Abstract
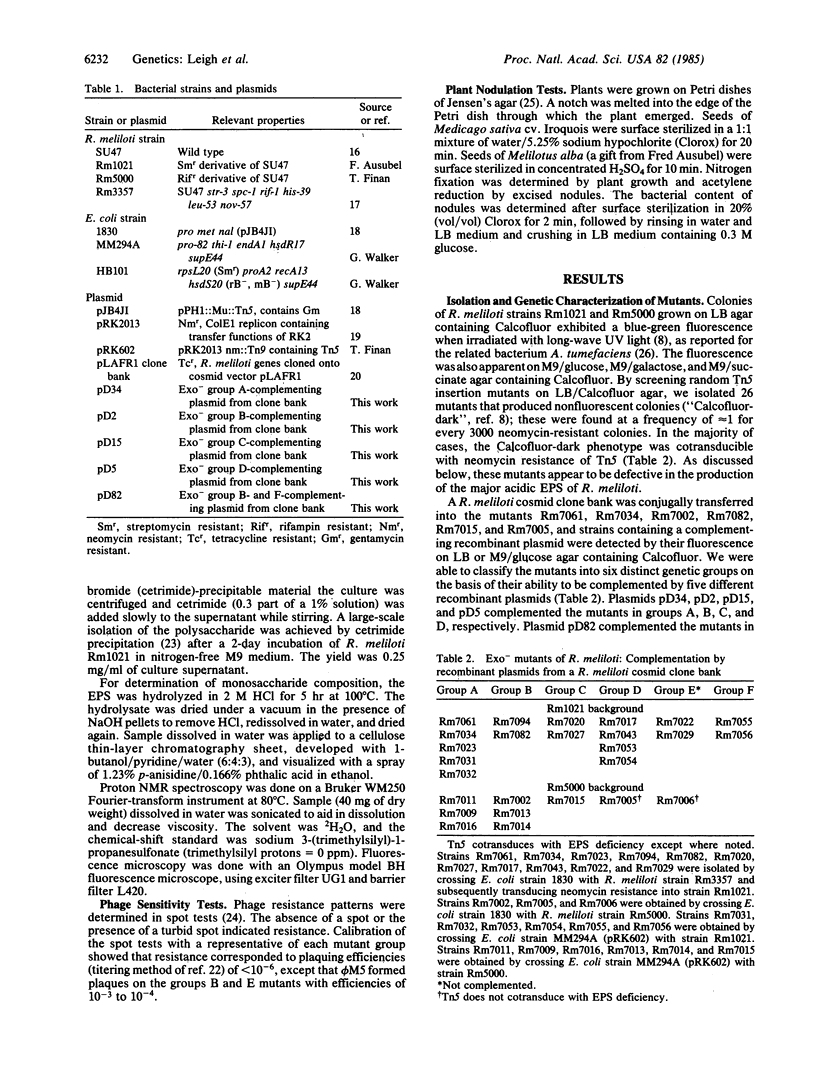
By screening with the fluorescent stain Calcofluor, we have isolated 26 independent transposon Tn5 insertion mutants of Rhizobium meliloti that are deficient in the production of a known extracellular polysaccharide (Exo-). The mutants belonged to six distinct genetic groups based on the ability of their Exo- phenotype to be complemented by different recombinant plasmids from a R. meliloti clone bank. With few exceptions, all of the mutants formed ineffective (non-nitrogen-fixing) nodules on alfalfa. For all but one group, the complementing plasmids restored effective nodulation. These results establish a firm and extensive correlation between the ability of Rhizobium to produce a particular polysaccharide and symbiotic proficiency. The ineffective nodules appeared to contain no bacteroids and to form without shepherds' crooks or infection threads; this symbiotic phenotype matches that described for a set of independently isolated mutants that belong phenotypically and genetically to the group B exopolysaccharide mutants described previously [Finan et al. (1985) Cell 40, 869-877]. Apparently the exopolysaccharide, although not required for nodule formation, is involved in wild-type nodule invasion.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe M., Sherwood J. E., Hollingsworth R. I., Dazzo F. B. Stimulation of clover root hair infection by lectin-binding oligosaccharides from the capsular and extracellular polysaccharides of Rhizobium trifolii. J Bacteriol. 1984 Nov;160(2):517–520. doi: 10.1128/jb.160.2.517-520.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björndal H., Erbing C., Lindberg B., Fåhraeus G., Ljunggren H. Studies on an extracellular polysaccharide from Rhizobium meliloti. Acta Chem Scand. 1971;25(4):1281–1286. doi: 10.3891/acta.chem.scand.25-1281. [DOI] [PubMed] [Google Scholar]
- Chakravorty A. K., Zurkowski W., Shine J., Rolfe B. G. Symbiotic nitrogen fixation: molecular cloning of Rhizobium genes involved in exopolysaccharide synthesis and effective nodulation. J Mol Appl Genet. 1982;1(6):585–596. [PubMed] [Google Scholar]
- Darzins A., Chakrabarty A. M. Cloning of genes controlling alginate biosynthesis from a mucoid cystic fibrosis isolate of Pseudomonas aeruginosa. J Bacteriol. 1984 Jul;159(1):9–18. doi: 10.1128/jb.159.1.9-18.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan T. M., Hartweig E., LeMieux K., Bergman K., Walker G. C., Signer E. R. General transduction in Rhizobium meliloti. J Bacteriol. 1984 Jul;159(1):120–124. doi: 10.1128/jb.159.1.120-124.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan T. M., Hirsch A. M., Leigh J. A., Johansen E., Kuldau G. A., Deegan S., Walker G. C., Signer E. R. Symbiotic mutants of Rhizobium meliloti that uncouple plant from bacterial differentiation. Cell. 1985 Apr;40(4):869–877. doi: 10.1016/0092-8674(85)90346-0. [DOI] [PubMed] [Google Scholar]
- Friedman A. M., Long S. R., Brown S. E., Buikema W. J., Ausubel F. M. Construction of a broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene. 1982 Jun;18(3):289–296. doi: 10.1016/0378-1119(82)90167-6. [DOI] [PubMed] [Google Scholar]
- Harada T., Amemura A., Jansson P. E., Lindberg B. Comparative studies of polysaccharides elaborated by Rhizobium, Alceligenes, and Agrobacterium. Carbohydr Res. 1979 Dec;77:285–288. doi: 10.1016/s0008-6215(00)83821-5. [DOI] [PubMed] [Google Scholar]
- Higashi S., Abe M. Promotion of infection thread formation by substances from Rhizobium. Appl Environ Microbiol. 1980 Feb;39(2):297–301. doi: 10.1128/aem.39.2.297-301.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch A. M., Drake D., Jacobs T. W., Long S. R. Nodules are induced on alfalfa roots by Agrobacterium tumefaciens and Rhizobium trifolii containing small segments of the Rhizobium meliloti nodulation region. J Bacteriol. 1985 Jan;161(1):223–230. doi: 10.1128/jb.161.1.223-230.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch A. M., Wilson K. J., Jones J. D., Bang M., Walker V. V., Ausubel F. M. Rhizobium meliloti nodulation genes allow Agrobacterium tumefaciens and Escherichia coli to form pseudonodules on alfalfa. J Bacteriol. 1984 Jun;158(3):1133–1143. doi: 10.1128/jb.158.3.1133-1143.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson P. E., Kenne L., Lindberg B., Ljunggren H., Lönngren J., Rudén U., Svensson S. Demonstration of an octasaccharide repeating unit in the extracellular polysaccharide of Rhizobium meliloti by sequential degradation. J Am Chem Soc. 1977 May 25;99(11):3812–3815. doi: 10.1021/ja00453a049. [DOI] [PubMed] [Google Scholar]
- Johansen E., Finan T. M., Gefter M. L., Signer E. R. Monoclonal antibodies to Rhizobium meliloti and surface mutants insensitive to them. J Bacteriol. 1984 Oct;160(1):454–457. doi: 10.1128/jb.160.1.454-457.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor A. N., Alexander M. Formation of tumor-like structures on legume roots by Rhizobium. J Bacteriol. 1971 Mar;105(3):728–732. doi: 10.1128/jb.105.3.728-732.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthysse A. G. Role of bacterial cellulose fibrils in Agrobacterium tumefaciens infection. J Bacteriol. 1983 May;154(2):906–915. doi: 10.1128/jb.154.2.906-915.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade H. M., Signer E. R. Genetic mapping of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1977 May;74(5):2076–2078. doi: 10.1073/pnas.74.5.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolmasky M. E., Staneloni R. J., Leloir L. F. Lipid-bound saccharides in Rhizobium meliloti. J Biol Chem. 1982 Jun 25;257(12):6751–6757. [PubMed] [Google Scholar]
- Truchet G., Rosenberg C., Vasse J., Julliot J. S., Camut S., Denarie J. Transfer of Rhizobium meliloti pSym genes into Agrobacterium tumefaciens: host-specific nodulation by atypical infection. J Bacteriol. 1984 Jan;157(1):134–142. doi: 10.1128/jb.157.1.134-142.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance C. P. Rhizobium infection and nodulation: a beneficial plant disease? Annu Rev Microbiol. 1983;37:399–424. doi: 10.1146/annurev.mi.37.100183.002151. [DOI] [PubMed] [Google Scholar]
- Wong C. H., Pankhurst C. E., Kondorosi A., Broughton W. J. Morphology of root nodules and nodule-like structures formed by Rhizobium and Agrobacterium strains containing a Rhizobium meliloti megaplasmid. J Cell Biol. 1983 Sep;97(3):787–794. doi: 10.1083/jcb.97.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Rijn I. Streptococcal hyaluronic acid: proposed mechanisms of degradation and loss of synthesis during stationary phase. J Bacteriol. 1983 Dec;156(3):1059–1065. doi: 10.1128/jb.156.3.1059-1065.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]



