Abstract
The craniometric linear dimensions of the posterior fossa have been relatively well studied, but angular craniometry has been poorly studied and may reveal differences in the several types of craniocervical junction malformation. The objectives of this study were to evaluate craniometric angles compared with normal subjects and elucidate the main angular differences among the types of craniocervical junction malformation and the correlation between craniocervical and cervical angles. Angular craniometries were studied using primary cranial angles (basal and Boogard’s) and secondary craniocervical angles (clivus canal and cervical spine lordosis). Patients with basilar invagination had significantly wider basal angles, sharper clivus canal angles, larger Boogard’s angles, and greater cervical lordosis than the Chiari malformation and control groups. The Chiari malformation group does not show significant differences when compared with normal controls. Platybasia occurred only in basilar invagination and is suggested to be more prevalent in type II than in type I. Platybasic patients have a more acute clivus canal angle and show greater cervical lordosis than non-platybasics. The Chiari group does not show significant differences when compared with the control, but the basilar invagination groups had craniometric variables significantly different from normal controls. Hyperlordosis observed in the basilar inavagination group was associated with craniocervical kyphosis conditioned by acute clivus canal angles.
Keywords: Platybasis, Arnold–Chiari malformation, Basilar invagination, Cephalometry
Introduction
Craniocervical junction malformation (CCJM) in adults is often described by Chiari malformation (CM) and/or basilar invagination (BI). These CCJMs are derived from mesodermal malformations that result in the underdevelopment of the occipital bone with subsequent herniation of the cerebellar tonsils and/or invagination of the upper cervical spine toward the base of the skull.
Studies correlating the spine and constitutional characteristics of the pelvis have been described. The sagittal balance of the spine around the plumb line has been classically measured from the upper C2 (or C7) to the sacrum [10].
Relations between the cranial and spinal angles have not been studied. Cranial angles have the potential to influence the angular geometry of the craniocervical junction and, consequently, all the spine [1]. Hypothetically, patients with larger basal angles (platybasics) have greater craniocervical kyphosis and more intense brainstem ventral compression.
Studies suggest that patients with basilar invagination are, among all types of malformations, those most heavily affected by malformation [2, 3, 6–9, 11–13]. The aim of this study was to evaluate cranial angulations and the correlation between craniocervical and cervical angles in different types of CCJM compared with normal subjects.
Patients and methods
We evaluated magnetic resonance imaging (MRI) scans of the craniocervical junction in T1 and T2 midline-weighted acquisitions from a CCJM patient sample treated by the authors between 1996 and 2012. A computed tomography scan was used only in specific cases, when necessary, to clarify details of bone anatomy.
The control group’s craniometric angles were obtained randomly from MRI scans that were considered normal by the Radiology Department of the Mandaqui Hospital. Patients with tumors, trauma, and any diagnostic pathology were excluded from this study. Patients with CM were those with symptomatic cerebellar tonsil herniation and/or posterior fossa structures and cisterna magna compressions.
Patients with BI were divided into two groups. Those with axis dens invagination into the foramen magnum were called type I (Fig. 1). Patients with invagination of the dens toward the base of the skull, not inside the foramen magnum, but with a Chamberlain’s line violation by the dens of at least 5 mm were classified as exhibiting type II basilar invagination (Fig. 2).
Fig. 1.
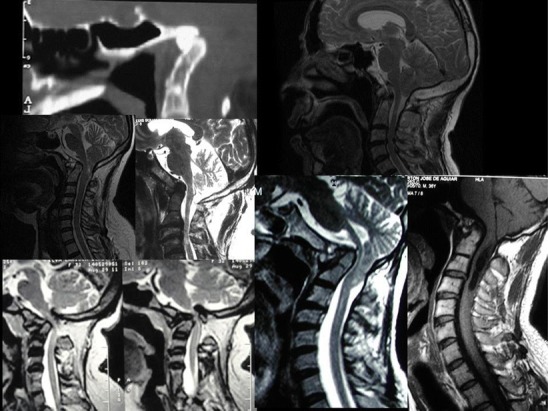
Examples of BI type I. The tip of the dens is inside the foramen magnum
Fig. 2.
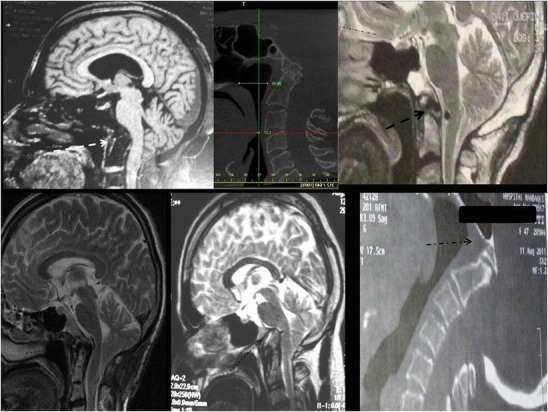
BI type II. The tip of the dens stops in osseous structures, preventing upward migration (arrows)
Angular craniometries were studied in the craniocaudal direction by primary cranial angles (basal and Boogard’s) and secondary craniocervical angles (clivus canal and cervical spine lordosis) as follows (Fig. 3):
Basal angle (BA): Defined by the angle measured from the nasion, top of the dorsum sellae, and the basion [4]
Clivus canal angle (CC): The angle between the line extending from the top of the dorsum sellae to the basion and the line between the inferodorsal portions of C2 to the most superodorsal part of the dens
Boogard’s angle (BOO): The angle between the top of the dorsum sellae, basion, and opisthion
Cervical lordosis angle (CL): The angle measured between a line drawn from the most inferodorsal to the most superodorsal part of C2 (dens of the axis) and another line drawn between the supero- and inferodorsal regions of the C7 posterior vertebral body. Note that the larger CL obtained resulted in a more straightened spine and that the shorter CL measured resulted in a more lordotic spine.
Fig. 3.
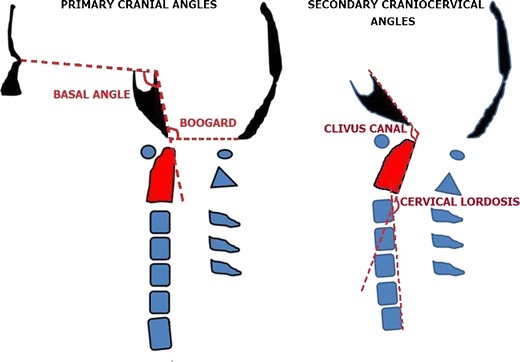
Left Primary angles: basal and Boogard’s angles. Right Secondary angles: clivus canal and cervical lordosis angles
Platybasia was defined as an enlargement of the basal angle. Measurements of the BA in the control group were used as standards of normality and compared with measurements from other groups to determine the prevalence of platybasia and any correlations with other craniometric angles.
The angles were measured with MeazureTM 2.0 software (C Thing Software). To eliminate variations in measures, all exams, from all groups, were evaluated by the same person in the same fashion (EDZF).
Statistics
Descriptive statistics are reported as means, ranges, and standard deviations. The adherence chi-squared test was used to analyze the difference in gender between the CCJM and control (CTRL) groups. Analysis of variance was used to compare the angles between the three groups studied. The homogeneity of variance between the groups was tested, and adjustments were made with the Brown–Forsythe test. Significant differences between groups were tested with multiple comparisons using Dunnett’s and the Bonferroni tests. For all comparisons, the significance level was considered to be 5 %. Correlation analysis between angles was performed with Spearman’s test.
Results
Craniometric values of 106 individuals, 73 patients with CCJM and 33 normal subjects, were studied and are presented in Fig. 4 and represented in Fig. 5. Among the 73 patients with malformations, 67 % (49) exhibited CM and 32 % (24) had BI.
Fig. 4.
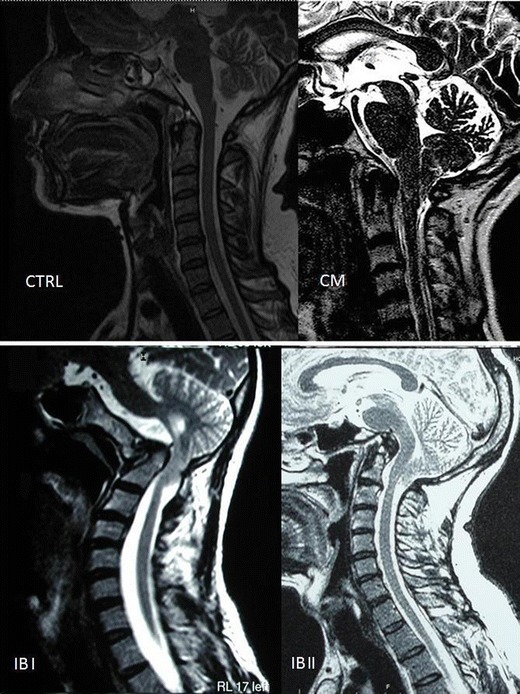
MRI image of the four studied groups. Upper left Normal subject. Upper right Chiari malformation. Lower left Basilar invagination I (BI I). Lower right BI II
Fig. 5.
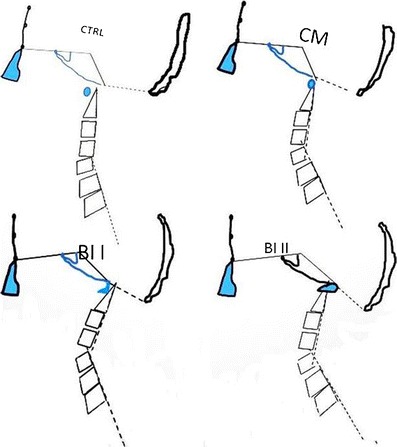
Illustration based on the average craniometric angles depicting the four CCJM subgroups. CTRL normal individuals, CM Chiari malformation, BI I basilar invagination type I, BI II basilar invagination type II
Eight patients had BI type I (10 % of CCJM) and 16 patients had BI type II (21 % of CCJM). The mean patient age with CCJM was 47 ± 13 years; the mean age of the control group was 45 ± 12 years (p = 0.42). Forty-two percent of CCJM and 57 % of the control group were males (chi-square test: p = 0.87).
The minimum, maximum, mean, and standard deviation values of the craniometric angles from the four separate groups are listed in Table 1.
Table 1.
Angle values of the four studied groups
| CCJM | CCJM angles (deg) | |||
|---|---|---|---|---|
| Minimum | Maximum | Mean | SD | |
| BA BI I | 104 | 155 | 128 | 19.35 |
| BA BI II | 115 | 158 | 130 | 11.11 |
| BA CM | 102 | 129 | 117 | 7.15 |
| BA CTRL | 107 | 132 | 119 | 7.11 |
| CC BI I | 77 | 145 | 123 | 24.50 |
| CC BI II | 80 | 144 | 117 | 16.46 |
| CC CM | 123 | 180 | 150 | 12.43 |
| CC CTRL | 129 | 175 | 148 | 9.88 |
| Boo BI I | 124 | 190 | 158 | 23.41 |
| Boo BI II | 153 | 236 | 183 | 24.72 |
| Boo CM | 105 | 175 | 136 | 15.26 |
| Boo CTRL | 103 | 148 | 126 | 9.40 |
| CL BI I | 125 | 157 | 138 | 11.81 |
| CL BI II | 113 | 159 | 137 | 14.89 |
| CL CM | 115 | 180 | 153 | 14.78 |
| CL CTRL | 130 | 189 | 158 | 13.89 |
BA basal angle, CC clivus canal angle, Boo Boogard’s angle, CL cervical lordosis angle, BI basilar invagination, CM Chiari malformation, CTRL control group
The average CCJM angle distribution is presented in Fig. 6.
Fig. 6.
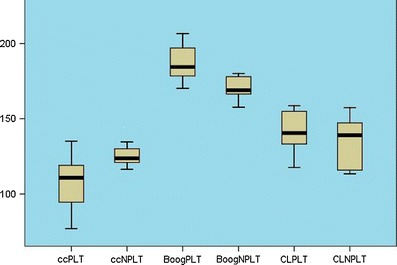
Graphic illustrations of basilar invagination angles with and without platybasia. ccPLT clivus canal angle of the platybasic group, ccNPLT clivus canal angle of the non-platybasic group, BooPLT Boogard’s angle of the non-platybasic group, BooNPLT Boogard’s angle of the platybasic group, CLPLT cervical lordosis of the platybasic group, CLNPLT cervical lordosis of the non-platybasic group
BI groups I and II were collectively called BI to avoid insufficient number of patients to reveal differences.
Basal angle
The comparison of the BA between groups showed that they were significantly different (Brown–Forsythe test: p < 0.001). The mean of the BI basal angle was greater than that of the CM group (X = 131.01 ± 13.77 vs. X = 117.35 ± 7.15, p = 0.001) and that of the CTRL group (X = 118.71 ± 7.11, p = 0.002). The CM BA was not different from that of the CTRL group (p = 0.813).
Clivus canal angle
Analysis of variance revealed significant differences in the CC among the four groups (p < 0.001). The BI CC was smaller (sharper) than that of the CM (119.31 ± 19.18 vs. 150.20 ± 12.43, p < 0.001) and smaller than that of the CTRL (148.42 ± 9.88, p < 0.001). There was no difference between the CC of the CM and CTRL groups (p = 0.844).
Boogard’s angle
Analysis of variance revealed significant differences in the BOO among the groups (p < 0.001).
The BI group showed a wider BOO angle than that of the CM group (172 ± 136 vs. 136 ± 15.26, p < 0.001), and was also wider than that of the CTRL group (126 ± 15.26, p < 0.001). The BOO in the CM group was wider than in the CTRL group (136 ± 15.26 vs. 126 ± 9.40, p = 0.003).
Cervical lordosis
The CCJM groups were significantly different when comparing cervical lordosis (p < 0.001).
Angles close to or >180° indicate that the spine is more straightened. Smaller angles indicate a more lordotic spine. The BI group showed higher lordosis (lower angle) than the CM (137.71 ± 13.49 vs. 153 ± 14.78, p < 0.001) and the CTRL groups (158.02 ± 13.89, p < 0.001). There were no differences between the CM and the CTRL groups (p = 0.347).
Platybasia
The mean, standard deviation, and the range of normal subjects’ BA were 118.71 ± 7.11 (106.80–132.10).
The CTRL BA’s sample distribution fit a t-distribution with 29 degrees of freedom. The upper limit of 99 % for the average angles of the basal group of normal individuals was initially considered a potential estimator of normality: 122.29. However, this angular value was invalid as the upper limit of BA normality because there were several normal subjects that had angular values above this limit.
As a result, we tested the greatest amplitude of the BA in normal individuals as the upper normality value. The maximum value obtained from the CTRL group was 132.10; 133° was then used as the upper BA normality limit, and anything above this value was considered to define platybasia. This threshold was tested in the entire set of CCJM groups; only eight patients, among the 106 patients with analyzed exams, were considered platybasics. All platybasic patients belonged to one of the two BI groups: six in the BI II group and two in the BI I group.
A comparison among four angles (primary and secondary) in platybasic patients is shown in Fig. 6. The platybasic subjects had a CC that was more acute than that of non-platybasics (104.53 ± 22 vs. 125.8 ± 13.65, p = 0.021) and flatter BOO (187.0625 ± 12.88 vs. 163, 5 ± 27.87, p = 0.036) than non-platybasics. There was no significant difference in CL measurements (p = 0.867).
Association between primary and secondary angles
The secondary angles were correlated with the other angles studied (Table 2). The CCA was significantly related to the BA (r = −58, p = 0.01), the BOO (R = −0.63, p = 0.01), and the CL (R = 0.43, p = 0.000). The CL was also significantly associated with Boogard’s angle (R = −0.38, p = 0.000).
Table 2.
Correlations between primary and secondary angles
| Correlations | ||||||
|---|---|---|---|---|---|---|
| CC | BA | BOO | CL | |||
| Spearman’s rho | CC | Correlation coefficient | 1.000 | −0.586a | −0.630a | 0.439a |
| Significance (two-tailed) | 0.000 | 0.000 | 0.000 | |||
| N | 104 | 94 | 104 | 94 | ||
| BA | Correlation coefficient | −0.586a | 1.000 | 0.489a | −0.148 | |
| Significance (two-tailed) | 0.000 | 0.000 | 0.178 | |||
| N | 94 | 94 | 94 | 84 | ||
| BOO | Correlation coefficient | −0.630a | 0.489a | 1.000 | −0.383a | |
| Significance (two-tailed) | 0.000 | 0.000 | 0.000 | |||
| N | 104 | 94 | 104 | 94 | ||
| CL | Correlation coefficient | 0.439a | −0.148 | −0.383a | 1.000 | |
| Significance (two-tailed) | 0.000 | 0.178 | 0.000 | |||
| N | 94 | 84 | 94 | 96 | ||
aCorrelation is significant at the 0.01 level (two-tailed)
Discussion
There are numerous CCJM configurations due to disorders of embryogenesis of the paraxial mesoderm, occipital bone, atlas, and axis [5, 7, 8, 11]. Primary mesodermal defects and alterations, as well as secondary alterations from collagen and bone metabolic diseases, can lead to CCJM [2]. We studied only primary (or typical) CCJM [8, 11].
In recent decades, several craniometric studies have been performed to produce linear cranial diameter measurements [1, 7, 13]. Historically, craniocervical angles have been studied individually in plain radiographies to identify abnormalities of the axis’s dens position in BI.
Basal angle values measurements were done by Boogard [4] in plain radiographies. Plain radiographs revealed significant variations in the results and values for the diagnosis of platybasia, ranging from 125° to 143° [4]. Both the tuberculum selae and the center of the pituitary fossa were independently used as landmarks in the pre-resonance era papers. In 2005, Koenigsberg evaluated measurement of BA by MRI. The floors of the anterior cranial fossa, dorsum sellae, and clivus were clearly identifiable and reproducible in midline MR imaging.
The use of only four cranial points (nasion, top of the sella turcica, basion, opisthion, and the vertical axis of C2) and cervical lordosis for all craniometric angle definitions in MRI standardizes angle measurements. This removes the variability seen in a plethora of preexisting historical craniometric values that were obtained in the era of plain radiography and conventional planigraphy [3, 4].
Two of the three cranial angles are structural, and they are not influenced by the craniocervical posture or balance (BA and BOO). The BA reflects a flattening of the anterior skull base, and the BOO is influenced by the flattening of the clivus.
The quantification of basal angle with MRI was done by Koenigsberg et al. [4] and Karagoz et al. [3]. The former studied 200 adults and 50 children classified as normal using the top of the dorsum sellae (rather than the center of the pituitary fossa) to measure the basal angle and found a normal value of 129 ± 6. Karagoz et al. [3] studied (with a technique similar to the one used in this study) 84 patients with CM. Of these, three had BI and two had platybasia. The BA of the control group was 121 ± 6 and that of the CM group was 126 ± 9.
Data using the top of the dorsum sellae as a reference for the BA showed values significantly smaller than those found by using the pituitary fossae center as a reference point. Our data suggest that values for basal angle above 133° are indicative of platybasia. Considering the BA values immediately above the normal range for diagnosis of platybasia, all platybasic patients identified were from the BI group. The comparison of craniometric data from non-platybasic and platybasic patients showed that they were significantly different (Fig. 6).
Platybasic patients had a more acute clivus canal angle and a wider Boogard’s angle. Platybasia was more frequent in patients with BI type II (37.5 %) than in those with BI type I (20 %). The CC measurement used was that suggested by Konigsberg et al. [4], between the top of the dorsum sellae, basion, and axis. The clivus canal angle reflects craniocervical kyphosis that, ultimately, is correlated to ventral brainstem compression. The CM group showed no difference in craniometric angular measurements compared to normal subjects.
Overall data analysis suggests that an enlarged BA is associated with a more acute CC and the largest BOO. An acute CC correlates with greater cervical lordosis (hyperlordosis). It is possible that the hyperlordosis observed in the BI group is secondarily associated with craniocervical kyphosis conditioned by an acute clivus canal angle. In fact, cervical lordosis was only correlated with the CC and with BOO. There were several differences between the two types of BI. Only two patients in BI type I were platybasic. Craniocervical instability was only present in BI type I.
Conclusions
The angular craniometry in craniocervical junction malformation differs significantly among the subgroups of patients and control patients.
Patients with basilar invagination have a significantly wider basal angle, sharper clivus canal angle, larger Boogard’s angle, and greater cervical lordosis than the Chiari malformation and control groups. The Chiari malformation group does not show significant differences when compared with normal controls. Platybasia occurred only in basilar invagination and is suggested to be more prevalent in type II than in type I. Platybasic patients have a more acute clivus canal angle and show greater cervical lordosis than non-platybasics.
Acknowledgments
The authors thank Dr. Andre Coutinho Castilla for his support with control group images.
Financial support
None declared.
Footnotes
Comments
Dachling Pang, Davis, USA
This paper describes a study of certain craniocervical angles using MRI data from normal controls and two groups of patients with craniovertebral junction malformations, namely, basilar invagination (BI) and Chiari I malformation without BI. The study generated some important data that are pertinent to the embryogenesis of these unwieldy malformations.
The four sets of angle data chosen are not random. They reciprocally influence each other and are frequently causally related. Understanding their relationships gives insight into the pathogeneses of certain craniovertebral junction malformations. For example, it has been suggested in other studies [1] that platybasia (with a wide basal angle) raises the basion, thereby obliging a lordotic tilt in the planes of the foramen magnum and the condyle-C1 joints and consequently increases Boogard’s angle. Because the dens anlagen are physically in continuity with the structures around the foramen magnum in the mesenchymal membranous stage, they are thus forced to rise up toward the brainstem in platybasia, producing the kind of basilar invagination that is associated with anterior skull base anomalies, sometimes called anterior basilar invagination [1]. For the same reason, the developing dens is also simultaneously “bent” backwards in line with the lordotic condylar-C1 joints into a retroflexed shape. This in turn induces the whole upper cervical spine to arch dorsally in lordosis, closing the cervical lordosis angle (CL in the article).
Also in platybasia, the flattened clival incline must logically narrow the clivus canal (clivus axis) angle. Since formation of the bony embryonic axis follows a craniocaudal temporal sequence, it is reasonable to theorize that clival anomalies such as platybasia and short clivus, which also raises the basion, must precede many odontoid malformations and likely underlie most forms of basilar invagination, especially those with a retroflex dens. It also explains why plain cerebellar tonsilar herniation without basilar invagination (the authors’ CM group), though impelled by a small posterior fossa, is not generally associated with clival deformities. If the authors had provided similar narratives to accompany their elegant data, the correlative packaging of the angle measurements should become even more cogent and the tremendous clinical applicability of their results can be better appreciated.
Lastly, the authors’ division of basilar invagination into two types seems artificial and unnecessary. If the basion–opisthion line is severely tilted up far enough, as in the type II case depicted in the lower middle image of their Fig. 2, the brainstem is just as badly compressed as any of the type I cases in Fig. 1, even though the dens is technically outside the foramen magnum. I see no embrylogical or clinical significance to this division or to the exact location of the odontoid tip relative to the basion.
Reference
[1] Pang D, Thompson DNP (2011) Embryology and bony malformations of the craniovertebral junction. Child’s Nerv Syst 27:523–564
References
- 1.Botelho RV, Neto EB, Patriota GC, Daniel JW, Cumont PA, Rotta JM. Basilar invagination: craniocervical instability treated with cervical traction and occipitocervical fixation. Case report. J Neurosurg Spine. 2007;7(4):444–449. doi: 10.3171/SPI-07/10/444. [DOI] [PubMed] [Google Scholar]
- 2.Burwood RJ, Watt I. Assimilation of the atlas and basilar impression: a review of skull and cervical spine 1.500 radiographs. Clin Radiol. 1974;25(3):327–333. doi: 10.1016/S0009-9260(74)80159-5. [DOI] [PubMed] [Google Scholar]
- 3.Karagoz F, Izgi N, Kapijcijoglu Sencer S. Morphometric measurements of the cranium in patients with Chiari type I malformation and comparison with the normal population. Acta Neurochir (Wien) 2002;144(2):165–171. doi: 10.1007/s007010200020. [DOI] [PubMed] [Google Scholar]
- 4.Konigsberg RA, Vakil N, Hong TA, Htaik T, Faerber E, Maiorano T, et al. Evaluation of platybasia with MR imaging. AJNR Am J Neuroradiol. 2005;26(1):89–92. [PMC free article] [PubMed] [Google Scholar]
- 5.Marin-Padilla M, Marin-Padilla TM. Morphogenesis of experimentally induced Arnold–Chiari malformation. J Neurol Sci. 1981;50(1):29–55. doi: 10.1016/0022-510X(81)90040-X. [DOI] [PubMed] [Google Scholar]
- 6.Milhorat TH, Nishikawa M, Kula RW, Dlugacz RW. Mechanisms of cerebellar tonsil herniation in patients with Chiari malformations the guide to clinical management. Acta Neurochir (Wien) 2010;152(7):1117–1127. doi: 10.1007/s00701-010-0636-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishikawa M, Sakamoto H, Hakuba A, Nakanishi N, Inoue Y. Pathogenesis of Chiari malformation: a morphometric study cranial fossa of the posterior. J Neurosurg. 1985;86(1):40–47. doi: 10.3171/jns.1997.86.1.0040. [DOI] [PubMed] [Google Scholar]
- 8.Pang D, Thompson DN. Embryology and bony malformations of the craniovertebral junction. Childs Nerv Syst. 2011;27(4):523–564. doi: 10.1007/s00381-010-1358-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Posner MD. Basilar impression—the value of the various radiological criteria. Proc Wkly Semin Neurol. 1963;15:26–35. [PubMed] [Google Scholar]
- 10.Roussouly P, Pinheiro-Franco JL. Biomechanical analysis of the spino-pelvic organization and adaptation in pathology. Eur Spine J. 2011;20(Suppl 5):609–618. doi: 10.1007/s00586-011-1928-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schady W, Metcalfe RA, Butler P. The incidence of craniocervical bony anomalies in the adult Chiari malformation. J Neurol Sci. 1987;82(1–3):193–203. doi: 10.1016/0022-510X(87)90018-9. [DOI] [PubMed] [Google Scholar]
- 12.Smoker WR. Craniovertebral junction: normal anatomy, craniometry, and congenital anomalies. Radiographics. 1994;14(2):255–277. doi: 10.1148/radiographics.14.2.8190952. [DOI] [PubMed] [Google Scholar]
- 13.Vega A, Quintana F, Berciano J. Basichondrocranium anomalies in adult Chiari type I malformation: a morphometric study. J Neurol Sci. 1990;99(2–3):137–145. doi: 10.1016/0022-510X(90)90150-L. [DOI] [PubMed] [Google Scholar]


