Abstract
Background
The aim of this retrospective study was to define the incidence of brain metastases as a first site of recurrence among women with triple receptor-negative breast cancer (TNBC).
Methods
2448 patients with stage I–III TNBC diagnosed between 1990 and 2010 were identified. We computed the cumulative incidence of developing brain metastases as a first site of recurrence at 2 and 5 years. Cox proportional hazards models were fitted to determine factors that could predict for the development of brain metastases as a first site of recurrence. Kaplan-Meier product limit method was used to compute survival following a diagnosis of brain metastases.
Results
At a median follow up of 39 months 115 (4.7%) patients had developed brain metastases as a first site of recurrence. The cumulative incidence at 2 and 5 years was 3.7% (95% CI 2.9%–4.5%) and 5.4% (95% CI 4.4%–6.5%), respectively. Among patients with stage I, II and III disease, the 2-year cumulative incidence of brain metastases was 0.8%, 3.1% and 8%, respectively (p<0.0001). 5-year cumulative incidence was 2.8%, 4.6% and 9.6% among patients with stage I, II and III disease, respectively (p<0.0001). In the multivariable model, patients with stage III disease had a significant increase in the risk of developing brain metastases as a first site of recurrence (HR = 3.51; 95% CI 1.85 – 6.67; p = .0001) compared to patients with stage I disease. Those with stage II disease had a non significant increased risk of developing brain metastases as a first site of recurrence (HR = 1.61; 95% CI 0.92 – 2.81; p = .10) compared to patients with stage I disease. Median survival following a diagnosis of brain metastases was 7.2 months (range 5.7 to 9.4 months).
Conclusion
Patients with non metastatic TNBC have a high early incidence of developing brain metastases as a first site of recurrence, which is associated with subsequent poor survival. Patients with stage III TNBC in particular would be an ideal cohort to research preventive strategies.
Introduction
It is estimated that in the year 2010 207,090 women were diagnosed with breast cancer in the USA, with approximately 39,840 deaths attributed to this disease (1). Of these women diagnosed with breast cancer, approximately 6% had de novo stage IV disease and 20% to 50% diagnosed with primary breast cancer will go on to develop metastatic disease with an estimated five year survival of 26% (2). One of the biggest clinical challenges faced by women with metastatic breast cancer is the development of brain metastases, with a reported incidence ranging from 10% to 30% (3–5) and typically associated with an approximately 80% mortality within one year of diagnosis (2). Over the last decade evidence has emerged that the incidence of brain metastases appears to be increasing, probably attributed to the fact that with the introduction of newer chemotherapeutic and targeted agents (e.g trastuzumab) women with breast cancer are living long enough to develop brain metastases coupled with superior imaging techniques leading to improved detection rates (6–9).
It has firmly been established that breast cancer is not a homogenous disease with gene expression profiling identifying at least four distinct subtypes including luminal A, luminal B, HER2-positive and basal-like subtypes (10–13). Each subtype is associated with a distinct prognostic outcome and unique predilections for sites of recurrence highlighted by the fact that the incidence of brain metastases among women with HER2-positive and basal-like subtype cancer exceeds one third of patients, while the incidence is notably lower among women with breast cancer of luminal subtype (2). Despite the high incidence of brain metastases among women with HER2-positive breast cancer, the recent introduction of trastuzumab into their treatment protocols has not only increased the time to development of brain metastases but has significantly improved survival following the development of brain metastases (14,15). Unfortunately the same trend has not been observed among women with triple receptor-negative breast cancer (TNBC) (a subtype not expressing estrogen receptors and progesterone receptors and not exhibiting over expression and/or gene amplification of HER-2/neu that typically accounts for approximately 85% of basal-like tumors).
TNBC is typically associated with an early risk of distant recurrence, a high incidence of brain metastases and an overall poor survival outcome (16, 17). Lin et al (16) reported that nearly 46% of women with metastatic TNBC developed brain metastasis that was associated with a median survival time of 4.9 months. Robust nomograms have also been developed that can help risk stratify women with metastatic breast cancer helping to identify groups most likely to develop brain metastases (18). However, with the relatively high overall incidence of brain metastases and the associated poor survival following its diagnosis among women with TNBC, a more practical and clinically meaningful research direction would be to identify groups of women with early stage TNBC who are at high risk of developing brain metastases. Our own group reported an overall 2- and 5-year cumulative incidence of brain metastases of 5.6% and 9.6%, respectively, among women with non metastatic TNBC (19). In this study we take this one step further, looking at a larger cohort of women with TNBC with longer follow up treated at the MD Anderson Cancer Center (MDACC), with the goal of determining the incidence of brain metastases as a first site of recurrence and identifying factors that can predict specific groups who are at particularly high risk.
Patients and Methods
Patient Population
Using an electronic database maintained at the Breast Medical Oncology department of the MDACC we identified a cohort of women with stage I to III TNBC. We excluded patients who were male, those with de novo stage IV disease and those with more than one primary cancer. Variables extracted from the database included those pertaining to patient demographics, tumor characteristics, stage of disease, and first site of disease recurrence. Stage of disease was coded based on the 6th of edition of the American Joint Committee on Cancer (20). Histological classification and grade of the primary tumor was coded according to the World Health Organization classification system (21) and the modified Black’s nuclear grading system (22), respectively. TNBC was defined as breast tumors that were negative for HER2, estrogen receptor and progesterone receptor. Negative status for HER2 was defined as those breast tumors that either did not exhibit gene amplification by FISH and/or were 0 or 1+ by immunohistochemistry.
Statistical analysis
Patient and tumor characteristics were tabulated and grouped by whether or not patients had developed brain metastases as a first site of recurrence and compared across groups using chi square test. Time to brain metastases was computed from the date of diagnosis of primary breast cancer to the date of development of brain metastases as a first site of recurrence or last follow up. The cumulative incidence of brain metastases as a first site of recurrence was calculated considering death or metastases at other sites as competing risks. Cumulative incidence of brain metastases as a first site of recurrence at 2 and 5 years were computed. Among patients who developed brain metastasis as a first site of recurrence, survival following brain metastases was computed from the date of diagnosis of brain metastases as a first site of recurrence to the date of death or last follow up. Both survival end points were computed using the Kaplan–Meier product limit method and compared across groups using log rank statistic. Cox proportional hazards models were then fitted to examine the association of factors with time to brain metastases. Variables included in the model were those deemed to be clinically important rather than statistically significant. The multivariable Cox proportional hazards model for time to brain metastases was adjusted for age, race, stage of disease, lymphovascular invasion, type of chemotherapy, and adjuvant radiation therapy. P-values less than 0.05 were considered statistically significant; all tests were two-sided. Statistical analyses were carried out using SAS 9.1 (SAS Institute Inc., Cary, NC), S-Plus 7.0 (Insightful Corporation, Seattle, WA) and the contributed package cmprsk (23) in R 2.9.0.
Results
Patient and tumor characteristics
We identified 2448 patients diagnosed between 1990 and 2010 with stage I to III TNBC. Table 1 summarizes patient demographics and tumor characteristics stratified by brain metastases development. Median age at diagnosis for the whole cohort was 50 years (range 21–97 years). Six hundred and sixteen (25.2%) patients had stage I disease, 1256 (51.3%) patients had stage II disease, and 576 (23.5%) patients had stage III disease. One thousand nine hundred and fifty (79.6%) patients received anthracyclines and 1614 (65.9%) patients received taxanes as part of pre operative and/or adjuvant treatment. One hundred and fifteen (4.7%) patients had brain metastases as a first site of recurrence and tended to have higher initial stage of disease and lymphovascular invasion in the primary.
Table 1.
Patient Characteristics stratified by brain metastases development as a first site of recurrence
| No Brain Metastases (N=2333) | Yes Brain Metastases (N=115) | ||||
|---|---|---|---|---|---|
|
| |||||
| N | % | N | % | P | |
|
| |||||
| Age, Median | 50 | 47 | |||
| Age | |||||
| < 50 | 1124 | 48.2 | 65 | 56.5 | |
| ≥ 50 | 1209 | 51.8 | 50 | 43.5 | .08 |
| Menopausal Status | |||||
| Pre | 970 | 41.8 | 53 | 46.1 | |
| Post | 1348 | 58.2 | 62 | 53.9 | .37 |
| Race | |||||
| White | 1514 | 64.9 | 82 | 71.3 | |
| Black | 422 | 18.1 | 15 | 13.0 | |
| Other | 397 | 17.0 | 18 | 15.7 | .31 |
| Histology | |||||
| Invasive ductal | 2108 | 91.6 | 108 | 93.9 | |
| Invasive lobular | 21 | 0.9 | 2 | 1.7 | |
| Mixed (ductal/lobular) | 24 | 1.0 | 2 | 1.7 | |
| Other | 149 | 6.5 | 3 | 2.6 | .27 |
| Stage | |||||
| I | 600 | 25.7 | 16 | 13.9 | |
| II | 1205 | 51.7 | 51 | 44.3 | |
| III | 528 | 22.6 | 48 | 41.7 | <.0001 |
| Nuclear Grade | |||||
| I | 13 | 0.6 | 0 | 0.0 | |
| II | 193 | 8.5 | 6 | 5.3 | |
| III | 2056 | 90.9 | 107 | 94.7 | .34 |
| Lymphovascular invasion | |||||
| Negative | 1642 | 72.0 | 66 | 58.9 | |
| Positive | 637 | 28.0 | 46 | 41.1 | .003 |
| Breast surgery | |||||
| Breast conservation | 1120 | 48.8 | 42 | 38.2 | |
| Mastectomy | 1150 | 50.2 | 68 | 61.8 | |
| None | 23 | 1.0 | 0 | 0.0 | .04 |
| Taxane | |||||
| No | 772 | 33.5 | 32 | 27.8 | |
| Yes | 1531 | 66.5 | 83 | 72.2 | .21 |
| Anthracycline | |||||
| No | 446 | 19.4 | 20 | 17.4 | |
| Yes | 1855 | 80.6 | 95 | 82.6 | .60 |
| Adjuvant radiation | |||||
| No | 831 | 35.6 | 38 | 33.0 | |
| Yes | 1502 | 64.4 | 77 | 67.0 | .57 |
Development of Brain metastases as a first site of disease recurrence
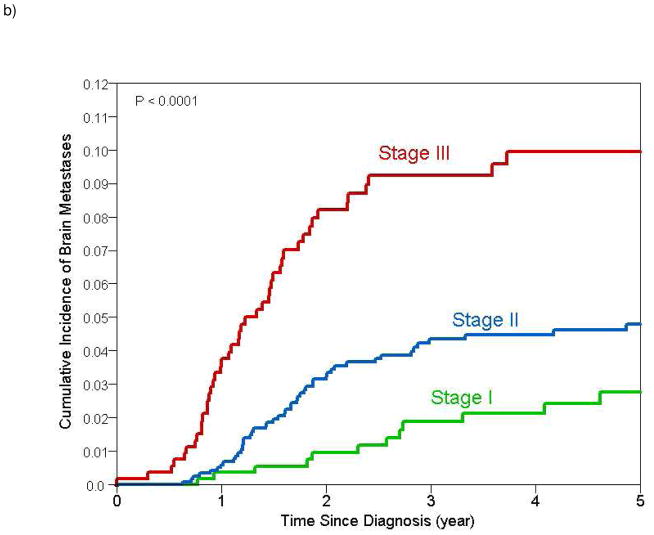
Median follow up for the whole cohort was 39 months (range 1 to 233 months). At the time of this analyses, overall 805 (32.9%) patients had developed distant recurrences of whom 115 (4.7%) had developed brain metastases as a first site of distant recurrence. Overall cumulative incidence of a distant recurrence at 2 and 5 years was 24.9% (95% CI 23.0% – 26.8%) and 38.5% (95% CI 36.2% – 40.8%), respectively. Table 2 summarizes the results of 2- and 5-year cumulative incidence of brain metastases as a first site of disease recurrence. Cumulative incidence of brain metastases as a first site of recurrence was 3.7% (95% CI 2.9%–4.5%) and 5.4% (95% CI 4.4%–6.5%) at 2 and 5 years, respectively. Among patients with stage I, II and III disease, the 2-year cumulative incidence of brain metastases was 0.8%, 3.1% and 8%, respectively (p<0.0001). 5-year cumulative incidence of brain metastases was 2.8%, 4.6% and 9.6% among patients with stage I, II and III disease, respectively (p<0.0001) (Figure 1). The presence of lymphovascular invasion was also found to be associated with a significantly higher cumulative incidence of brain metastases.
Table 2.
Cumulative Incidence of Brain Metastases at 2 and 5 Years by Patient and Clinical Characteristics
| N Patients | N Events | 2-Year cumulative incidence | 95% CI | 5-Year cumulative incidence | 95% CI | P | |
|---|---|---|---|---|---|---|---|
| All | 2448 | 115 | 3.7% | 2.9% to 4.5% | 5.4% | 4.4% to 6.5% | |
| Age | |||||||
| < 50 | 1189 | 65 | 4.1% | 2.9% to 5.3% | 6% | 4.5% to 7.5% | |
| ≥ 50 | 1259 | 50 | 3.4% | 2.3% to 4.4% | 4.7% | 3.4% to 6% | .11 |
| Menopausal Status | |||||||
| Pre | 1023 | 53 | 4% | 2.7% to 5.3% | 5.8% | 4.2% to 7.5% | |
| Post | 1410 | 62 | 3.6% | 2.5% to 4.6% | 5% | 3.7% to 6.3% | .40 |
| Race | |||||||
| White | 1596 | 82 | 3.9% | 2.9% to 4.9% | 5.8% | 4.5% to 7.1% | |
| Black | 437 | 15 | 2.6% | 1% to 4.3% | 3.3% | 1.4% to 5.1% | |
| Other | 415 | 18 | 3.9% | 1.8% to 6% | 4.6% | 2.3% to 7% | .38 |
| Stage | |||||||
| I | 616 | 16 | 0.8% | 0% to 1.5% | 2.8% | 1.2% to 4.4% | |
| II | 1256 | 51 | 3.1% | 2.1% to 4.2% | 4.6% | 3.3% to 5.9% | |
| III | 576 | 48 | 8% | 5.5% to 10.4% | 9.6% | 6.9% to 12.3% | <.0001 |
| Nuclear Grade | |||||||
| I/II | 212 | 6 | 0.5% | 0% to 1.5% | 1.7% | 0% to 3.6% | |
| III | 2163 | 107 | 4.1% | 3.2% to 5% | 5.7% | 4.6% to 6.8% | .07 |
| Lymphovascular invasion | |||||||
| Negative | 1708 | 66 | 3.1% | 2.2% to 4% | 4.3% | 3.2% to 5.3% | |
| Positive | 683 | 46 | 5.3% | 3.5% to 7% | 7.6% | 5.4% to 9.8% | .009 |
| Breast surgery | |||||||
| Breast conservation | 1162 | 42 | 2.5% | 1.5% to 3.4% | 3.8% | 2.6% to 5% | |
| Mastectomy | 1218 | 68 | 4.8% | 3.5% to 6.1% | 6.7% | 5.1% to 8.3% | .06 |
| Taxane | |||||||
| No | 804 | 32 | 2.1% | 1.1% to 3.2% | 4.2% | 2.7% to 5.8% | |
| Yes | 1614 | 83 | 4.5% | 3.4% to 5.6% | 5.8% | 4.6% to 7.1% | .12 |
| Anthracycline | |||||||
| No | 466 | 20 | 2.4% | 0.8% to 4% | 5.3% | 2.8% to 7.8% | |
| Yes | 1950 | 95 | 4% | 3% to 4.9% | 5.3% | 4.2% to 6.4% | .80 |
| Adjuvant radiation | |||||||
| No | 869 | 38 | 3.4% | 2% to 4.7% | 5.1% | 3.4% to 6.9% | |
| Yes | 1579 | 77 | 3.9% | 2.9% to 4.9% | 5.3% | 4.1% to 6.5% | .89 |
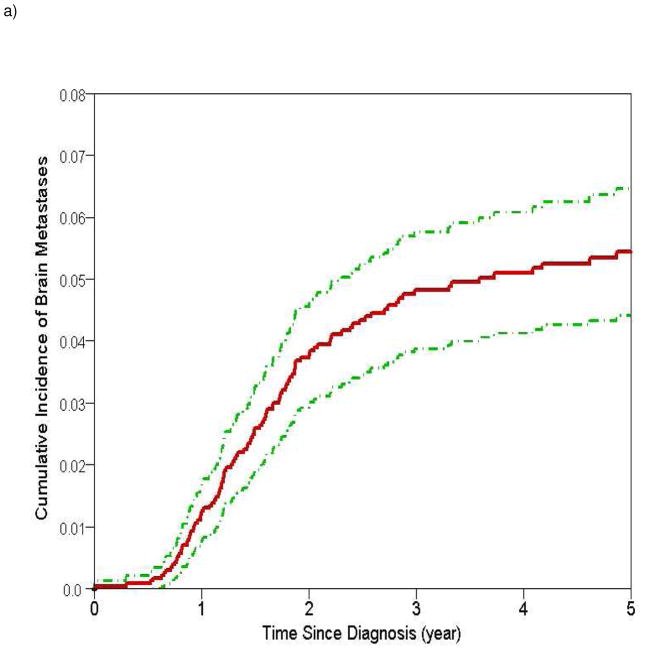
Figure 1.
Estimates of cumulative incidence of brain metastases with 95% confidence intervals for a) all patients (cumulative incidence at 2 and 5 years 3.7% and 5.4% respectively) and b) stratified by stage (cumulative incidence at 2 and 5 years for stage I 0.8% and 2.8% respectively; stage II 3.1% and 4.6% respectively; stage III 8% and 9.6% respectively)
Median time to brain metastases was 31 months (range 0 to 214 months). After adjusting for age, race, stage of disease, lymphovascular invasion, type of chemotherapy, and adjuvant radiation therapy, patients with stage III disease had a significant increase in the risk of developing brain metastases as a first site of recurrence (HR = 3.51; 95% CI 1.85 – 6.67; p = .0001) compared to patients with stage I disease. Those with stage II disease had a non significant increased risk of developing brain metastases (HR = 1.61; 95% CI 0.92 – 2.81; p = .10) compared to patients with stage I disease (Table 3). Compared to patients who were of white race the risk of developing brain metastases as first site metastases was not significantly different among those of black (HR = 0.61, 95%CI 0.34–1.09, p=0.09) and other race (HR = 0.80, 95% CI 0.48–1.35, p=0.41).
Table 3.
Multivariable Cox proportional hazards model for time to brain metastases
| HR | 95% CI | P | |
|---|---|---|---|
| Age: ≥ 50 v. < 50 | 0.75 | 0.52 – 1.10 | 0.14 |
| Race: black v. white | 0.61 | 0.34 – 1.09 | 0.09 |
| Race: other v. white | 0.80 | 0.48 – 1.35 | 0.41 |
| Stage: II v. I | 1.61 | 0.92 – 2.81 | 0.10 |
| Stage: III v. I | 3.51 | 1.85 – 6.67 | 0.0001 |
| LVI: positive v. negative | 1.29 | 0.87 – 1.93 | 0.21 |
| Adjuvant radiation: yes v. no | 0.93 | 0.61 – 1.42 | 0.74 |
| Taxane: yes v. no | 1.04 | 0.66 – 1.65 | 0.86 |
| Anthracycline: yes v. no | 0.71 | 0.40 – 1.24 | 0.23 |
Survival following brain metastases
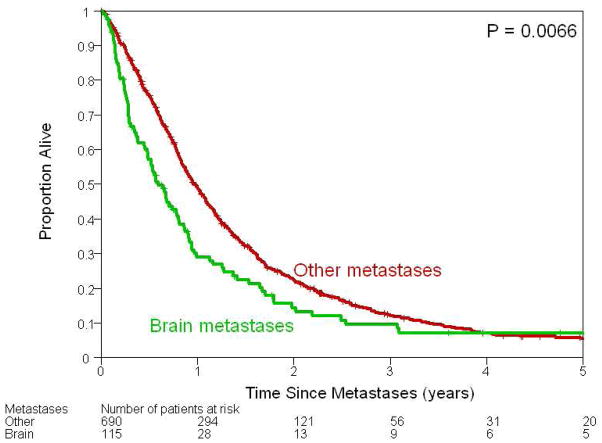
Median follow up following a diagnosis of brain metastases was 6.5 months (0 – 165 months). At the time of these analyses 94 (81.7%) of the 115 patients with brain metastases had died. Median survival following a diagnosis of brain metastases based on the Kaplan-Meier product limit method was 7.2 months (95% CI 5.7 – 9.4 months) (Figure 2). Fifty-three percent (n=61) of patients with brain metastases had a median survival greater than 6 months. Patients with distant metastases at sites other than the brain had a significantly longer median survival than patients with brain metastases as a first site of recurrence (11.6 months vs. 7.2 months, p =0.006). Table 4 summarizes factors associated with survival following a diagnosis of brain metastases as a first site of recurrence. Initial stage of breast cancer did not significantly affect survival following a diagnosis of brain metastases.
Figure 2.
Kaplan–Meier plots illustrating survival following development of metastases among patients who developed brain metastases and those who developed metastases at other sites. Median survival among those who developed brain and other metastases was 7.2 months [95% CI 5.7–9.4 months] and 11.6 months (95% CI 10.6 – 13.0 months), respectively.
Table 4.
Univariate survival estimates following brain metastases
| N Patients | N Events | Median survival (months) | Lower 95% CI | Upper 95% CI | P | |
|---|---|---|---|---|---|---|
| All | 115 | 94 | 7.2 | 5.7 | 9.4 | |
| Age | ||||||
| < 50 | 65 | 54 | 8.5 | 6.6 | 11.3 | |
| ≥ 50 | 50 | 40 | 5.5 | 3.5 | 9.4 | .11 |
| Menopausal Status | ||||||
| Pre | 53 | 44 | 9.8 | 6.6 | 11.9 | |
| Post | 62 | 50 | 5.8 | 3.7 | 8.9 | .13 |
| Race | ||||||
| White | 82 | 69 | 8.9 | 5.8 | 10.8 | |
| Black | 15 | 11 | 7.7 | 4.1 | 8.1 | |
| Other | 18 | 14 | 5.5 | 2.8 | 19.8 | .97 |
| Stage | ||||||
| I | 16 | 13 | 7.7 | 5.7 | 18.4 | |
| II | 51 | 42 | 9.8 | 5.5 | 11.6 | |
| III | 48 | 39 | 5.8 | 3.4 | 6.9 | .61 |
| Nuclear Grade | ||||||
| II | 6 | 5 | 20.5 | 3.1 | 30.5 | |
| III | 107 | 88 | 6.9 | 5.7 | 9.4 | .32 |
| Lymphovascular invasion | ||||||
| Negative | 66 | 54 | 8.1 | 5.8 | 11.0 | |
| Positive | 46 | 38 | 6.4 | 4.2 | 9.7 | .20 |
| Breast surgery | ||||||
| Breast conservation | 42 | 32 | 7.7 | 4.6 | 10.8 | |
| Mastectomy | 68 | 59 | 6.9 | 5.4 | 9.7 | .63 |
| Adjuvant chemotherapy | ||||||
| No | 64 | 49 | 5.8 | 3.7 | 8.2 | |
| Yes | 51 | 45 | 8.9 | 6.9 | 11.9 | .57 |
| Taxane | ||||||
| No | 32 | 25 | 10.4 | 7.7 | 20.5 | |
| Yes | 83 | 69 | 6.4 | 4.1 | 8.5 | .051 |
| Anthracycline | ||||||
| No | 20 | 14 | 10.4 | 6.9 | 26.3 | |
| Yes | 95 | 80 | 6.5 | 4.6 | 8.5 | .14 |
| Adjuvant radiation | ||||||
| No | 38 | 35 | 6.7 | 3.2 | 11.0 | |
| Yes | 77 | 59 | 7.7 | 5.8 | 9.8 | .61 |
Discussion
The goal of this large, single-institution retrospective study was to define the incidence of brain metastases as a first site of recurrence among women with stage I to III TNBC. Our results confirm what is already known about early stage TNBC in that this subtype has a high early incidence of distant recurrence with a cumulative incidence at 2 and 5 years of 24.9% and 38.5% observed in our cohort. The cumulative incidence of brain metastases as a first site of recurrence for the whole cohort at 2 years was observed to be 3.7% and was 8% for women with stage III TNBC.
Several studies have looked at the incidence of brain metastases among women with early stage breast cancer and have attempted to identify risk factors for this site of metastases (7, 24–29). Looking at a cohort of more than 9000 women with early stage breast cancer who were enrolled into the International Breast Cancer Study Group clinical trials between 1978 and 1999 Pestalozzi et al (24) reported an overall 2-year incidence of CNS metastases as a first site of recurrence of 0.5% with women who had estrogen receptor negative disease (2-year incidence of 1.1%) and those having HER2 positive disease (2-year incidence 1.2%) being at higher risk. In a single institution study of over 3000 women Hietz et al (29) reported a higher odds of developing brain metastases among women with TNBC (OR:4.16; 95%CI:2.26–7.64; p<0.001) compared to other breast tumor subtypes. Following a diagnosis of brain metastases, several studies have documented poor survival outcomes across all subtypes of breast cancer with TNBC exhibiting a worse outcome (16, 19, 30–33). Looking at a cohort of 99 patients with breast cancer and brain metastases, Carey et al (33) reported that patients with TNBC had the shortest survival (0.24 years) compared to other subtypes. Our own group recently reported an overall survival of 2.9 months following a diagnosis of brain metastases at any time point among women with TNBC and 5.8 months among women with this subtype who developed brain metastases as a first site of recurrence (19).
It is obvious from the studies discussed that TNBC is a subtype of breast cancer that is associated with high risk of developing brain metastases, with an associated subsequent poor survival outcome making it an ideal cohort to look at exploring newer targeted agents and other modalities geared to preventing the development of brain metastases. In order to explore such options it will first be important to further identify a specific subgroup of patients with TNBC at highest risk of developing brain metastases. Graesslin et al (18) developed a robust nomogram that is able to identify women with metastatic breast cancer at high risk of developing brain metastases. However, the current evidence, although retrospective in nature, indicates that women with TNBC have a high early risk of relapse including the development of brain metastases. Indeed, in this study we report a 2–year cumulative incidence of brain metastases of 3.7% as a first site of recurrence. These results allow us to hypothesize that the better group to target preventive strategies would be early stage TNBC rather than established metastatic TNBC. We further report that the women with non metastatic TNBC at highest risk of developing brain metastases as a first site recurrence are those with stage III disease at diagnosis, with a cumulative incidence of 8% at 2-years. Our results further indicate that the risk there after plateaus with a cumulative incidence of brain metastases increasing only slightly to 9.6% at five years for women with stage III TNBC. Other variables such as age at diagnosis, menopausal status, race, lymphovascular invasion and type of chemotherapy received for non metastatic TNBC did not significantly impact the risk of developing brain metastases.
Prior studies have reported that compared to Caucasian women African American women with breast cancer have an overall worse prognostic outcome and have a higher risk of developing TNBC (34). In our study we noted that among women with non metastatic TNBC race did not significantly impact the incidence of brain metastases (2-year cumulative incidence of 3.9% vs. 2.6% among white vs. black women) nor did it impact survival following a diagnosis of brain metastases (median survival 8.9 months vs. 7.7 months among white vs. black women). This is consistent with results recently reported by Carey et al (33) where ethnicity was not found to be associated with a differential prognostic outcome following a diagnosis of brain metastases across all subtypes of breast cancer.
Having identified a subgroup of women with TNBC at highest risk of developing brain metastases the next question is whether we can further risk stratify. In terms of clinical variables, the presence of residual disease following pre operative chemotherapy has been shown to predict a higher risk of recurrence among women with TNBC compared to women with non TNBC who did not achieve a pathological complete response rate (17, 35). Although the terms TNBC and basal-like subtype are often used interchangeably, approximately 25% of TNBC are not basal-like on gene expression arrays (36). Basal-like tumors haves been shown to be better characterized by using five immunohistochemistry markers that include, in addition to the estrogen, progesterone and HER2 receptors, cytokeratin 5/6 and EGFR (36). Using this method, Cheang et al (37) were able to identify a group of women with TNBC who had inferior prognostic outcome when their tumors in addition were positive for cytokeratin 5/6 and/or EGFR. A subset of TNBC has also been identified that although not exhibiting over expression of HER2 by immunohistochemistry, exhibits gene expression signatures of the HER2 enriched subtype on gene expression profiling and may thus identify a subgroup of TNBC that portends a worse prognostic outcome. The interesting question for this subtype is whether agents directed against HER2 would be of any benefit (38). Another recently identified subgroup of TNBC is the claudin low subgroup that typically represents 5% of tumors, is characterized by expression of stem cell features and is associated with poor prognostic outcome (36, 38).
In summary, our results indicate that women with stage III TNBC have a high early risk of developing brain metastases as the first site recurrence. We acknowledge that the study has several limitations including the fact that it is retrospective in design and is from a single institution and thus subject to referral bias. However, our results are hypothesis generating, indicating that perhaps the best group of women with TNBC to target preventive strategies is the group of women with stage III disease. As described above, TNBC in itself is a heterogeneous disease encompassing a number of poor prognostic subgroups including the basal-like, HER2 enriched and claudin low subtypes that could perhaps further refine high-risk TNBC groups. Regardless, preventive strategies such as prophylactic cranial radiation therapy and/or novel targeted agents that can cross the blood barrier need to be explored.
Acknowledgments
Supported, in part, by 2P30 CA016672 33 and the Nellie B. Connally Breast Cancer Research Fund
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Lu J, Steeg PS, Price JE, et al. Breast cancer metastasis: challenges and opportunities. Cancer Res. 2009;69(12):4951–3. doi: 10.1158/0008-5472.CAN-09-0099. [DOI] [PubMed] [Google Scholar]
- 3.Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004;22(17):3608–17. doi: 10.1200/JCO.2004.01.175. [DOI] [PubMed] [Google Scholar]
- 4.Tsukada Y, Fouad A, Pickren JW, Lane WW. Central nervous system metastasis from breast carcinoma. Autopsy study Cancer. 1983;52:2349–2354. doi: 10.1002/1097-0142(19831215)52:12<2349::aid-cncr2820521231>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 5.Patanaphan V, Salazar OM, Risco R. Breast cancer: Metastatic patterns and their prognosis. Southern Medical Journal. 1988;81:1109–1112. [PubMed] [Google Scholar]
- 6.Leyland-Jones B. Human epidermal growth factor receptor 2-positive breast cancer and central nervous system metastases. J Clin Oncol. 2009;27(31):5278–86. doi: 10.1200/JCO.2008.19.8481. [DOI] [PubMed] [Google Scholar]
- 7.Pelletier EM, Amonkar MM, Shim B. Incidence and prevalence of brain metastases among patients with advanced breast cancer in a US managed-care population. J Clin Oncol. 2007;25(suppl 18):353s. abstr 6624. [Google Scholar]
- 8.Carey LA, Ewend MG, Metzger R, et al. Central nervous system metastases in women after multimodality therapy for high risk breast cancer. Breast Cancer Res Treat. 2004;88:273–280. doi: 10.1007/s10549-004-0999-3. [DOI] [PubMed] [Google Scholar]
- 9.Schouten LJ, Rutten J, Huveneers HA, et al. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94:2698–2705. doi: 10.1002/cncr.10541. [DOI] [PubMed] [Google Scholar]
- 10.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumors. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 11.Sorlie T. Molecular portraits of breast cancer: Tumour subtypes as distinct disease entities. Eur J Cancer. 2004;40:2667–2675. doi: 10.1016/j.ejca.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 12.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brenton JD, Carey LA, Ahmed AA, et al. Molecular classification and molecular forecasting of breast cancer: Ready for clinical application? J Clin Oncol. 2005;23:7350–7360. doi: 10.1200/JCO.2005.03.3845. [DOI] [PubMed] [Google Scholar]
- 14.Eichler AF, Kuter I, Ryan P, et al. Survival in patients with brain metastases from breast cancer: The importance of HER-2 status. Cancer. 2008;112:2359–2367. doi: 10.1002/cncr.23468. [DOI] [PubMed] [Google Scholar]
- 15.Dawood SS, Broglio K, Esteva FJ, et al. Defining prognosis for women with breast cancer and CNS metastases by HER2 status. Ann Oncol. 2008;19:1242–1248. doi: 10.1093/annonc/mdn036. [DOI] [PubMed] [Google Scholar]
- 16.Lin NU, Claus E, Sohl J, et al. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer. 2008;113(10):2638–45. doi: 10.1002/cncr.23930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carey LA, Dees EC, Sawyer L, Gatti L, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13(8):2329–34. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 18.Graesslin O, Abdulkarim BS, Coutant C, et al. Nomogram to predict subsequent brain metastasis in patients with metastatic breast cancer. J Clin Oncol. 2010;28(12):2032–7. doi: 10.1200/JCO.2009.24.6314. [DOI] [PubMed] [Google Scholar]
- 19.Dawood S, Broglio K, Esteva FJ, et al. Survival among women with triple receptor-negative breast cancer and brain metastases. Ann Oncol. 2009;20(4):621–7. doi: 10.1093/annonc/mdn682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singletary SE, Allred C, Ashley P, et al. Staging system for breast cancer: revisions for the 6th edition of the AJCC Cancer Staging Manual. Surg Clin North Am. 2003;83:803–819. doi: 10.1016/S0039-6109(03)00034-3. [DOI] [PubMed] [Google Scholar]
- 21.The World Health Organization Histological Typing of Breast Tumors—Second Edition. The World Organization. Am J Clin Pathol. 1982;78:806–816. doi: 10.1093/ajcp/78.6.806. [DOI] [PubMed] [Google Scholar]
- 22.Black MM, Speer FD. Nuclear structure in cancer tissues. Surg Gynecol Obstet. 1957;105:97–102. [PubMed] [Google Scholar]
- 23.Gray B. The cmprsk package. Vienna, Austria: The Comprehensive R Archive Network, Vienna University of Economics and Business Administration; 2007. [Google Scholar]
- 24.Pestalozzi BC, Zahrieh D, Price KN, et al. Identifying breast cancer patients at risk for Central Nervous System (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG) Ann Oncol. 2006;17:935–944. doi: 10.1093/annonc/mdl064. [DOI] [PubMed] [Google Scholar]
- 25.Hicks DG, Short SM, Prescott NL, et al. Breast cancers with brain metastases are more likely to be estrogen receptor negative, express the basal cytokeratin CK 5/6, and overexpress HER2 or EGFR. American Journal of Surgical Pathology. 2006;30:1097–1104. doi: 10.1097/01.pas.0000213306.05811.b9. [DOI] [PubMed] [Google Scholar]
- 26.Tham YL, Sexton K, Kramer R, Hilsenbeck S, Elledge R. Primary breast cancer phenotypes associated with propensity for central nervous system metastases. Cancer. 2006;107:696–704. doi: 10.1002/cncr.22041. [DOI] [PubMed] [Google Scholar]
- 27.Stemmler HJ, Kahlert S, Siekiera W, Untch M, Heinrich B, Heinemann V. Characteristics of patients with brain metastases receiving trastuzumab for HER2 overexpressing metastatic breast cancer. Breast. 2006;15:219–225. doi: 10.1016/j.breast.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 28.Samaan NA, Buzdar AU, Aldinger KA, et al. Estrogen receptor: A prognostic factor in breast cancer. Cancer. 1981;47:554–560. doi: 10.1002/1097-0142(19810201)47:3<554::aid-cncr2820470322>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 29.Heitz F, Harter P, Traut A, Lueck HJ, Beutel B, du Bois a. Cerebral metastases (CM) in breast cancer (BC) with focus on triple-negative tumors. J Clin Oncol. 2008;26(May 20 suppl):abstr 1010. [Google Scholar]
- 30.Nam B-H, Kim SY, Han HS, et al. Breast cancer subtypes and survival in patients with brain metastases. Breast Cancer Res. 2008;10:R20. doi: 10.1186/bcr1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hines SL, Vallow LA, Tan WW, McNeil RB, Perez EA, Jain A. Clinical outcomes after a diagnosis of brain metastases in patients with estrogen- and/or human epidermal growth factor receptor 2-positive versus triple-negative breast cancer. Ann Oncol. 2008;19(9):1561–5. doi: 10.1093/annonc/mdn283. [DOI] [PubMed] [Google Scholar]
- 32.Dawood S, Broglio K, Esteva FJ, et al. Defining prognosis for women with breast cancer and CNS metastases by HER2 status. Ann Oncol. 2008;19(7):1242–8. doi: 10.1093/annonc/mdn036. [DOI] [PubMed] [Google Scholar]
- 33.Anders CK, Deal AM, Miller CR, et al. The prognostic contribution of clinical breast cancer subtype, age, and race among patients with breast cancer brain metastases. Cancer. 2011;117(8):1602–11. doi: 10.1002/cncr.25746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Brien KM, Cole SR, Tse CK, et al. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clin Cancer Res. 2010;16(24):6100–10. doi: 10.1158/1078-0432.CCR-10-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26(8):1275–81. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 36.Carey LA. Directed therapy of subtypes of triple-negative breast cancer. Oncologist. 2011;16 (Suppl 1):71–8. doi: 10.1634/theoncologist.2011-S1-71. [DOI] [PubMed] [Google Scholar]
- 37.Cheang MC, Voduc D, Bajdik C, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008;14:1368–76. doi: 10.1158/1078-0432.CCR-07-1658. [DOI] [PubMed] [Google Scholar]
- 38.Perou CM. Molecular stratification of triple-negative breast cancers. Oncologist. 2011;16 (Suppl 1):61–70. doi: 10.1634/theoncologist.2011-S1-61. [DOI] [PubMed] [Google Scholar]