Abstract
Calcium oxalate monohydrate (COM) crystals bind avidly to the surface of proliferating and migrating renal endothelial cells, perhaps a key event in kidney stone formation. Oxalate-induced pre-oxidative stress can further promote crystal attachment cells. Natural products including gallotannins found in green teas have been studied as potentially novel treatments to prevent crystal retention and kidney stone formation. Gallotannin significantly inhibited COM crystal growth and binding to MDCK I renal epithelial cells at non-toxic concentrations and also delayed renal cell migration in a wound healing assay. Reverse transcription polymerase chain reaction (RT-PCR) analysis revealed that gallotannin significantly attenuated oxalate-induced mRNA expression of monocyte chemoattractant protein 1 (MCP-1), osteopontin (OPN), nicotinamide adenine dinucleotide phosphate (NADPH) oxidase subunit p22phox and p47phox in human primary renal epithelial cells (HRCs). Gallotannin also reduced HRC production of reactive oxygen species (ROS) and malondialdehyde (MDA) as well as enhanced antioxidant enzyme superoxide dismutase (SOD) activity in response to oxalate. Taken together, our findings suggest that gallotannin can contribute to nephrolithiasis prevention via direct effects on renal epithelial cells including suppression of COM binding and MCP-1 and OPN expression, along with augmenting antioxidant activity.
Keywords: gallotannin, renal epithelial cells, calcium oxalate monohydrate, MCP-1, osteopontin, ROS, SOD
INTRODUCTION
Nephrolithiasis or kidney stones are a common, painful condition caused by precipitation and retention of poorly soluble salts in the kidney. Calcium oxalate (CaOx) kidney stones are the most common type, accounting for up to 70%. Adhesion of newly formed CaOx monohydrate (COM) crystals to the apical surface of renal tubular epithelial cells could be an important initiating event, at least in the subset with more marked hyperoxaluria as a contributing factor (i.e., primary hyperoxaluria or enteric hyperoxaluria). Interaction of renal epithelial cells with COM crystals has been shown to stimulate expression of monocyte chemoattractant protein-1 (MCP-1) and osteopontin (OPN)1) as well as increase generation of reactive oxygen species (ROS) from nicotinamide adenine dinucleotide phosphate (NADPH) as a second messenger system2).
Recently, several studies have reported that natural products can prevent renal calcification in animal models of nephrolithiasis, in part by preventing hyperoxaluira-induced oxidative stress. Powdered green tea leaves significantly decreased CaOx deposit formation and increased superoxide dismutase (SOD) activity in an ethylene glycol (EG)-induced hyperoxaluric rat model3) while Bergenia ligulata also ameliorated free radical formation and lipid peroxidation4). We recently reported that 1,2,3,4,6-penta-o-galloyl-beta-D-glucose (PGG) from gallnut of Rhus chinensis MILL reduced renal crystallization and oxidative renal injury in a hyperoxaluric rat model5). Gallotannin, polyphenolic hydrolysable tannin found in green tea, was also previously demonstrated to effectively block renal calcification in an ethylene glycol rat model. In the current study we investigated the effect of this natural product on the interaction of renal cells, oxalate, and COM crystals.
MATERIALS AND METHODS
Chemicals
Gallotannin (molecular weight = 1701.2, Fig. 1A) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Sodium oxalate, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and heparin were purchase from Sigma Chemicals (St. Louis, MO).
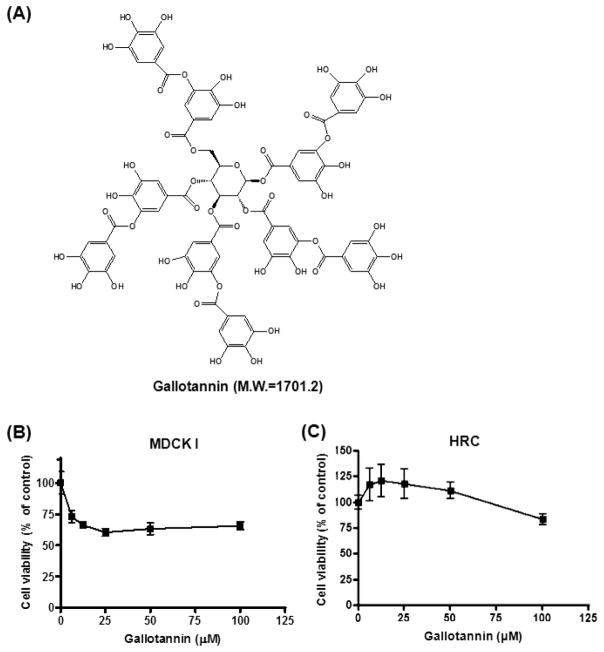
Fig. 1. Cytotoxic effect of gallotannin in MDCK I cells.
(A) Chemical structure of gallotannin. Molecular weight = 1701.2. (B and C) Cytotoxicity of gallotannin was evaluated in renal epithelial cells MDCK I (B) and HRCs (C) by MTT assay. Cells were plated onto 96-well microplates (1×104 cells/well) and treated with various concentrations of gallotannin (0, 6.25, 12.5, 25, 50 or 100 μM) for 24 h. Data were expressed as means ± SD of three independent experiments.
Cell culture
Human primary renal epithelial cells (HRCs)6) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Welgene, Deagu, Korea) supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/ml) and streptomycin (100 μg/ml). Madin-Darby Canine Kidney Cells type I (MDCK I), derived from the distal nephron, were kindly provided by Dr. John C Lieske at Mayo Clinic. The cells were maintained in DMEM containing 25 mM glucose supplemented with 10% FBS, penicillin (100 U/ml) and streptomycin (100 μg/ml).
Cytotoxicity assay
The cytotoxicity of gallotannin was measured by MTT colorimetric assay. HRCs or MDCK I cells were seeded onto 96-well microplates at a density of 1 × 104 cells per well and treated with various concentrations of gallotannin for 24 h. MTT working solution (5 mg/ml in PBS) was added to each well and incubated at 37°C for 3 h. The optical density (OD) was then measured at 570 nm using a microplate reader (Sunrise, TECAN, Männedorf, Switzerland). Cell viability was calculated as a percentage of viable cells in gallotannin-treated group versus untreated control by the following equation. .
Crystal growth inhibition assay
Crystal growth inhibition (CGI) was assayed using the method of Asplin and colleagues7). COM seed crystal slurries (0.8 mg/ml.) were prepared by adding 32 mg of crystals to 40 ml of 90 mM NaCl, 10 mM Tris buffer, pH 7.2. The slurry was stirred for 48 h at approximately 1,500 rpm. Between 80 and 150 μl of seed slurry (amount varied daily to give consistent growth signal) was added to 2 ml of calcium oxalate solution consisting of 0.5 mM calcium, 0.5 mM oxalate, 90 mM NaCl and 10 mM Tris (pH 7.2) in a quartz cuvette at 37°C and magnetically stirred at 1,100 rpm. Oxalate consumption was initiated by the addition of seed crystals and monitored for 420 seconds with a Varian UV-visible, continuously recording spectrophotometer (Varian Cary 3E UC-Visible spectrophotometer, Melbourne, Australia) at 214 nm. Data for analysis were ported from the spectrophotometer to a personal computer using data capture software (Cary Win UV (version 3.00) 2002 Varian Australia Pty Ltd, Melbourne, Australia). Buffer-exchanged totally urinary protein (20 μg) was added to experimental samples and an equal volume of buffer to the controls. The rate of oxalate consumption in a seeded crystal growth system followed second order kinetics8). Oxalate consumption was fitted to a nonlinear function (Eq 1):
where Ct is the measured cuvette optical density at 214 nm, Ci is the initial and C∞ the final stable reading after 4 h of incubation, a is a rate constant and t is time of incubation. The function was fitted to the data for Ct and allowed to calculate best fit for Ci and a. C∞ was measured experimentally as 0.16 in the system and given as a constant. The rate constant a was calculated for the buffer-exchanged urine protein and concomitant controls, and growth rate (G) in the presence of urine proteins was expressed as a percentage of control growth (Eq 2):
where e and c refer to the experimental and control cuvette results, respectively. To obtain the experimental rate constant ae we used the average of the 2 experimental cuvette values. The units were a measure of residual growth after urine inhibition. Each sample and control was run in duplicates.
Calcium oxalate monohydrate (COM) crystal binding assay
The COM crystal binding assay was performed as previously described9,10). MDCK I cells were grown to confluency in 35-mm tissue culture dishes and serum-starved for 24 h. [14C] COM crystals were incubated with gallotannin to pre-coat crystals in microcentrifuge tubes subjected to end-over-end rotation for 15 min at room temperature, and then washed 3 times with PBS. To assess the capacity to bind crystals, a [14C] COM crystal suspension (8 μg/ml, 50 μl) was distributed homogeneously across the monolayer, where it settled on top of the cells under the force of gravity (41.6 μg/cm2 of cells) for 2 min and rinsed three times with phosphate buffered saline (PBS). The monolayer was scraped and directly added into a 5.0-ml scintillation vial filled with 3.0 ml of scintillation cocktail (Emulsifier Safe Scintillation mixture; PerkinElmer). Radioactivity of adherent crystals was counted using a beta scintillation counter (Beckman Coulter, Fullerton, CA)10)
Total RNA isolation and Reverse Transcription –Polymerase Chain Reaction (RT-PCR) analysis
Total RNA was prepared by using Trizol reagent according to the manufacturer’s instructions. Total RNA (1.0 μg) was reverse transcribed using MMLV reverse transcriptase (Promega, Madison, WI) by incubation at 25°C for 10 min, at 42°C for 60 min and at 99C for 5 min. The synthesized cDNA was amplified using TaKaRa Taq DNA polymerase (TaKaRa Biotechnology, Shiga, Japan) and the following specific primers: p22phox (sense 5′-GTTTGTTTTGTGCCTGCTGGAGT-3′; antisense 5′-TGGGCGGCTGCTTGATGGT-3′), p47phox (sense 5′-ACCCAGCCAGCACTATGTGT-3′; antisense 5′-AGTAGCCTGTGACGTCGTCT-3′), MCP-1 (sense 5′-GCTCGCTCAGCCAGATGCAAT-3′, antisense 5′-TGGGTTGTGGAGTGAGTGTTC-3′, OPN (sense 5′-TGAGTCTGGAAATAACTAATGTGTTTGA-3′, antisense 5′-GAACATAGACATAACCCTGAAGCTTTT-3′, and GAPDH (sense 5′-GTGGATATTGTTGCCATCA-3′, antisense 5′-ACTCATACAGCACCTCAG-3′. PCR conditions were 30 cycles of 94°C for 30 sec, 59°C for 30 sec and 72°C for 30 sec, followed by 5 min incubation at 72°C. PCR products were run on 2% agarose gel and then stained with ethidium bromide (EtBr).
Western Blot Analysis
Cells were lysed in radio immunoprecipitation assay (RIPA) buffer (50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 0.25% sodium deoxycholic acid, 1 M ethylene diaminetetraacetic acid (EDTA), 1 mM Na3VO4, 1 mM NaF and protease inhibitors cocktail). Protein samples were quantified by using a Bio-Rad DC protein assay kit II (Bio-Rad, Hercules, CA, U.S.A.), separated by electrophoresis on 8 to 15% sodium dodecyl sulfatepolyacrylamide gel electrophoresis (SDS-PAGE) gel and electrotransferred onto a Hybond ECL transfer membrane (Amersham Pharmacia, Piscataway, NJ, U.S.A.). After blocking with 5% nonfat skim milk, the membrane was probed with antibodies for MCP-1, OPN, p22phox, p47phox (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.), and β-actin (Sigma Aldrich Co., St. Louis, MO, U.S.A.) followed by exposing to horseradish peroxidase (HRP)-conjugated secondary anti-mouse or rabbit antibodies (AbD serotec, kidlington, U.K.). Protein expression was determined by using enhanced chemiluminescence (ECL) system (Amersham Pharmacia, Piscataway, NJ, U.S.A.).
Measurement of reactive oxygen species (ROS) production
ROS level was measured using 2,7-dichlorofluorescein diacetate (DCFDA) fluorescence dye. Cells were incubated with 1 μM DCFDA at 37°C for 30 min. Fluorescence intensities were measured by BD FACSCalibur flow cytometry (Becton Dickinson, Franklin Lakes, NJ).
Superoxide dismutase (SOD) and malondialdehyde (MDA) assays
SOD enzyme activity and MDA content were measured by using Bioxytech SOD-525 and MDA-586 Assay kits (OXIS International, Portland, OR), respectively.
Statistical analyses
All data were expressed as means ± SD. The statistically significant differences between control and gallotannin-treated cells were calculated by analysis of variance (ANOVA) test followed by a post hoc analysis (Tukey’s or Dunnett’s multiple comparison test) using Prism software 5 (GraphPad Software, Inc., San Diego, CA, U.S.A.).
RESULTS
Cytotoxicity of gallotannin in MDCK I cells and HRCs
To evaluate the cytotoxic effect of gallotannin on MDCK I cells and HRCs, an MTT assay was conducted. The cells were treated with increasing concentrations of gallotannin (0, 6.25, 12.5, 25, 50 or 100 μM) for 24 h. As shown in Fig. 1, gallotannin exerted weak cytotoxicity against MDCKs to 60% of untreated control at the concentration of ca. 12.5 μM, while it did not show any cytotoxicity against HRCs at the concentrations of <60 μM, indicating that MDCKs originated from mouse distal nephron were more susceptible to gallotannin compared to HRCs originated from human nephron. We also think the susceptibility of two cell lines can be different by origin, type and gene specificity.
Effects of gallotannin on COM crystal binding in MDCK I cells
To examine whether gallotannin can prevent the growth of CaOx crystals, a crystal growth inhibition (CGI) assay was performed. Gallotannin significantly increased the inhibitory activity to 0.05, 0.24 and 0.48 at the concentrations of 0.1, 1.0 and 10 μM, respectively (Fig. 2A) while the positive control molecule heparin showed an inhibitory activity of 0.47 at 10.29 U/ml. Furthermore, gallotannin significantly reduced the binding of pre-coated calcium oxalate monohydrate (COM) crystals to MDCK I cells in a dose-dependent manner (Fig. 2B). However, gallotannin-pretreatment of cells for 15 min or 24 h had no significant effect on the crystal binding (data not shown).
Fig. 2. Effects of gallotannin on COM crystal binding in MDCK I cells.
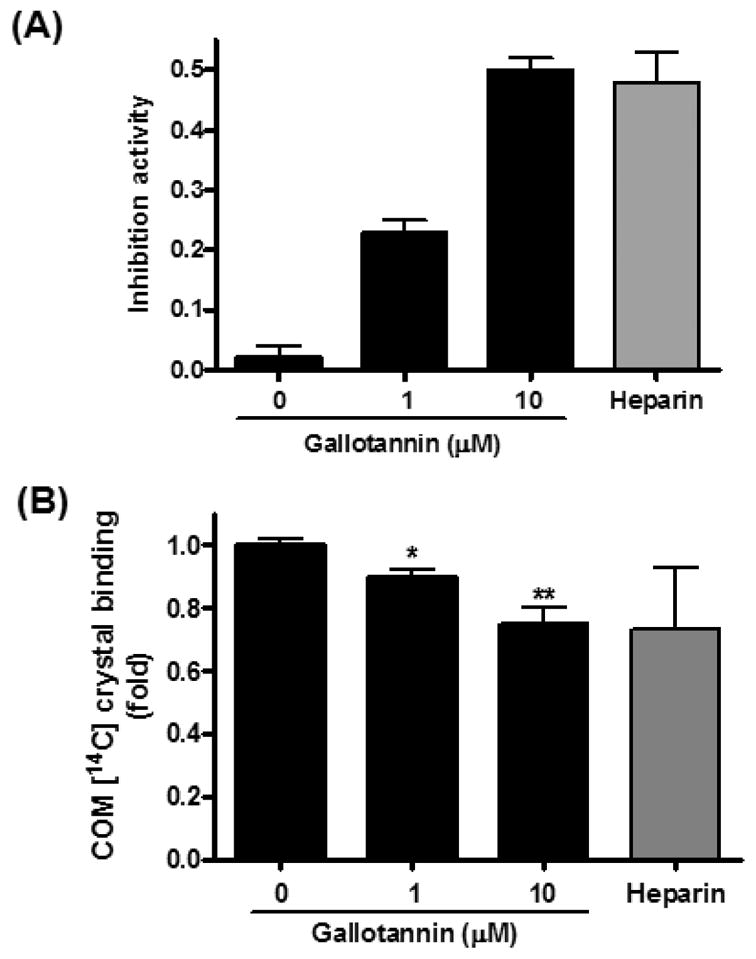
(A) COM seed crystal slurries were incubated with gallotannin and added to calcium oxalate solution consisting of 0.5 mM calcium, 0.5 mM oxalate, 90 mM NaCl and 10 mM Tris (pH 7.2) in a quartz cuvette at 37°C and magnetically stirred at 1100 rpm. Oxalate consumption was initiated by the addition of seed crystals and monitored for 420 s with a Varian UV-Visible, continuously recording spectrophotometer (Varian Cary 3E UC-Visible spectrophotometer, Melbourne, Australia) at 214 nm. (B) [14C] COM crystals were incubated with gallotannin to pre-coat crystals and distributed homogeneously across the monolayer of MDCK I for 2 min and rinsed three times with PBS. Radioactivity of adherent crystals was counted using a beta scintillation counter (Beckman Coulter, Fullerton, CA, U.S.A.).10) Data were expressed as mean±S.D. of three independent experiments. *p<0.05 and **p<0.01 vs. untreated control, one-way analysis of variance followed by a post hoc analysis.
Effect of gallotannin on MCP-1 and osteopontin expression in HRCs
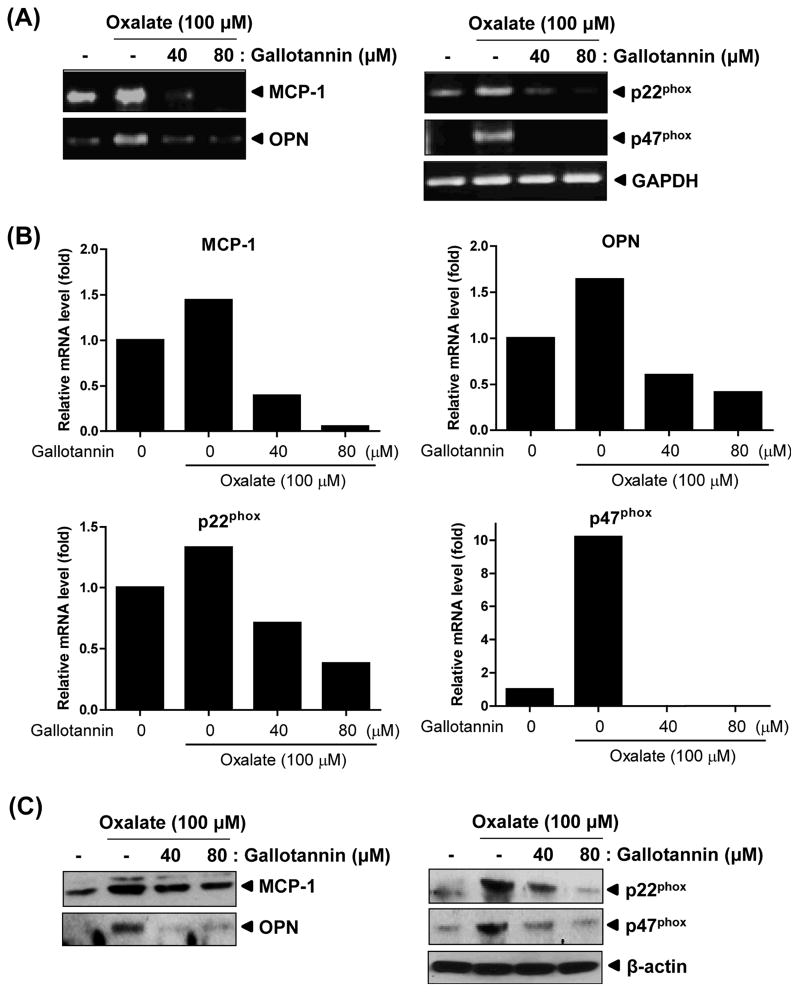
COM crystals were previously shown to promote the renal epithelial cell production of monocyte chemoattractant protein 1 (MCP-1) and osteopontin (OPN) 11,12). Therefore we analyzed MCP and OPN mRNA expression in oxalate-treated human primary renal epithelial cells (HRCs) with or without gallotannin. As expected, the mRNA levels of MCP-1 and OPN were clearly increased in oxalate-treated cells (lane 2) compared with untreated control (lane 1) (Fig. 4). In contrast, gallotannin treatment remarkably suppressed oxalate-mediated expression of MCP-1 and OPN in a dose-dependent manner (Fig. 4, lanes 3 and 4). Nicotinamide adenine dinucleotiide phosphate (NADPH) oxidase has been proposed as a second messenger that signals expression of OPN and MCP-12). In our study, gallotannin significantly reduced the expression of p22phox and p47phox, major subunits of NADPH oxidase, in oxalate-treated HRCs (Figs. 3A, B). Consistently, gallotannin decreased oxalate-mediated overexpression of MCP-1, OPN, p22phox and p47phox at protein level by Western blotting (Fig. 3C). However, oxalate did not exert significant cytotoxicity against HRCs (data not shown), despite significant regulation of NADPH oxidase system. Also, regarding some gene expression less than basal level at mRNA level, we have to confirm the results in different cell lines and experimental design in the future.
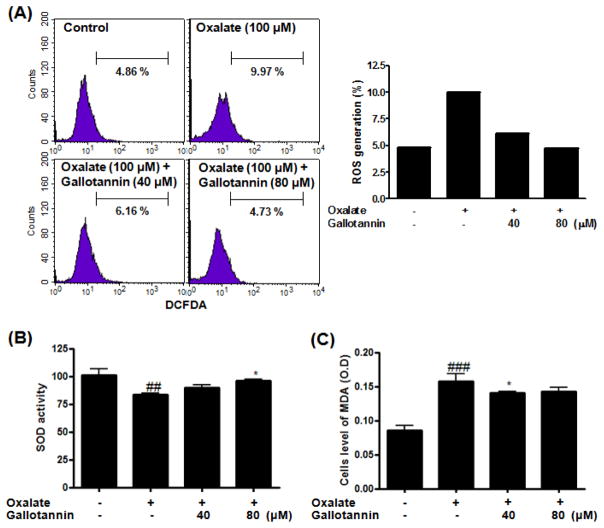
Fig. 4. Anti-oxidative effect of gallotannin in oxalate-treated HRCs.
Cells were exposed to oxalate (100 μM) with or without gallotannin (40 or 80 μM) for 24 h. (A) ROS generation (%) was measured using ROS-sensitive fluorometric probe 2,7-dichlorofluorescein diacetate (DCFDA) by flow cytometric analysis. (B) SOD activity was measured by using Bioxytech SOD-525 Assay kit. (C) MDA content was measured by using Bioxytech MDA-586 Assay kit. Data were expressed as mean±S.D. of three independent experiments. ##p<0.01 and ###p<0.001 vs. untreated control *p<0.05 vs. oxalate control, two-way analysis of variance followed by a post hoc analysis.
Fig. 3. Effect of gallotannin on mRNA expressions of MCP-1, OPN, p22phox and p47phox in oxalate-treated HRCs.
Cells were exposed to oxalate (100 μM) with or without gallotannin (40 or 80 μM) for 24 h. (A) Gene expressions of MCP-1, OPN, p22phoxand p47phox were analyzed by semi-quantitative RT-PCR. (B) Graphs represent fold changes of mRNA expression of indicated genes adjusted by GAPDH. (C) Cell lysates were prepared and subjected to Western blotting for MCP-1, OPN, p22phox and p47phox
Anti-oxidant activity of gallotannin in oxalate-treated HRCs
NADPH oxidase is an important source of reactive oxygen species (ROS) in renal diseases13). To assess the effect of gallotannin on oxalate-induced oxidative stress, HRCs were exposed to oxalate in the absence or presence of gallotannin for 24 h. As shown in Fig. 4A, ROS levels were significantly raised in the presence of oxalate. In contrast, gallotannin significantly reduced the production of ROS from 9.97% to 6.16 and 4.73% at 40 and 80 μM, respectively (Fig. 4A). Activity of the antioxidative enzyme superoxide dismutase (SOD) was significantly increased in oxalate/gallotannin-treated cells compared with oxalate control (Fig. 4B). In contrast, malondialdehyde (MDA) levels significantly declined after gallotannin treatment in a dose-dependent manner (Fig. 4C).
DISCUSSION
The incidence of nephrolithiasis has been increasing in the US over the past three decades14). Nephrolithiasis has multifactorial causes, including contribution by diet and genetics. One important result is supersaturation of the urine with salts that produce kidney stones, including CaOx. CaOx in turn can induce oxidative renal cell injury15). Recently, phytotherapeutic agents were proposed as useful alternative or a complementary therapies for the management of urolithiasis, in part due to anti-oxidative effects16). Gallotannins, hydrolysable tannins with polyesters of a sugar moiety (or other non-aromatic polyhydroxy compounds) and organic acids are chiefly contained in cheakpeas, cowpeas, mangos, persimmons, star fruit (Averrhoa carambola L), pecans and rhubarbs17). Despite their multi-biological activities including anticancer, anti-inflammatory and hepatoprotective effects, the potential anti-nephrolithic activities of gallotannin (MW=1701.2) have not been investigated until now.
COM crystals bind avidly to the surface of renal endothelial cells, and oxalate-induced pre-oxidative stress can promote crystal attachment to renal epithelial cells18,19). Massive accumulation of COM crystals in kidney tissues can produce renal tubular necrosis that leads to kidney failure20,21). In the current study, the potential for gallotannins to protect renal cells against CaOx and/or oxalate was assessed. Gallotannin significantly inhibited COM crystal growth and binding to MDCK I renal epithelial cells at non-toxic concentrations in vitro, implying a potential for gallotannin to exert positive effects in vivo to protect against stone formation.
There is previous evidence to suggest that increased expression of MCP-1 and OPN play an important role in kidney stone formation2,22–24). In this study, RT-PCR analysis confirmed that gallotannin attenuated MCP-1 and OPN mRNA expression in oxalate-treated HRCs. In general renal cell injury, perhaps secondary to oxalate-induced free radical generation, appears to favor CaOx crystal retention, 25–28). In our study, gallotannin significantly attenuated the mRNA expression of NADPH oxidase subunit p22phox and p47phox in oxalate-treated HRCs. Gallotannin consistently reduced the production of ROS and MDA and also well as enhanced activity of the antioxidant enzyme SOD in oxalate-treated HRCs. These antioxidant effects of gallotannin should block pathways that could lead to crystal retention in the kidney.
In summary, gallotannin inhibited COM crystal growth and adhesion to renal epithelial cells, proliferation and migration of renal epithelial cells in a wound healing assay, and attenuated mRNA expression of MCP-1, OPN, NADPH oxidase subunit p22phox and p47phox after oxalate treatment in HRCs. Furthermore, gallotannin reduced oxalate-induced ROS and MDA generation while enhancing antioxidant enzyme activity. Overall, our findings suggest that gallotannin is a candidate for further study as a natural product that could prevent kidney stones by suppressing adhesion and retention of COM crystals within nephrons. However, additional in vivo studies of gallotannin are required to confirm these in vitro results.
Acknowledgments
This work was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MEST) (No. 2011-0017956) to S-H K, the Mayo Clinic O’Brien Urology Research Center P50 DK083007 funded by NIDDK and the Rare Kidney Stone Consortium U54 DK082908 funded by NIDDK and the NIH Office of Rare Diseases Research to JCL, and NIH Postdoctoral Training Grant T32 DK 07013 to MPL.
References
- 1.Khan SR. Crystal-induced inflammation of the kidneys: results from human studies, animal models, and tissue-culture studies. Clin Exp Nephrol. 2004;8:75–88. doi: 10.1007/s10157-004-0292-0. [DOI] [PubMed] [Google Scholar]
- 2.Umekawa T, Tsuji H, Uemura H, Khan SR. Superoxide from NADPH oxidase as second messenger for the expression of osteopontin and monocyte chemoattractant protein-1 in renal epithelial cells exposed to calcium oxalate crystals. BJU Int. 2009;104:115–120. doi: 10.1111/j.1464-410X.2009.08374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Itoh Y, Yasui T, Okada A, Tozawa K, Hayashi Y, Kohri K. Preventive effects of green tea on renal stone formation and the role of oxidative stress in nephrolithiasis. J Urol. 2005;173:271–275. doi: 10.1097/01.ju.0000141311.51003.87. [DOI] [PubMed] [Google Scholar]
- 4.Bashir S, Gilani AH. Antiurolithic effect of Bergenia ligulata rhizome: an explanation of the underlying mechanisms. J Ethnopharmacol. 2009;122:106–116. doi: 10.1016/j.jep.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Lee HJ, Jeong SJ, Lee HJ, Lee EO, Bae H, Lieske JC, Kim SH. 1,2,3,4,6-Penta-O-galloyl-beta-D-glucose reduces renal crystallization and oxidative stress in a hyperoxaluric rat model. Kidney Int. 2011;79:538–545. doi: 10.1038/ki.2010.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dörrenhaus A, Müller JI, Golka K, Jedrusik P, Schulze H, Föllmann W. Cultures of exfoliated epithelial cells from different locations of the human urinary tract and the renal tubular system. Arch Toxicol. 2000;74:618–626. doi: 10.1007/s002040000173. [DOI] [PubMed] [Google Scholar]
- 7.Asplin JR, Parks JH, Chen MS, Lieske JC, Toback FG, Pillay SN, Nakagawa Y, Coe FL. Reduced crystallization inhibition by urine from men with nephrolithiasis. Kidney Int. 1999;56:1505–1516. doi: 10.1046/j.1523-1755.1999.00682.x. [DOI] [PubMed] [Google Scholar]
- 8.Meyer JL, Smith LH. Growth of calcium oxalate crystals. I A model for urinary stone growth. Invest Urol. 1975;13:31–35. [PubMed] [Google Scholar]
- 9.Lee JH, Yehl M, Ahn KS, Kim SH, Lieske JC. 1,2,3,4,6-Penta-Ogalloyl-beta-D-glucose attenuates renal cell migration, hyaluronan expression, and crystal adhesion. Eur J Pharmacol. 2009;606:32–37. doi: 10.1016/j.ejphar.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 10.Lieske JC, Leonard R, Toback FG. Adhesion of calcium oxalate monohydrate crystals to renal epithelial cells is inhibited by specific anions. Am J Physiol. 1995;268:F604–F612. doi: 10.1152/ajprenal.1995.268.4.F604. [DOI] [PubMed] [Google Scholar]
- 11.Kleinman JG, Wesson JA, Hughes J. Osteopontin and calcium stone formation. Nephron Physiol. 2004;98:43–47. doi: 10.1159/000080263. [DOI] [PubMed] [Google Scholar]
- 12.Kumar V, Lieske JC. Protein regulation of intrarenal crystallization. Curr Opin Nephrol Hypertens. 2006;15:374–380. doi: 10.1097/01.mnh.0000232877.12599.f4. [DOI] [PubMed] [Google Scholar]
- 13.Peixoto EB, Pessoa BS, Biswas SK, Lopes de Faria JB. Antioxidant SOD mimetic prevents NADPH oxidase-induced oxidative stress and renal damage in the early stage of experimental diabetes and hypertension. Am J Nephrol. 2009;29:309–318. doi: 10.1159/000163767. [DOI] [PubMed] [Google Scholar]
- 14.Marengo SR, Romani AM. Oxalate in renal stone disease: the terminal metabolite that just won’t go away. Nat Clin Pract Nephrol. 2008;4:368–377. doi: 10.1038/ncpneph0845. [DOI] [PubMed] [Google Scholar]
- 15.Rashed T, Menon M, Thamilselvan S. Molecular mechanism of oxalate-induced free radical production and glutathione redox imbalance in renal epithelial cells: effect of antioxidants. Am J Nephrol. 2004;24:557–568. doi: 10.1159/000082043. [DOI] [PubMed] [Google Scholar]
- 16.Butterweck V, Khan SR. Herbal medicines in the management of urolithiasis: alternative or complementary? Planta Med. 2009;75:1095–1103. doi: 10.1055/s-0029-1185719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serrano J, Puupponen-Pimiä R, Dauer A, Aura AM, Saura-Calixto F. Tannins: current knowledge of food sources, intake, bioavailability and biological effects. Mol Nutr Food Res. 2009;53 (Suppl 2):S310–S329. doi: 10.1002/mnfr.200900039. [DOI] [PubMed] [Google Scholar]
- 18.Hall PM. Nephrolithiasis: treatment, causes, and prevention. Cleve Clin J Med. 2009;76:583–591. doi: 10.3949/ccjm.76a.09043. [DOI] [PubMed] [Google Scholar]
- 19.Wendt-Nordahl G, Evan AP, Spahn M, Knoll T. Calcium oxalate stone formation. New pathogenetic aspects of an old disease. Urologe A. 2008;47:538, 540–544. doi: 10.1007/s00120-008-1707-4. [DOI] [PubMed] [Google Scholar]
- 20.McMartin K. Are calcium oxalate crystals involved in the mechanism of acute renal failure in ethylene glycol poisoning? Clin Toxicol (Phila) 2009;47:859–869. doi: 10.3109/15563650903344793. [DOI] [PubMed] [Google Scholar]
- 21.Okamoto M, Kohjimoto Y, Iba A, Saji F, Hara I, Shigematsu T. Calcium oxalate crystal deposition in metabolic syndrome model rat kidneys. Int J Urol. 2010;17:996–1003. doi: 10.1111/j.1442-2042.2010.02661.x. [DOI] [PubMed] [Google Scholar]
- 22.Kleinman JG, Beshensky A, Worcester EM, Brown D. Expression of osteopontin, a urinary inhibitor of stone mineral crystal growth, in rat kidney. Kidney Int. 1995;47:1585–1596. doi: 10.1038/ki.1995.222. [DOI] [PubMed] [Google Scholar]
- 23.Miyajima J, Hayashi T, Saito K, Iida S, Matsuoka K. The Interaction between female sex hormone receptors and osteopontin in a rat hyperoxaluric model. Kurume Med J. 2010;57:73–80. doi: 10.2739/kurumemedj.57.73. [DOI] [PubMed] [Google Scholar]
- 24.Boonla C, Hunapathed C, Bovornpadungkitti S, Poonpirome K, Tungsanga K, Sampatanukul P, Tosukhowong P. Messenger RNA expression of monocyte chemoattractant protein-1 and interleukin-6 in stone-containing kidneys. BJU Int. 2008;101:1170–1177. doi: 10.1111/j.1464-410X.2008.07461.x. [DOI] [PubMed] [Google Scholar]
- 25.Thamilselvan S, Byer KJ, Hackett RL, Khan SR. Free radical scavengers, catalase and superoxide dismutase provide protection from oxalate-associated injury to LLC-PK1 and MDCK cells. J Urol. 2000;164:224–229. [PubMed] [Google Scholar]
- 26.Thamilselvan S, Hackett RL, Khan SR. Lipid peroxidation in ethylene glycol induced hyperoxaluria and calcium oxalate nephrolithiasis. J Urol. 1997;157:1059–1063. [PubMed] [Google Scholar]
- 27.Thamilselvan V, Menon M, Thamilselvan S. Selective Rac1 inhibition protects renal tubular epithelial cells from oxalate-induced NADPH oxidase-mediated oxidative cell injury. Urol Res. 2011 doi: 10.1007/s00240-011-0405-7. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selvam R. Calcium oxalate stone disease: role of lipid peroxidation and antioxidants. Urol Res. 2002;30:35–47. doi: 10.1007/s00240-001-0228-z. [DOI] [PubMed] [Google Scholar]