Abstract
Objectives
Although biofeedback therapy is effective in the short term management of dyssynergic defecation, its long term efficacy is unknown. Our aim was to compare the one year outcome of biofeedback (manometric- assisted pelvic relaxation, and simulated defecation training), with standard therapy (diet, exercise, laxatives) in patients who completed 3 months of either therapy.
Methods
Stool diaries, visual analog scales (VAS), colonic transit, anorectal manometry, and balloon expulsion time were assessed at baseline, and at one year after each treatment. All subjects were seen at 3 month intervals and received reinforcement. Primary outcome measure (ITT analysis) was a change in the number of complete spontaneous bowel movements (CSBM) per week. Secondary outcome measures included bowel symptoms, changes in dyssynergia and anorectal function.
Results
Of 44 eligible patients with dyssynergic defecation, 26 agreed to participate in the long term study. All 13 subjects who received biofeedback, and 7 of 13 who received standard therapy completed one year; 6 failed standard therapy. The number of CSBMs/week increased significantly (p<0.001) in the biofeedback but not in the standard group. Dyssynergia pattern normalized (p<0.001), balloon expulsion time improved (p=0.0009), defecation index increased (p<0.001) and colonic transit time normalized (p=0.01) only in the biofeedback group.
Conclusions
Biofeedback therapy provided sustained improvement of bowel symptoms and anorectal function in constipated subjects with dyssynergic defecation while standard therapy was largely ineffective.
Keywords: Biofeedback therapy, constipation, dyssynergia, anorectal function, treatment
INTRODUCTION
About one-third of patients with chronic constipation have an evacuation disorder, and most have dyssynergic defecation (1–4). Also known as anismus (5) or pelvic floor dyssynergia (6), dyssynergia is characterized by a failure of the abdominal, rectal, pelvic floor and anal sphincter muscles to effectively coordinate and complete the process of defecation (6–10). Consequently, these patients complain of excessive straining, incomplete evacuation and hard stools together with infrequent stooling (3,4,11), and about 40% use digital maneuvers to assist defecation (11). Most of them are refractory to traditional approaches of management of constipation. The impaired propulsion of stool from the rectum, paradoxical anal contraction or inadequate anal relaxation together with impaired rectal sensation or a combination of these mechanisms leads to dyssynergic defecation (7,9,10).
Recently, three randomized controlled trials have concluded that biofeedback therapy is superior to sham feedback or standard therapy (12) or laxatives (13) or diazepam (14) in the management of patients with dyssynergic defecation. However, these trials were short term (3 months), while constipation with dyssynergic defecation is a chronic disorder with a prevalence of symptoms of at least 2 years (11,13). Whether biofeedback therapy is effective in the long-term management of patients with dyssynergic defecation has not been systematically assessed.
In two uncontrolled long term studies, biofeedback therapy was felt to improve symptoms (15,16). In the only other long term controlled study, patients with normal colonic transit and dyssynergia were found to show greater improvement in bowel function after biofeedback but not after polyethylene glycol (13). However, a majority of patients with dyssynergic defecation have coexisting slow transit constipation (3,7,8,17). Thus, whether biofeedback therapy is effective in the long term management of all subjects with dyssynergic defecation, irrespective of their colonic transit is not known.
In this study, we hypothesized that patients with dyssynergic defecation who receive biofeedback therapy will demonstrate greater long-term improvement in bowel symptoms and in colonic and anorectal physiology when compared to those who receive standard therapy. Our aim was to prospectively compare the one year symptomatic and physiologic outcome of biofeedback therapy with standard therapy in patients with constipation and dyssynergic defecation who completed three months of either therapy. Specifically, we tested whether subjects who received biofeedback therapy were more likely to show an increase in the number of complete spontaneous bowel movements and to correct a dyssynergic pattern of defecation when compared to those who received standard therapy.
MATERIALS AND METHODS
We recruited subjects with chronic constipation who had failed routine management of constipation (>1 year) and fulfilled Rome II criteria for functional constipation (18). In addition, all subjects fulfilled the following criteria for dyssynergic defecation: they demonstrated a dyssynergic pattern of defecation during attempted defecation (13), and either had prolonged difficulty with expelling a 50 ml water filled balloon (> 1 minute) or prolonged delay (> 20% marker retention) in colonic transit time (6,8,12). They were also required to have no evidence of structural or metabolic diseases that could cause constipation, as assessed by colonoscopy/barium enema and routine hematological, biochemical and thyroid function tests. Patients taking constipating drugs, for example opioids, were excluded or were asked to discontinue the drug two weeks before enrollment. Other exclusion criteria included: previous gastrointestinal, spinal or pelvic surgery except cholecystectomy, hysterectomy or appendectomy, alternating constipation and diarrhea rectal prolapse, anal fissure, neurologic diseases such as multiple sclerosis, stroke or spinal injury, severe cardiac or renal disease, impaired cognizance (mini-mental score < 15), pregnancy, and legal blindness.
Subjects were randomized using the permuted blocks method with 1:1 assignment into the two study groups (Biofeedback and Standard). Random numbers generated in advance were placed into sequentially numbered opaque envelopes, sealed and used for subject assignment. While the therapist and patient could not be blinded, the investigators performing the subjective and manometry analyses were blinded to the patient assignment or previous data. Standard protocols were employed for each group to ensure that all patients received similar general guidelines for management of their constipation.
We assessed several objective and subjective outcome measures at baseline and after treatment because a diagnosis of dyssynergic defecation requires both symptomatic and physiological parameters (6,8,10). These included anorectal physiology, balloon expulsion test (8,19,20), and colonic transit study in which three different shaped radioopaque markers (Sitzmark ®, Konsyl Pharmaceuticals Fort Worth, Texas) were administered on three consecutive days and a plain abdomen x-ray was taken on day 6 (12). All subjects were required to maintain a prospective stool diary, starting one week before enrollment in which they recorded the time, consistency (Bristol stool scale; type 1=hard pellets and 7 = watery stools), straining effort (1=normal, 2=moderately excessive, 3=severe) of each bowel movement and whether a bowel movement was complete, and whether they needed digital assistance (12). Also, they rated the overall satisfaction with bowel function on a 100 mm visual analog scale (VAS). The study was approved by the Institutional Review Board and all participants gave written informed consent.
Standard treatment
During an initial visit, a gastroenterologist, nurse therapist and dietitian provided advice regarding bowel habits, exercise, laxatives, dietary fiber and fluid intake, and timed-toilet training (12). This was reinforced by the nurse therapist during follow up visits. All patients were advised to attempt bowel movement for 5 minutes, twice a day, 30 minutes after eating, irrespective of their urge to defecate. The nurse therapist taught subjects how to improve their push effort by using postural and diaphragmatic breathing techniques, and instructed them to practice these maneuvers at home for 15 minutes, three times a day (12). Magnesium gluconate (Magonate ® 500 mg, Fleming & Company, St. Louis, MO) 2–4 tablets daily was recommended daily as the standard laxative, and subjects were instructed to titrate the dose. All subjects were advised to refrain from using digital maneuvers to assist defecation, and if employed its use was recorded. Patients having no bowel movement for 48 hours were instructed to use one glycerin suppository, then after 72 hours, a tap water enema and after 96 hours two Bisacodyl tablets orally (rescue laxatives). The dietitian advised subjects to consume a balanced, adequate calorie diet, increase fruit and vegetable intake to five servings per day and consume 25 g of dietary fiber from natural food sources daily. After completing their initial treatment for 3 months (short term therapy), subjects were invited to enroll in the long term phase of this study and to return for 3 follow up visits at 3 month intervals with the last scheduled visit at one year after starting therapy. During these one hour follow up visits, their symptoms and bowel habits were assessed and additional advice was provided as needed. All subjects were required to maintain a one week stool diary prior to each visit, which was used for symptom analysis. Manometric and colonic transit measurements and balloon expulsion tests were performed at the end of one year.
Biofeedback treatment
In addition to receiving the instructions described under standard therapy, subjects randomized to biofeedback therapy had biofeedback training sessions by a nurse therapist. Biofeedback therapy consisted of placing a solid state manometry probe into the rectum (Koningsberg Instruments, Pasadena, CA), and using software (Gaeltec Ltd. Dunvegan, Isle of Skye) for displaying the manometric data. Biofeedback therapy consisted of three components. The goal of rectoanal coordination was to increase the push effort as reflected by a rise in intra-abdominal/intra-rectal pressures and synchronized with anal relaxation as reflected by a decrease in anal sphincter pressure. While sitting on a commode, subjects watched the manometric tracings on a computer monitor and received training using visual and verbal feedback techniques (6,12). The goal of simulated defecation training was to train subjects over three consecutive trials to expel a silicone-filled artificial stool-FECOM. The subjects’ posture and breathing techniques were continuously monitored and appropriate advice and feedback was provided to improve defecatory effort. Patients with impaired rectal sensation received sensory conditioning by repeated inflations/deflations of a rectal balloon (6,12). After completing 3 months (short term therapy), subjects who agreed to participate in the long term phase of our study were asked to return for 3 follow up visits at 3 month intervals with the last visit scheduled at one year. During these visits, they received general advice and biofeedback therapy. Bowel symptoms and stool patterns were assessed from a prospectively maintained one week stool diary prior to each visit. Manometric and colonic transit measurements and balloon expulsion tests were repeated at one year.
Data analysis and outcome measures
The primary outcome measure was the number of complete spontaneous bowel movements (CSBM) per week. A spontaneous bowel movement was defined as a bowel movement that occurred naturally or without use of rescue laxatives, suppositories or enemas within the previous 24 hrs. A CSBM was defined as a spontaneous bowel movement reported on a stool diary without a feeling of incomplete evacuation. Secondary outcome measures included the global bowel satisfaction as recorded on VAS, stool frequency, stool consistency, straining effort, proportion of patients needing digital assistance for stooling and a laxative consumption score per week [none= no laxatives, Type I= high fiber diet ± bran and stool softeners, Type II= oral laxatives [magnesium oxide, 17 g polyethylene glycol (Miralax ® Braintree Labs, MA), Type III= stimulants (Bisacodyl), Type IV= enemas, suppositories, magnesium citrate, 236 g polyethylene glycol solutions (Golytely ® Braintree Labs, MA)]. The physiologic outcome measures included the presence of dyssynergia during attempted defecation, balloon expulsion time, anal residual pressure, % anal relaxation, intrarectal pressure and defecation index during attempted defecation (19,20), thresholds for first perception and urge to defecate (20), and the proportion of subjects with slow colonic transit time. All subjects who signed a consent form and agreed to participate in the long term study irrespective of whether they dropped out or they completed the study were included in the data analyses and an intention to treat analyses were performed.
Statistical Analysis
The primary outcome measure was the number of CSBM/week, and this was analyzed using repeated-measure negative binomial regression analysis. The number of stools and CBMs per week and the balloon expulsion time were also analyzed using negative binomial regression. As these analyses involve log-transformed data, the results are expressed as means with their asymmetrical mean-SEM and mean±SEM confidence intervals. Mixed-model ANOVAs were used for assessment of the bowel satisfaction VAS, the stool consistency and stool strain scores, and the physiological anorectal manometric data. These quantitative data are expressed as mean ± SEM. The use of laxatives and of digital assistance and the presence of slow colonic transit were analyzed using exact probabilities from Fisher’s and McNemar’s tests. The Fisher’s test was also used for analysis of the proportion of subjects with dyssynergia at the one year assessment. These data are expressed as subject counts. For the 6 subjects in the standard therapy arm who did not complete the one year assessment, the results from their last previous assessments were carried forward to one year. We compared the data for baseline versus post- treatment at one year for each intervention as well as testing for baseline versus post-treatment differences between the two study arms.
Count data that detail the number of events over a certain period of time (e.g. number of CSBMs over 7 days) or the time delay to an event (e.g. number of seconds needed to expel a balloon) often tend to have distributions that are highly skewed towards the right with many zeros. In such circumstances, analyses based on the Gaussian (normal) distribution are inappropriate. Hence, we have analyzed our count data with repeated-measures regression models that are based on the negative binomial distribution, involving estimation of an additional dispersion parameter that allows for inequality of mean and variance and for correlation among the observations.
RESULTS
Subject demographics
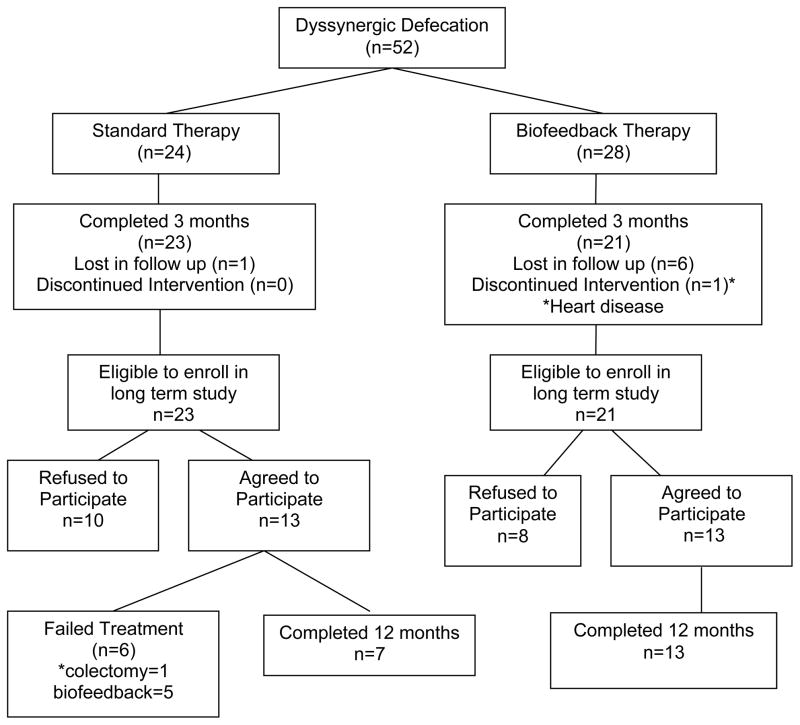
Fifty two subjects with dyssynergic defecation were randomized, of whom forty four subjects (21 in biofeedback, 23 in standard) completed the initial phase of treatment (3 months). The outcome of this phase of our study has been reported previously (12). Of the 21 patients who completed the short term biofeedback treatment and were eligible for the long term study, 13 (m/f=1/12, mean age=48 years) elected to participate in the long term assessment, and all of these subjects completed one year of treatment whereas eight subjects declined to participate, although 7 had improved (Fig 1). The reasons for declining further participation were personal including transportation issues (4), relocation (1), other medical problems (2) and hip surgery (1). Of the 23 subjects who completed the standard treatment 10 declined [personal reasons (7), relocation (1), pelvic surgery (1), medical problems (1)], and 7/10 had improved and 3/10 had failed therapy. The remaining 13 subjects (m/f=2/11, mean age=45 years) elected to participate in the long term study. Among these, 6 subjects failed standard therapy with one requiring colectomy at 6 months and 5 choosing behavioral therapy between 6–9 months after initial enrollment. Seven subjects completed one year of standard therapy. All 13 subjects who agreed to participate in the long term study of standard therapy were included in the ITT analysis (Fig 1).
Figure 1.
Consort diagram of the long term clinical trial with biofeedback therapy.
Symptom profiles
The baseline bowel symptom profiles including stool frequency, stool consistency, and straining effort were comparable and similar between the two groups, including the percentage of subjects needing digital assistance to defecate (Table 1).
Table 1.
| p between treatments | Biofeedback ( n = 13 ) | Standard ( n = 13 ) | |||||
|---|---|---|---|---|---|---|---|
| Baseline | One Year | p | Baseline | One Year | p | ||
| BMs per Week | 0.023 | 4.87 (4.00; 5.92) | 7.70 (6.56; 9.03) | 0.003 | 5.72 (4.83; 6.79) | 5.71 (4.99; 6.55) | 0.99 |
| CBMs per Week | 0.005 | 1.91 (1.49; 2.46) | 4.85 (3.55; 6.64) | 0.0003 | 1.66 (0.99; 2.78) | 1.43 (0.97; 2.11) | 0.60 |
| Bowel Satisfaction VAS | 0.61 | 17.3 ±6.0 | 67.1 ±6.0 | <0.001 | 13.1 ±6.0 | 56.8 ± 6.0 | <0.001 |
| Use of Digital Maneuvers | 0.47 | 6 of 13 | 2 of 13 | 0.05 | 5 of 13 | 4 of 13 | 0.7 |
| Mean Strain score per Week | 0.56 | 1.8 ±0.16 | 1.8 ±0.16 | 1.0 | 2.0 ±0.16 | 1.8 ±0.16 | 0.42 |
OUTCOME MEASURES
Primary outcome measure
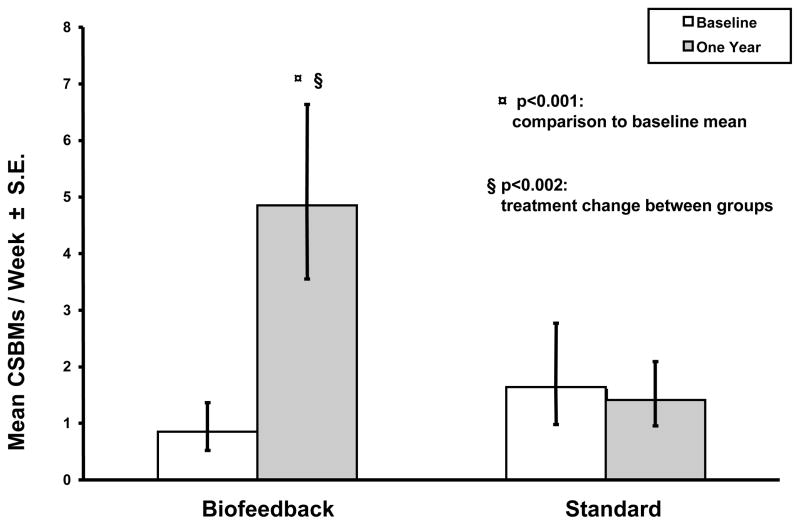
Subjects who were randomized to biofeedback therapy demonstrated a significant increase in the number of CSBMs per week when compared to the baseline period (p<0.001) and when compared to the standard group ( p<0.002) whereas there was no change in the standard group (Fig 2).
Figure 2.
This shows the number of complete spontaneous bowel movements per week in each of the two treatment groups, before and after treatment.
Secondary symptomatic outcome measures
The mean number of bowel movements per week (the mean number of complete bowel movements per week) also increased significantly in the biofeedback group when compared to standard group (p=0.005) and baseline (p=0.0003), (Table 1). The bowel satisfaction score increased significantly (p<0.0001) after biofeedback therapy and after standard therapy (p<0.001) when compared to baseline (Table 1). Although subjects receiving biofeedback reported more improvement, the difference was not significant. There was no change in the mean straining effort score in both groups (Table 1).
There was a non-significant trend towards softer stools in both the biofeedback ( 3.7 ± 0.3 vs 4.0 ± 0.3, p=0.4) and standard (3.2 ± 0.3 vs 3.6 ± 0.3, p=0.4) therapy groups, but there was no difference between groups (p=0.4). The laxative usage did not significantly change in either group. All subjects were using a Type I or Type II laxative at baseline. A Type III or Type IV laxative was used by 1/13 and 3/13 subjects randomized to biofeedback therapy, both at baseline and at one year (p=1.0). Similarly, 2/13 used a Type III laxative and 1/13 subjects used a Type IV laxative at baseline, and 1/13 and 2/13 subjects respectively at one year in the standard therapy group. Approximately 30 % of subjects in the biofeedback group discontinued all laxative usage, where as none of the subjects in the standard group stopped using laxatives in one year. A need for digital assistance with stooling lessened significantly (p=0.05) in the biofeedback group but not in standard group (Table 1).
Physiological Outcome measures
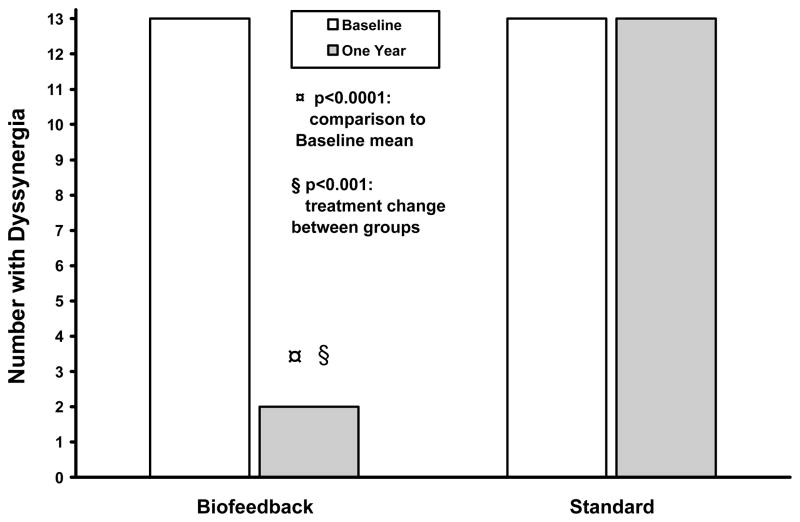
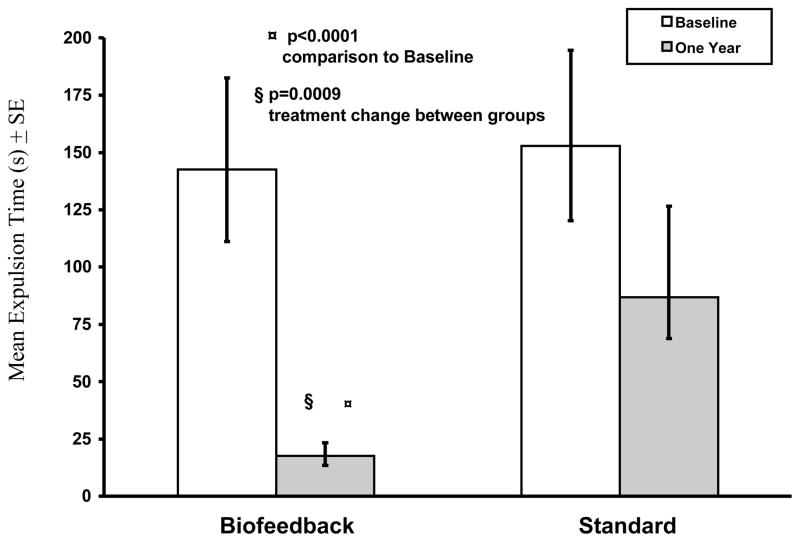
The dyssynergia pattern was corrected in 12/13 subjects who received biofeedback, and in none of the subjects who received standard treatment (Fig 3). Biofeedback was superior to baseline and standard treatment (p<0.0001). The balloon expulsion time decreased significantly in subjects who received biofeedback when compared to their baseline (p<0.0001), but not in standard group (p=012), (Fig 4). Also, the balloon expulsion time improved significantly in those who received biofeedback when compared to the standard group (p=0.0009).
Figure 3.
This shows the number of subjects who exhibited a dyssynergic pattern of defecation on anorectal manometry, before and after each treatment.
Figure 4.
This shows the effect of each treatment on the balloon expulsion time in subjects with dyssynergic defecation.
The anal residual pressure decreased (p<0.0001), the intrarectal pressure increased (p<0.01), and defecation index increased significantly (p<0.0001) in the biofeedback group when compared to baseline, but was unchanged in the standard treatment group (Table 2). Also, all of these parameters improved significantly in the biofeedback group (Table 2), when compared to those who received standard therapy.
Table 2.
| p between treatments | Biofeedback ( n= 13 ) | Standard ( n = 13 ) | |||||
|---|---|---|---|---|---|---|---|
| Baseline | One Year | p | Baseline | One Year | p | ||
| Anal Residual Pressure (mmHg) | 0.002 | 81.0 ± 7.5 | 32 ± 8 | <0.001 | 62.1 ± 7.5 | 59.8 ± 7.5 | 0.83 |
| Anal Relaxation% | <0.0001 | 4.5 ± 5 | 46 ± 5 | <0.0001 | 7.7 ± 4.5 | 10.1 ± 4.5 | 0.71 |
| Rectal Pressure (mmHg) | 0.01 | 31± 5 | 57 ± 5 | 0.001 | 35.5 ± 5.2 | 33.9 ± 5.2 | 0.83 |
| Defecation Index | <0.001 | 0.4 ± 0.14 | 1.9 ± 0.1 | <0.0001 | 0.68 ± 0.14 | 0.66 ± 0.14 | 0.92 |
| First Sensation (cc) | 0.12 | 49 ± 8 | 18 ± 8 | 0.01 | 29.2 ± 7.9 | 24.6 ± 7.9 | 0.68 |
| No. of subjects with Slow Colonic Transit (%) | 0.33 | 7 of 13 (54%) | 1 of 13 (7.7%) | 0.01 | 7 of 13 (54%) | 4 of 13 (30%) | 0.09 |
In the biofeedback group, the threshold for first sensory perception decreased significantly at one year when compared to baseline (p=0.01) (Table 2). There was no difference in other sensory thresholds between the two groups. At baseline, 54% (7/13) and after treatment 7.7% (1/13) of subjects who received biofeedback had slow colonic transit (p=0.01), when compared to 54% (7/13) and 30 % (4/13), respectively (p=0.09) with standard therapy (Table 2).
DISCUSSION
In this study, we found that subjects who were treated with biofeedback therapy showed a sustained improvement in bowel symptoms, as reflected by a significant increase in the number of complete spontaneous bowel movements per week (the primary outcome measure) when compared to baseline and when compared to standard therapy. Also, these subjects showed a greater increase in the total number of bowel movements and were more likely to discontinue the use of digital maneuvers than subjects who received standard treatment. Thus, our long term randomized controlled trial revealed that at one year, biofeedback therapy was more likely to restore normal bowel function in subjects with chronic constipation and dyssynergic defecation than standard therapy.
In parallel with the symptomatic improvement, we also observed a significant improvement in colonic and anorectal function as revealed by measurements of colonic transit and anorectal physiology. After biofeedback, colonic transit time improved significantly and normalized in almost all subjects with co-existing slow transit constipation, but mean transit time did not significantly improve in subjects after standard therapy. The lack of statistical difference between the two treatment arms could be due to a Type II error. At baseline, all subjects demonstrated a dyssynergic pattern of defecation during an attempted defecation. After treatment, all subjects randomized to standard treatment continued to exhibit dyssynergia whereas 92% of subjects who received biofeedback therapy showed that the dyssynergic pattern of defecation was corrected. Likewise, other manometric indices that reflect a coordinated bowel movement such as the defecation index, and the time taken to expel a balloon also improved significantly in the biofeedback group but were unchanged in the standard group. These findings confirm previous observations (8,11,13) that dyssynergic defecation is a chronic disorder and will persist unless a behavioral intervention is instituted to correct the underlying dysfunction. Also, biofeedback therapy is effective in reversing these dysfunctions.
Because all patients received a similar degree of attention and advice regarding coping strategies, it appears that the clinical improvement in subjects who received biofeedback therapy was mainly achieved by modifying the underlying physiological dysfunction. In contrast, those who received standard therapy failed to demonstrate any improvement in a range of primary and secondary outcome measures and had many drop outs. The lack of any change in anorectal function after standard therapy suggests that treatment with laxatives, exercise, diet and advice alone was unlikely to correct the chronic bowel problem.
The global bowel satisfaction score improved after biofeedback as well as after standard therapy when compared to baseline. This finding further confirms our previous study (12) and suggests that carefully monitored therapy with coping strategies may improve satisfaction with bowel symptoms in some patients with chronic bowel problems, but may not produce real and effective change in bowel function. This observation underscores the need for performing randomized controlled trails and for using objective measures of assessing bowel function; reliance on subjective measures such as global satisfaction as a sole measure of improvement may be inadequate as it can be influenced either by other factors such as a desire to please the investigators or by there overall wellbeing and not necessarily by their bowel function (21,22).
Our findings extend and confirm the only other long term controlled study (13) but there were methodological differences that merit discussion. We included all subjects with dyssynergic defecation whereas the previous study excluded patients with dyssynergia and coexisting slow transit constipation. Because nearly 60% of patients with dyssynergic defecation have coexisting slow transit constipation (3,7,17), we believe that our study population represents the broad group of dyssynergic subjects who are commonly encountered in clinical practice. Furthermore, colonic transit time normalized in almost all subjects with dyssynergic defecation after biofeedback therapy. This reaffirms that the transit abnormalities were secondary to outlet dysfunction and that dyssynergics with slow transit will respond favorably to biofeedback therapy. Also, in our study, the biofeedback and the standard therapy were administered by a nurse, under supervision of a physician, whereas, in their study two separate physicians, one a gastroenterologist and another a skilled biofeedback therapist provided all of the therapy including laxatives to the biofeedback group while another physician provided laxatives to the control group raising concerns for equitable management. Finally, after 3 months of treatment they told all patients who received biofeedback, irrespective of their outcome that they had improved. Although motivating patients is important, doing so by elevating patient’s expectations especially in a disorder with significant psychological comorbidity (23) could bias the clinical outcome, especially when the primary outcome measure was the subjects’ rating of symptom improvement. No such advice was given to our patients. Nonetheless, both studies found that biofeedback therapy was superior to laxatives in the long term.
The limitations of our study include the smaller sample size. Our screen failure and drop out rate is similar to those reported in previous biofeedback studies and reflects issues with recruitment and retention of subjects in a long term labor-intensive clinical trial (12,14,24). However, approximately equal number of subjects agreed to participate in both arms of the long term trial, and a similar number declined after completion of the initial three months treatment. The refusal to participate in the long term study was not because of a failure to respond to treatment in either group, but largely due to personal reasons or co-morbidity issues. In the standard therapy group, 6/13 (48%) subjects failed long-term treatment. One subject developed significant worsening of symptoms and elected to have a colectomy and five others felt that their symptoms had not improved, usually after another few months of long-term therapy and declined further participation. These five subjects successfully completed biofeedback therapy outside the clinical trial. In contrast, all 13 subjects assigned to the biofeedback group completed one year of treatment. Our results may require further validation through a larger, multi-center clinical trial.
In conclusion, our prospective randomized controlled trial shows that biofeedback therapy appears to be efficacious in the long term management of patients with chronic constipation and dyssynergic defecation, and is more likely to restore normal bowel function than standard therapy with laxatives.
Supplementary Material
What is current knowledge
Short term controlled trails have shown that biofeedback therapy is effective in the treatment of patients with constipation and dyssynergic defecation.
However, dyssynergic defecation is a chronic problem and whether biofeedback therapy is effective in the long term management of these patients is unclear.
What is new here
This prospective, one year, randomized controlled trial demonstrates that biofeedback therapy is effective in improving bowel symptoms and correcting the underlying pathophysiology of dyssynergia, and in providing sustained improvement in anorectal and colonic function.
Biofeedback therapy is the preferred treatment for the management of subjects with dyssynergic defecation and is superior to laxatives.
Acknowledgments
Portions of this work were presented at the annual meeting of the American College of Gastroenterology and published as an abstract; Am J Gastroenterol 2005; 100:386.
Financial Support: This work was supported by NIH grant RO1 DK 57100-05 and grant RR00059 from the General Clinical Research Centers program, National Center for Research Resources. We sincerely acknowledge the expert technical and nursing assistance of Mrs. Mary Stessman, RN, and Mrs. Kara Seaton, BS, dietary consult and advise of Mrs. Phyllis Stumbo, PhD, and secretarial assistance of Mrs. Kimberly Klein.
Footnotes
Satish SC Rao, MD, PhD, FRCP, FACG – Study concept and design, data acquisition, data collection, study recruitment, data analysis and interpretation, manuscript preparation, critical revision, important intellectual content and final approval.
Jessica Valestin, BS- Data acquisition, data collection and study recruitment.
C Kice Brown, MS - Data analysis and interpretation and manuscript preparation.
Bridget Zimmerman, PhD - Data analysis & interpretation and critical revision.
Konrad Schulze, MD, FRCP - Study concept and design, manuscript preparation and critical revision
Competing Interest: None.
References
- 1.Mertz H, Naliboff B, Mayer E. Physiology of refractory chronic constipation. Am J Gastroenterol. 1999;94:609–15. doi: 10.1111/j.1572-0241.1999.922_a.x. [DOI] [PubMed] [Google Scholar]
- 2.Koch A, Voderholzer WA, Klauser AG, et al. Symptoms in chronic constipation. Dis Colon Rectum. 1998;40:902–6. doi: 10.1007/BF02051196. [DOI] [PubMed] [Google Scholar]
- 3.Grotz RL, Pemberton JH, Talley NJ, et al. Discriminant value of psychological distress, symptom profiles, and segmental colonic dysfunction in outpatients with severe idiopathic constipation. Gut. 1994;35:798–802. doi: 10.1136/gut.35.6.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Surrenti E, Rath DM, Pemberton JH, et al. Audit of constipation in a tertiary referral gastroenterology practice. Am J Gastroenterol. 1995;90:1471–5. [PubMed] [Google Scholar]
- 5.Preston DM, Lennard-Jones J. Anismus in chronic constipation. Dig Dis Sci. 1985;30:413–8. doi: 10.1007/BF01318172. [DOI] [PubMed] [Google Scholar]
- 6.Rao SSC. Dyssynergic defecation and biofeedback therapy. Gastroenterol Clin North Am. 2008;37:569–86. doi: 10.1016/j.gtc.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao SSC, Welcher K, Leistikow J. Obstructive Defecation: A failure of rectoanal coordination. Am J Gastroenterol. 1998;93:1042–50. doi: 10.1111/j.1572-0241.1998.00326.x. [DOI] [PubMed] [Google Scholar]
- 8.Rao SSC, Mudipalli RS, Stessman M, et al. Investigation of the utility of colorectal function test and rome II criteria in dyssynergic defecation (Anismus) Neurogastroenterol Motil. 2004;16:589–96. doi: 10.1111/j.1365-2982.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 9.Karlbom U, Eeg-Olofsson EK, Graf W, et al. Paradoxical puborectalis contraction is associated with impaired rectal evacuation. Int J Colorect Dis. 1998;13:141–7. doi: 10.1007/s003840050152. [DOI] [PubMed] [Google Scholar]
- 10.Bharucha AE, Croak AJ, Gebhart JB, et al. Comparison of rectoanal axial forces in health and functional defecatory disorders. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1164–9. doi: 10.1152/ajpgi.00487.2005. [DOI] [PubMed] [Google Scholar]
- 11.Rao SSC, Tuteja AK, Vellema T, et al. Dyssynergic defecation: Demographics, symptoms, stool patterns and quality of life. J Clinical Gastroenterol. 2004;38:680–5. doi: 10.1097/01.mcg.0000135929.78074.8c. [DOI] [PubMed] [Google Scholar]
- 12.Rao SS, Seaton K, Miller M, et al. Randomized controlled trial of biofeedback, sham feedback, and standard therapy for dyssynergic defecation. Clin Gastroenterol Hepatol. 2007;5:331–8. doi: 10.1016/j.cgh.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 13.Chiarioni G, Whitehead WE, Pezza V, et al. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia. Gastroenterology. 2006;130:657–64. doi: 10.1053/j.gastro.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Heymen S, Scarlett Y, Jones K, et al. Randomized, controlled trial shows biofeedback to be superior to alternative treatments for patients with pelvic floor dyssynergia-type constipation. Dis Colon Rectum. 2007;50:428–41. doi: 10.1007/s10350-006-0814-9. [DOI] [PubMed] [Google Scholar]
- 15.Chiotakakou-Faliakou E, Kamm MA, Roy AJ, et al. Biofeedback provides long term benefit for patients with intractable, slow and normal transit constipation. Gut. 1998;42:517–521. doi: 10.1136/gut.42.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Battaglia E, Serra AM, Buonafede G, et al. Long-term study on the effects of visual biofeedback and muscle training as a therapeutic modality in pelvic floor dyssynergia and slow-transit constipation. Dis Colon Rectum. 2004;47:90–5. doi: 10.1007/s10350-003-0010-0. [DOI] [PubMed] [Google Scholar]
- 17.Karlbom U, Pahlman L, Nilsson S, et al. Relationships between defecographic findings, rectal emptying and colonic transit time in constipated patients. Gut. 1995;36:907–12. doi: 10.1136/gut.36.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson WG, Longstreth GF, Drossman DA, et al. Functional bowel disorders and functional abdominal pain. Gut. 1999;45:1143–7. doi: 10.1136/gut.45.2008.ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao SSC, Harfield R, Leistikow J, et al. Manometric tests of anorectal function in healthy humans. Am J Gastroenterol. 1999;94:773–83. doi: 10.1111/j.1572-0241.1999.00950.x. [DOI] [PubMed] [Google Scholar]
- 20.Rao SSC, Azpiroz F, Diamant N, et al. Minimum standards of anorectal manometry. Neurogastroenterol Motil. 2002;14:553–9. doi: 10.1046/j.1365-2982.2002.00352.x. [DOI] [PubMed] [Google Scholar]
- 21.Corney RH, Stanton R, Newell R, et al. Behavioral psychotherapy in the treatment of irritable bowel syndrome. J Psychosom Res. 1991;35:461–9. doi: 10.1016/0022-3999(91)90041-l. [DOI] [PubMed] [Google Scholar]
- 22.Patel SM, Stason WB, Legedza A, et al. The placebo effect in irritable bowel syndrome trials: a meta-analysis. Neurogastroenterol Motil. 2005;17:332–40. doi: 10.1111/j.1365-2982.2005.00650.x. [DOI] [PubMed] [Google Scholar]
- 23.Rao SSC, Seaton K, Miller MJ, et al. Psychological profiles and quality of life (QOL) differ between patients with dyssynergia and slow transit constipation. J Psychosomatic Res. 2007;63:441–9. doi: 10.1016/j.jpsychores.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 24.Norton C, Chelvanayagam S, Wilson-Barnett J, et al. Randomized controlled trial of biofeedback for fecal incontinence. Gastroenterology. 2003;125:1320–9. doi: 10.1016/j.gastro.2003.09.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.