, to smooth muscle | Acetylcholine/tachykinins |
| Interneurons (various types) (16%) | Oral and anal to other neurons | Acetylcholine/tachykinins/serotonin/neuropeptide Y/somatostatin |
| Neurons that project out of the gut (<1%) | Other autonomic ganglia | Acetylcholine |
| Submucosal plexus | ||
| Secreto-motor/vasodilator neurons (non-cholinergic) (45%) | Mucosal epithelium and intestinal blood vessels | Vasoactive intestinal peptide |
| Secreto-motor/vasodilator neurons (44%) | Mucosal epithelium and blood vessels | Acetylcholine |
| Intrinsic sensory neurons (11%) | Myenteric and submucosal neurons, and epithelium | Thought to be tachykinin (express calbindin) |
Broadly similar proportions of the main neuronal types have been found in all species studied to date
Adapted from Saffrey MJ. Ageing of the enteric nervous system. Mechanisms of ageing and development. 2004; 125(12): 899–906.
The submucosal plexus, which plays an important role in secretion and motility, has also been found to be impaired with age.[32] Phillips et al showed age-related increases in alpha-synuclein expression in the submucosal plexus[33], as well as significant decreases of glial cells, in Fischer 344 rats. This occurred in every region of the small and large intestine, except for the rectum, which showed a nonsignificant decrease. The reason for the preferential sparing of the rectum is unclear. An interdependency between loss of glial cells and the myenteric neuronal cell death was also found.[34] With age, the number and volume of interstitial cells of Cajal also decline, which reduces propulsive activity and sensorimotor functions within the gastrointestinal tract.[35]
Physiologic properties of isolated rat colonic smooth muscle cells demonstrate decreased average total Ca2+ current density with aging.[36] The decreased contractile properties of gastrointestinal smooth muscle with aging may be mediated by altered signal transduction pathways. Studies in aged rat colonic smooth muscle show signal transduction may be affected by decreases in caveolin-1, a caveolae-specific protein which helps permit rapid and efficient coordination of signal cascades that lead to smooth muscle contraction of the colon.[23] Deletion of growth hormone or its receptors has been shown to extend longevity, and expression is associated with smooth muscle cell proliferation and collagen synthesis in intestinal smooth muscle in rat models of intestinal growth and colonic inflammation.[37]
The distribution of elastin and collagen has also been found to be increased around the myenteric plexus in the aging human colon, and may affect the ability to accommodate gut contents.[38]
In summary, significant alterations in age-related enteric neuronal structure and function have been observed and could explain some of the disorders seen in the elderly.
Implications of Aging Neuromuscular Function on Anorectal Disorders
Although gastrointestinal functions are generally well preserved with aging, the progressive neuronal loss, and extrinsic factors such as diet, immobility, co-morbidity and the effects of medication as well as previous trauma, such as obstetric sphincter injury or back injury, may predispose the elderly to an impairment of colorectal sensorimotor function. Additionally, the clinical impact of co-morbid conditions has a significant effect on gastrointestinal muscular function, though these effects are heterogeneous and not clearly defined.[39] (See Table 2)
Table 2.
Table of Physiologic Changes in the Elderly
| Structure | Proposed change | Pathophysiologic Significance | Clinical problem |
|---|---|---|---|
| Number of HAPCs | Decreased | Decreased colonic propulsion | Constipation |
| Colonic Transit Time | Prolonged | Slow colon transit | Constipation |
| Internal Anal Sphincter | Thinning/atrophy | Weak sphincter | Fecal seepage/incontinence |
| External Anal Sphincter | Thiining/atrophy | Weak sphincter | Urgency/Incontinence |
| Pudendal Nerve Function | Decreased | Impairment of colorectal sensorimotor function | Fecal seepage/incontinence |
| Rectal Sensation | Decreased | Impairment of colorectal sensorimotor function | Fecal seepage/incontinence |
| Rectal Compliance | Decreased | Impaired reservoir function | Urgency/Incontinence |
| Anal Sphincter Length | Decreased | Weak sphincter | Fecal seepage/Urgency/Incontinence |
| Rectal Capacity | Decreased | Impaired reservoir function | Urgency/Incontinence |
Aging is associated with a variety of effects on anorectal function. Healthy elderly women have demonstrated thinning of the internal anal sphincter resulting in decreased resting and maximum squeeze pressure in the anal canal. Thickening of the external sphincter is also observed, but does not correlate with increased continence. In the absence of disease, age-related changes in the sphincter function of aging males appears minimal.[40, 41] Increasing age is also associated with a more positive rectoanal gradient during simulated evacuation and a shorter balloon expulsion time in asymptomatic women.[42] There is an increase in collagen in the colon wall that is accompanied by a decrease in tensile strength, which may predispose to mucosal herniation, and decreased reservoir function.[8] Age- related reductions in basal and maximum anal sphincter tone, decreased compliance of the rectal vault, reduced rectal sensation, and increased perineal descent also occur.
Constipation and Dyssynergic Defecation
Slow colonic transit and increased frequency of segmenting contractions may result in increased water resorption and hard feces. Decreased fiber intake also predisposes to the production of hard feces and excessive straining. When traditional approaches, including a high fiber diet and over the counter laxatives have not helped, and obstruction, secondary to things such as colon cancer, has been excluded, an assessment of colonic transit time (CTT) with radiopaque markers (ROM) and/or anorectal manometry should be performed. Although widely available, the utility of ROM’s in assessing CTT for slow transit constipation (STC) in the elderly is limited. Furthermore, there is significant overlap between STC and dyssynergia with approximately 60% of patients with dyssynergic defecation showing delayed CTT. A newer technology, the wireless motility capsule (WMC), which measures pH, temperature, and pressure, has emerged as a useful test in the evaluation of slow transit constipation. It has better sensitivity compared with ROM (86% versus 28%), and good specificity (89%). A recent study using this modality showed that older constipated patients had slower transit than older healthy controls.[43]
Fecal Incontinence
In the American College of Gastroenterology (ACG) Practice Guidelines, Rao proposed three clinical subtypes of FI (See Table 3).[10] There is overlap between these three groups, but making a clinical distinction could help to assess the underlying etiology and guide investigation(s) and management.
Table 3.
Fecal Incontinence Subtypes
| Type | Clinical Description | Potential Mechanism(s) |
|---|---|---|
| Passive Incontinence | The involuntary discharge of stool or gas without awareness. | Weak IAS and EAS Neuropathy Rectal hyposensitivity |
| Urge Incontinence | The discharge of fecal matter in spite of active attempts to retain bowel contents. | Weak IAS and EAS Rectal hypersensitivty Impaired rectal compliance |
| Fecal Seepage | The leakage of stool following otherwise normal evacuation. | Dyssynergia Rectal hyposensitivity Neuropathy |
IAS, Internal Anal Sphincter. EAS, External Anal Sphincter.
FI is defined as the involuntary passage of fecal matter through the anus, or the inability to control this discharge. In elderly hospitalized patients, contributing factors to FI include fecal loading (57%), functional disability (83%), loose stools (67%), and cognitive impairment (43%). The distribution of factors appears to differ in the nursing home setting, where cognitive impairment is significantly more common (up to 87%).[44] Contributing factors in both inpatients and outpatients include traumatic anal injury, neurologic deficits, inflammatory conditions, and defecatory disturbances associated with constipation and diarrhea. There is an important connection between FI and urinary incontinence (UI), or dual incontinence, and the strongest association is age older than 80 years, followed by depression, neurologic disease, functional limitation, multiparity, and heavier fetal birth weight.[45] One study found that in women who had suffered a third or fourth degree obstetric tear, 21% were incontinent at a mean of 18 months follow-up.[46] A survey-based study showed at long-term follow-up in women who had suffered a third degree obstetric tear (at least 10 years), 53% of respondents complained of fecal incontinence.[47] UI is also an independent risk factor for developing FI in both community-dwelling and institutionalized adults.[48, 49] Retrospective and survey data suggest that sacral nerve neuromodulation can improve dual incontinence (FI and UI), suggesting a common etiology.[50, 51]
Constipation plays an integral role in the development of FI, which can result from fecal impaction and subsequent overflow FI, internal anal sphincter incompetence, decrease rectal or anal sensation, and from other structural pelvic floor or anorectal neuromuscular dysfunction caused by prior trauma from surgery or irradiation.[41] Loss of the endovascular cushions, impaired anorectal sensation, poor rectal compliance, compromised accommodation, or neuropathy affecting the pudendal, sacral, spinal, or central nervous system may also contribute to incontinence. Failure to perceive stool in the rectum may produce severe urgency to defecate or leakage of stool, especially when access to toileting is limited. Other problems that are common in the elderly and need more study include neuropathy, excessive perineal descent, rectocele, rectal mucosal intussusception and prolapse.[52, 53]
CONCLUSION
There are exciting advances in our understanding of the physiology and cellular biology of aging and anorectal neuromuscular function. However, much work remains: this includes a better characterization of common disorders and their phenotypes, a better understanding of the clinical factors that contribute to the burden of anorectal disorders, and more knowledge of the underlying genetic, molecular and biologic changes that occur with aging. There is also an urgent need for further longitudinal physiologic, structural, and neurophysiologic studies in women following obstetric trauma and/or pregnancy to better understand the impact of trauma and the aging process. Continued progress in this field, and a clear understanding of what is currently known, will pave the way for more accurate diagnosis and a rational approach to the treatment of these disorders.
Key Points.
The key mechanisms underlying age-related cellular dysfunction include oxidative damage affecting the nucleotide pool and biochemical pathways associated with cell structure and function, and epigenetic alterations in gene expression that affect the plasticity of senescent cells.
In the colons of human patients, age-related neuronal loss is also associated with an increased proportion of abnormal appearing myenteric ganglia with cavities, which may contribute to disturbed colonic motility with aging.[27]
Aging is associated with a variety of effects on anorectal function. Healthy elderly women have demonstrated thinning of the internal anal sphincter resulting in decreased resting and maximum squeeze pressure in the anal canal. Thickening of the external sphincter is also observed, but does not correlate with increased continence. In the absence of disease, age-related changes in the sphincter function of aging males appears minimal.[40, 41]
Acknowledgments
Grant Support:
Dr Rao is supported by a grant from National Institutes of Health NIH grant RO1 DK 57100-05NIHRO-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Campion EW. The oldest old. The New England journal of medicine. 1994;330(25):1819–1820. doi: 10.1056/NEJM199406233302509. [DOI] [PubMed] [Google Scholar]
- 2.Belsey J, et al. Systematic review: impact of constipation on quality of life in adults and children. Aliment Pharmacol Ther. 2010;31(9):938–949. doi: 10.1111/j.1365-2036.2010.04273.x. [DOI] [PubMed] [Google Scholar]
- 3.Goode PS, et al. Prevalence and correlates of fecal incontinence in community-dwelling older adults. J Am Geriatr Soc. 2005;53(4):629–635. doi: 10.1111/j.1532-5415.2005.53211.x. [DOI] [PubMed] [Google Scholar]
- 4.Santos-Eggimann B, Cirilli NC, Monachon JJ. Frequency and determinants of urgent requests to home care agencies for community-dwelling elderly. Home Health Care Serv Q. 2003;22(1):39–53. doi: 10.1300/J027v22n01_03. [DOI] [PubMed] [Google Scholar]
- 5.Rao SS, et al. Ambulatory 24-h colonic manometry in healthy humans. Am J Physiol Gastrointest Liver Physiol. 2001;280(4):G629–G639. doi: 10.1152/ajpgi.2001.280.4.G629. [DOI] [PubMed] [Google Scholar]
- 6.Rao SS, et al. Ambulatory 24-hour colonic manometry in slow-transit constipation. The American journal of gastroenterology. 2004;99(12):2405–2416. doi: 10.1111/j.1572-0241.2004.40453.x. [DOI] [PubMed] [Google Scholar]
- 7.Schuster M, Mendeloff AJ. Chracteristics of rectosigmoid motor function Their relationship to continence, defecation, and disease. In: Glass C, editor. Progress in gastroenterology. New York: Grune & Stratton; 1970. [Google Scholar]
- 8.Schouten W, Gordon PH. Physiology. In: Gordon PH NS, editor. Principles and Practice of Surgery for the Colon, Rectum, and Anus. New York, London: Informa healthcare; 2007. [Google Scholar]
- 9.Rao SS. Advances in diagnostic assessment of fecal incontinence and dyssynergic defecation. Clin Gastroenterol Hepatol. 2010;8(11):910–919. doi: 10.1016/j.cgh.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rao SS. Diagnosis and management of fecal incontinence. American College of Gastroenterology Practice Parameters Committee. The American journal of gastroenterology. 2004;99(8):1585–1604. doi: 10.1111/j.1572-0241.2004.40105.x. [DOI] [PubMed] [Google Scholar]
- 11.Chase S, et al. Anal sphincter repair for fecal incontinence: experience from a tertiary care centre. Indian J Gastroenterol. 2010;29(4):162–165. doi: 10.1007/s12664-010-0037-9. [DOI] [PubMed] [Google Scholar]
- 12.Kumar D, et al. Prolonged anorectal manometry and external anal sphincter electromyography in ambulant human subjects. Digestive diseases and sciences. 1990;35(5):641–648. doi: 10.1007/BF01540414. [DOI] [PubMed] [Google Scholar]
- 13.Gutierrez J, Shank AN. Fifth International Symposium on Gastrointestinal Motility. Herentals, Belgium: Typoff Press; 1975. Autonomic control of the internal anal sphincter in man. [Google Scholar]
- 14.Marcello P. Diseases of the Anorectum. In: Feldman M FL, Brandt LJ, editors. Sleisenger and Fordtran's Gastrointestinal and Liver Disease. Philadelphia: Saunders Elsevier; 2010. [Google Scholar]
- 15.Ferrara A, et al. Relationship between anal canal tone and rectal motor activity. Dis Colon Rectum. 1993;36(4):337–342. doi: 10.1007/BF02053935. [DOI] [PubMed] [Google Scholar]
- 16.Cintron J, Abacarian H. Benign anorectal: hemorrhoids. In: Wolff B, Fleshman JW, editors. The ASCRS of COlon and Rectal Surgery. New York, NY: Springfer-Verlag; 2007. pp. 156–177. [Google Scholar]
- 17.Cheeney G, et al. Topographic and manometric characterization of the recto-anal inhibitory reflex. Neurogastroenterol Motil. 2012;24(3):e147–e154. doi: 10.1111/j.1365-2982.2011.01857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheeney G, et al. Investigation of anal motor characteristics of the sensorimotor response (SMR) using 3-D anorectal pressure topography. Am J Physiol Gastrointest Liver Physiol. 2011;300(2):G236–G240. doi: 10.1152/ajpgi.00348.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Ocampo S, et al. Rectoanal sensorimotor response in humans during rectal distension. Dis Colon Rectum. 2007;50(10):1639–1646. doi: 10.1007/s10350-007-0257-y. [DOI] [PubMed] [Google Scholar]
- 20.Remes-Troche JM, et al. Rectoanal reflexes and sensorimotor response in rectal hyposensitivity. Dis Colon Rectum. 2010;53(7):1047–1054. doi: 10.1007/DCR.0b013e3181dcb2d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nivatvongs S, Gordon PH. Surgical Anatomy. In: Gordon PH NS, editor. Principles and Practice of Surgery for the Colon, Rectum, and Anus. New York London: Informa Healthcare; 2007. [Google Scholar]
- 22.Gladman MA, et al. Rectal hyposensitivity: a disorder of the rectal wall or the afferent pathway? An assessment using the barostat. The American journal of gastroenterology. 2005;100(1):106–114. doi: 10.1111/j.1572-0241.2005.40021.x. [DOI] [PubMed] [Google Scholar]
- 23.Bitar K, et al. Aging and gastrointestinal neuromuscular function: insights from within and outside the gut. Neurogastroenterol Motil. 2011;23(6):490–501. doi: 10.1111/j.1365-2982.2011.01678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camilleri M, Cowen T, Koch TR. Enteric neurodegeneration in ageing. Neurogastroenterol Motil. 2008;20(3):185–196. doi: 10.1111/j.1365-2982.2007.01072.x. [DOI] [PubMed] [Google Scholar]
- 25.Wade PR. Aging and neural control of the GI tract. I Age-related changes in the enteric nervous system. Am J Physiol Gastrointest Liver Physiol. 2002;283(3):G489–G495. doi: 10.1152/ajpgi.00091.2002. [DOI] [PubMed] [Google Scholar]
- 26.Asuzu DT, et al. Generalized neuromuscular hypoplasia, reduced smooth muscle myosin and altered gut motility in the klotho model of premature aging. Neurogastroenterol Motil. 2011;23(7):e309–e323. doi: 10.1111/j.1365-2982.2011.01730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanani M, et al. Age-related changes in the morphology of the myenteric plexus of the human colon. Auton Neurosci. 2004;113(1–2):71–78. doi: 10.1016/j.autneu.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Koch TR, et al. Inhibitory neuropeptides and intrinsic inhibitory innervation of descending human colon. Digestive diseases and sciences. 1991;36(6):712–718. doi: 10.1007/BF01311226. [DOI] [PubMed] [Google Scholar]
- 29.Lagier E, et al. Influence of age on rectal tone and sensitivity to distension in healthy subjects. Neurogastroenterol Motil. 1999;11(2):101–107. doi: 10.1046/j.1365-2982.1999.00145.x. [DOI] [PubMed] [Google Scholar]
- 30.Saffrey MJ. Ageing of the enteric nervous system. Mech Ageing Dev. 2004;125(12):899–906. doi: 10.1016/j.mad.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Wang C, et al. Changes in the innervation of the mouse internal anal sphincter during aging. Neurogastroenterol Motil. 2013;25(7):e469–e477. doi: 10.1111/nmo.12144. [DOI] [PubMed] [Google Scholar]
- 32.Phillips RJ, Pairitz JC, Powley TL. Age-related neuronal loss in the submucosal plexus of the colon of Fischer 344 rats. Neurobiol Aging. 2007;28(7):1124–1137. doi: 10.1016/j.neurobiolaging.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 33.Phillips RJ, et al. Alpha-synuclein expression patterns in the colonic submucosal plexus of the aging Fischer 344 rat: implications for biopsies in aging and neurodegenerative disorders? Neurogastroenterol Motil. 2013;25(9):e621–e633. doi: 10.1111/nmo.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillips RJ, Kieffer EJ, Powley TL. Loss of glia and neurons in the myenteric plexus of the aged Fischer 344 rat. Anat Embryol (Berl) 2004;209(1):19–30. doi: 10.1007/s00429-004-0426-x. [DOI] [PubMed] [Google Scholar]
- 35.Gomez-Pinilla PJ, et al. Changes in interstitial cells of cajal with age in the human stomach and colon. Neurogastroenterol Motil. 2011;23(1):36–44. doi: 10.1111/j.1365-2982.2010.01590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiong Z, et al. Changes in calcium channel current densities in rat colonic smooth muscle cells during development and aging. Am J Physiol. 1993;265(3 Pt 1):C617–C625. doi: 10.1152/ajpcell.1993.265.3.C617. [DOI] [PubMed] [Google Scholar]
- 37.Mahavadi S, et al. Amelioration of excess collagen IalphaI, fibrosis, and smooth muscle growth in TNBS-induced colitis in IGF-I(+/−) mice. Inflamm Bowel Dis. 2011;17(3):711–719. doi: 10.1002/ibd.21437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gomes OA, de Souza RR, Liberti EA. A preliminary investigation of the effects of aging on the nerve cell number in the myenteric ganglia of the human colon. Gerontology. 1997;43(4):210–217. doi: 10.1159/000213852. [DOI] [PubMed] [Google Scholar]
- 39.Firth M, Prather CM. Gastrointestinal motility problems in the elderly patient. Gastroenterology. 2002;122(6):1688–1700. doi: 10.1053/gast.2002.33566. [DOI] [PubMed] [Google Scholar]
- 40.Gundling F, et al. Influence of gender and age on anorectal function: normal values from anorectal manometry in a large caucasian population. Digestion. 2010;81(4):207–213. doi: 10.1159/000258662. [DOI] [PubMed] [Google Scholar]
- 41.Hall K. Effect of Aging on Gastrointestinal Function. In: Halter JB OJ, Tinetti ME, Studenski S, High KP, Asthana S, Hazzard WR, editors. Hazzard's Geriatric Medicine and Gerontology. New York, US: McGraw Hill; 2009. [Google Scholar]
- 42.Noelting J, et al. Normal values for high-resolution anorectal manometry in healthy women: effects of age and significance of rectoanal gradient. The American journal of gastroenterology. 2012;107(10):1530–1536. doi: 10.1038/ajg.2012.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rao SS, et al. Evaluation of constipation in older adults: radioopaque markers (ROMs) versus wireless motility capsule (WMC) Arch Gerontol Geriatr. 2012;55(2):289–294. doi: 10.1016/j.archger.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 44.Akpan A, Gosney MA, Barret J. Factors contributing to fecal incontinence in older people and outcome of routine management in home, hospital and nursing home settings. Clin Interv Aging. 2007;2(1):139–145. doi: 10.2147/ciia.2007.2.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matthews CA, et al. Risk factors for urinary, fecal, or dual incontinence in the nurses' health study. Obstet Gynecol. 2013;122(3):539–545. doi: 10.1097/AOG.0b013e31829efbff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tjandra JJ, et al. Predictive factors for faecal incontinence after third or fourth degree obstetric tears: a clinico-physiologic study. Colorectal Dis. 2008;10(7):681–688. doi: 10.1111/j.1463-1318.2007.01467.x. [DOI] [PubMed] [Google Scholar]
- 47.Samarasekera DN, et al. Long-term anal continence and quality of life following postpartum anal sphincter injury. Colorectal Dis. 2008;10(8):793–799. doi: 10.1111/j.1463-1318.2007.01445.x. [DOI] [PubMed] [Google Scholar]
- 48.Halland M, et al. Prevalence correlates and impact of fecal incontinence among older women. Dis Colon Rectum. 2013;56(9):1080–1086. doi: 10.1097/DCR.0b013e31829203a9. [DOI] [PubMed] [Google Scholar]
- 49.Ditah I, et al. Prevalence, Trends, and Risk Factors for Fecal Incontinence in US Adults, 2005–2010. Clin Gastroenterol Hepatol. 2013 doi: 10.1016/j.cgh.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 50.Faucheron JL, Chodez M, Boillot B. Neuromodulation for fecal and urinary incontinence: functional results in 57 consecutive patients from a single institution. Dis Colon Rectum. 2012;55(12):1278–1283. doi: 10.1097/DCR.0b013e31826c7789. [DOI] [PubMed] [Google Scholar]
- 51.Caremel R, et al. Can sacral neuromodulation improve minor incontinence symptoms in doubly incontinent patients successfully treated for major incontinence symptoms? Urology. 2012;79(1):80–85. doi: 10.1016/j.urology.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 52.Leung FW, Rao SC SJ. Fecal Incontinence. In: Capezuti EA SE, Mezey MD, editors. Encyclopedia of Elder Care. New York, US: Springer Publishing Company; 2008. [Google Scholar]
- 53.Leung FW, Rao SS. Approach to fecal incontinence and constipation in older hospitalized patients. Hosp Practice. 2011;39(1):97–104. doi: 10.3810/hp.2011.02.380. [DOI] [PubMed] [Google Scholar]