Abstract
The expanding nasal septal cartilage is believed to create a force that powers midfacial growth. In addition, the nasal septum is postulated to act as a mechanical strut that prevents the structural collapse of the face under masticatory loads. Both roles imply that the septum is subject to complex biomechanical loads during growth and mastication. The purpose of this study was to measure the mechanical properties of the nasal septum to determine (1) whether the cartilage is mechanically capable of playing an active role in midfacial growth and in maintaining facial structural integrity and (2) if regional variation in mechanical properties is present that could support any of the postulated loading regimens. Porcine septal samples were loaded along the horizontal or vertical axes in compression and tension, using different loading rates that approximate the in vivo situation. Samples were loaded in random order to predefined strain points (2–10%) and strain was held for 30 or 120 seconds while relaxation stress was measured. Subsequently, samples were loaded until failure. Stiffness, relaxation stress and ultimate stress and strain were recorded. Results showed that the septum was stiffer, stronger and displayed a greater drop in relaxation stress in compression compared to tension. Under compression, the septum displayed non-linear behavior with greater stiffness and stress relaxation under faster loading rates and higher strain levels. Under tension, stiffness was not affected by strain level. Although regional variation was present, it did not strongly support any of the suggested loading patterns. Overall, results suggest that the septum might be mechanically capable of playing an active role in midfacial growth as evidenced by increased compressive residual stress with decreased loading rates. However, the low stiffness of the septum compared to surrounding bone does not support a strut role. The relatively low stiffness combined with high stress relaxation under fast loading rates suggests that the nasal septum is a stress dampener, helping to absorb and dissipate loads generated during mastication.
Introduction
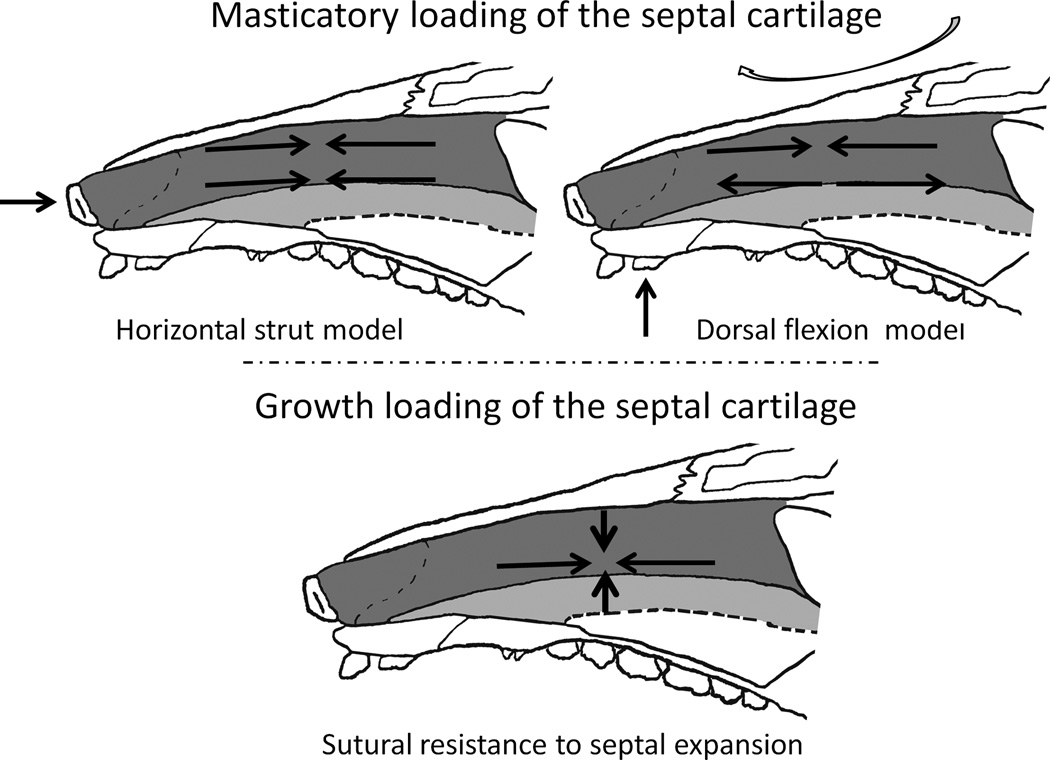
The nasal septal cartilage is a midline plate extending from the perpendicular plate of the ethmoid bone to the external nose. It is hypothesized that the septal cartilage acts as a primary growth center of the midface, with expansion of the cartilage separating the facial bones at the connecting sutures, resulting in stimulation of bone formation along the sutural edges (Scott, 1953, Kvinnsland, 1974; Sarnat and Wexler, 1966). In addition, it is believed that the nasal septum acts as a vertical strut supporting the face against masticatory loads (Moss et al., 1968; Stenström and Thilander, 1970, Badoux, 1966, 1968). Recently, the vertical strut role was challenged by the finding of anteroposterior compression in the dorsal half of the septum in pigs during mastication (Al Dayeh et al., 2009), however, the septum might still be mechanically necessary as an anteroposterior strut in resisting dorsal flexion of the snout (Figure 1).
Figure 1.
Medial view of the pig nasal septum (gray; the light gray part sits within the vomer bone) illustrating possible patterns of loading during mastication (top row) and during growth (bottom). Both the horizontal strut model and the dorsal flexion model are consistent with in vivo data indicating that the dorsal region of the septum and snout are compressed anteroposteriorly during mastication (Al Dayeh et al., 2009; Rafferty et al., 2003). Mechanical adaptation to a horizontal strut model would suggest a relatively high anteroposterior compressive stiffness and strength with vertical adaptation to resist tensile strain. Dorsal flexion of the snout predicts horizontal adaptation to compression dorsally and to tension ventrally. Because growth expands the snout both horizontally and vertically, the resistance would be to compression in both axes, but at a very slow loading rate.
If the septum is a growth center, then it should be able to resist the compressive loads generated by the resistance and recoil of the sutures. If the septum has a mechanical role during chewing, it should show directional and regional adaptations to its loading regime (Figure 1). Alternatively, the septum may act as a stress dampener, absorbing and dissipating loads generated during mastication. Furthermore, finite element analysis of trauma to the human nose also suggests a significant role of the septum in stress absorption (Lee et al., 2010).
Cartilage is an almost avascular tissue that includes type II collagen and aggrecans. This complex composition gives rise to a well documented viscoelastic mechanical behavior (Langelier and Buschmann, 2003, Huang et al., 2001). The compressive properties are dictated by the aggrecans and water content while the tensile properties are dictated by the collagen content. Thus, direction of loading, loading rate (DiSilvestro et al., 2001; Langelier and Buschmann, 2003; Li and Herzog, 2004), loading time and hydration (Race et al., 2000) are important determinants of the mechanical properties of cartilage. Loading rate is especially important in considering the possible roles of the septal cartilage in growth (slow loading) and mastication (fast loading). Although the septum, including the porcine septum, is often used as a source for the measurement of the mechanical properties of cartilage (Chao et al., 2003; Gaon et al., 2003; Grellmann et al., 2006; Naumann et al., 2002; Richmon et al., 2005, 2006;; Rotter et al., 2002; Westreich et al., 2007; Wong et al., 2001; Xia et al., 2012; Zemek et al., 2012), these studies have not systematically investigated directional and regional differences in relation to function. Additionally, the perichondrium was excised in most cases, a procedure that results in a 50% decrease in the elastic modulus of auricular cartilage (Roy et al., 2004).
The overall aim of this study was to measure the mechanical properties of the composite structure of the septal cartilage and the covering perichondrium in compression and tension, with particular attention to regional variation and loading rate. The perichondrium was left intact to approximate the mechanical properties of the nasal septum as it exists in vivo. The ultimate goal is to shed light on whether the mechanical properties of the nasal septum support an active role in facial growth and in supporting the face during dynamic loading.
Materials and Methods
Compression tests
Pig heads (n=18) were obtained from an abattoir (Kapowsin Meat, Graham, WA), age, sex and breed unknown. Nasal septa were extracted and stored frozen in 4% phosphate buffered saline (PBS).
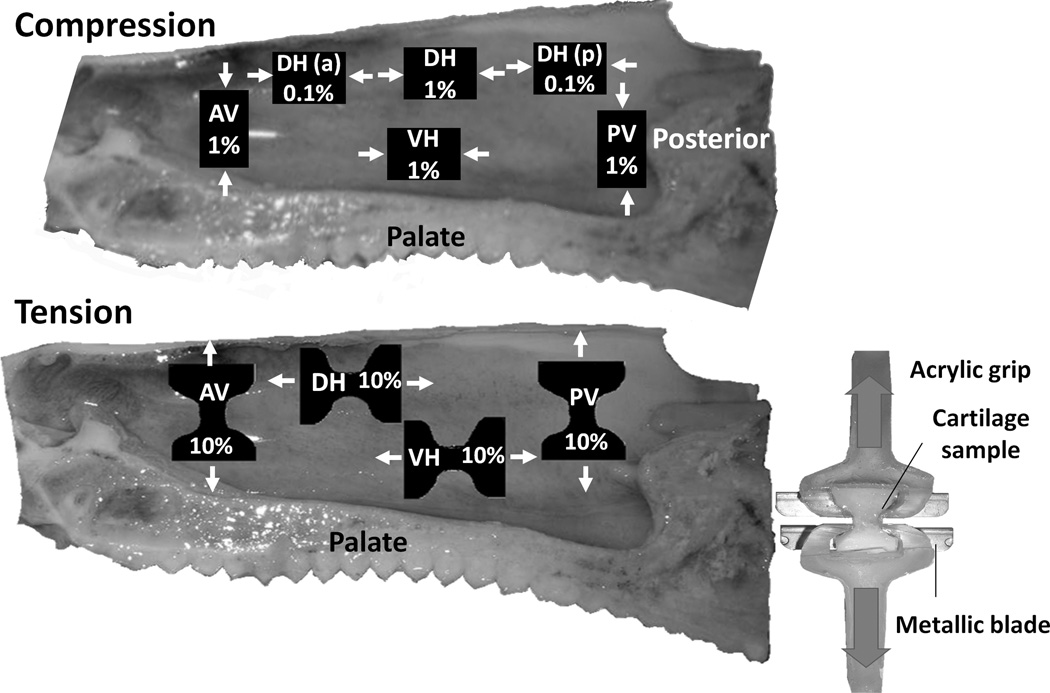
On the day of measurement, septa were thawed and six 9–10 by 9 mm rectangular samples were cut (Figure 2): 2 vertical (anterior and posterior) and 4 horizontal (three dorsal and one ventral). Natural thickness with the perichondrium in place was preserved and measured. The thickness of the porcine septum was relatively uniform, averaging 5.4±0.8 mm; thus, samples were rectangular in section. Samples were compressed in the sagittal plane in the long-axis orientation (arrows in Figure 2). The 2 vertical and 2 of the horizontal samples (dorsal middle and ventral) were loaded at 1%/s (~0.1 mm/sec) while the remaining 2 horizontal (dorsal anterior and posterior) samples were loaded at 0.1%/s (~0.01 mm/sec). The faster strain rate was chosen to mimic the low end of masticatory loading in pigs (Al Dayeh et al., 2009), while the slower rate was the closest approximation to the growth rate of the cartilage (approximately 2×10−5 %/sec, calculated from Al Dayeh et al., 2013) that could be achieved with our testing machine (MTS/Sintech 2, Raleigh, NC). Sandpaper was glued to the compression platens in order to keep samples in place, and samples were immersed in saline during preloading and testing.
Figure 2.
Medial view of the septum illustrating the location of the tested samples in compression (top) and in tension (bottom). Loading direction is designated by arrows. Numbers indicate loading speed in %length/sec. AV: anterior vertical, VH: ventral horizontal, DH: dorsal horizontal, PV: posterior vertical. DH (a): dorsal horizontal (anterior), DH (p): dorsal horizontal (posterior). Right: During tension experiments the cartilage samples were placed in acrylic grips and kept aligned by metallic blades.
Samples were preloaded to 1.5 or 2.5N, a process that was completed within 1 sec for the 1%/sec samples and 7 sec for the 0.1%/sec samples. The resultant deformation was defined as 0% strain. Subsequently, samples were loaded to strain points 2, 4, 6, 8 and 10% in random order, and the strain was held for a relaxation time of 120 sec. The low strain point of 2% approximates the strains generated on the septum during mastication (0.1–1%, calculated from Al Dayeh et al., 2009). Growth strains are much lower (Al Dayeh et al., 2013), but were impossible to approximate experimentally. The high strain point of 10% approximates some traumatic loads and is a typical lower end of values used by other workers (Richmon et al., 2005, 2006; Zemek et al., 2012). The varying strain levels provide a comparison to the literature and illustrate general patterns of septal mechanical behavior. A two-minute rest period separated each loading to allow rehydration. Finally, samples were loaded until failure.
Deformation was converted to strain by dividing by the length of the sample between compression platens before loading. Load was converted to stress by dividing by the sample cross-sectional area perpendicular to the compression axis, calculated from the thickness and width before the test. Throughout each test, stress and strain were continuously recorded at 50 Hz. Stress-strain and stress-time graphs were plotted. Samples that showed buckling or instability were discarded.
For each test, the stress-strain curve (Figure 3) was plotted, and stiffness was calculated as the slope of the least-squares linear regression line (line of best fit) through the data points. Relaxation stress was measured at 0, 10, 30 and 120 seconds (Figure 3). The percentage drop in the relaxation stress between each time interval was calculated as an indication of stress absorption by the cartilage. The final failure test was used to measure the ultimate stress and strain of the septum.
Figure 3.
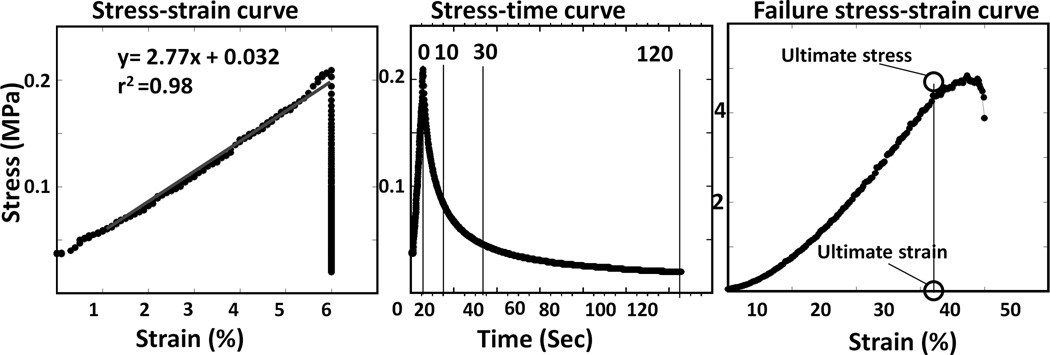
Examples of mechanical tests. Left: Stress-strain curve illustrating stiffness calculation. This sample was vertically compressed at 1%/s until 6% strain was reached. Center: Stress-time plot of the same sample. After 6% strain was reached, strain was held for 120 seconds (30 seconds in tension experiments). Relaxation stress was measured at 0, 10, 30, and in compression 120 seconds. The drop in stress between these time points was used to indicate stress dissipation. Right: Failure stress-strain curve for same sample.
The effect of loading rate was tested by comparing dorsal samples loaded at 1 and 0.1%/sec (Figure 2), using repeated measures ANOVA (RM-ANOVA).To assess whether the septum is adapted as a vertical or anteroposterior strut (Figure 1), stiffness and strength (ultimate stress) of vertically and horizontally loaded samples (Figure 2) were compared using RM-ANOVA followed by post-hoc paired t-tests to identify the source of significance. Statistical tests were performed using SPSS v13.0.
Tension tests
Pig heads (n=22) were obtained as before; nasal septa were extracted and stored in saline at 4°C. On the day of testing, four hourglass-shaped samples approximately 10×20mm were cut (Figure 2), 2 vertical (anterior and posterior) and 2 horizontal (dorsal and ventral).
Each sample was fitted into a pair of acrylic grips. Metallic blades and rubber bands were used to allow compression-free mechanical retention of the samples (Figure 2). The length and thickness of the exposed rectangular waist were measured prior to each experiment; final dimensions averaged 4.2×5.1 mm. Samples were mounted in the MTS/Sintech2 and kept hydrated during preloading and testing using saline immersion. As with compression, samples were loaded in the sagittal plane either in the vertical or horizontal orientation as illustrated in Figure 2. Samples were preloaded to 1.5 or 2.5N (about 0.1 sec at 10%/sec), and the resultant deformation was defined as 0% strain. Subsequently, samples were loaded at 10%/sec until predefined strain points were reached. The same strain points of 2, 4, 6, 8 and 10% were used in random order separated by ten-minute rest periods for rehydration. Finally, samples were loaded until failure. Recording parameters were the same as for compression testing, except that the time points for relaxation were 0, 10 and 30 seconds.
Masticatory loading could cause some regions of the septum to undergo tensile strain either directly as in dorsal flexion (Figure 1), or orthogonal to compressive loading owing to a Poisson effect. Thus adaptation to loading in the form of increased tensile stiffness might be present. This was tested by comparing vertically and horizontally loaded samples using RM-ANOVA followed by paired t-tests to detect the source of significance.
Results
Compression
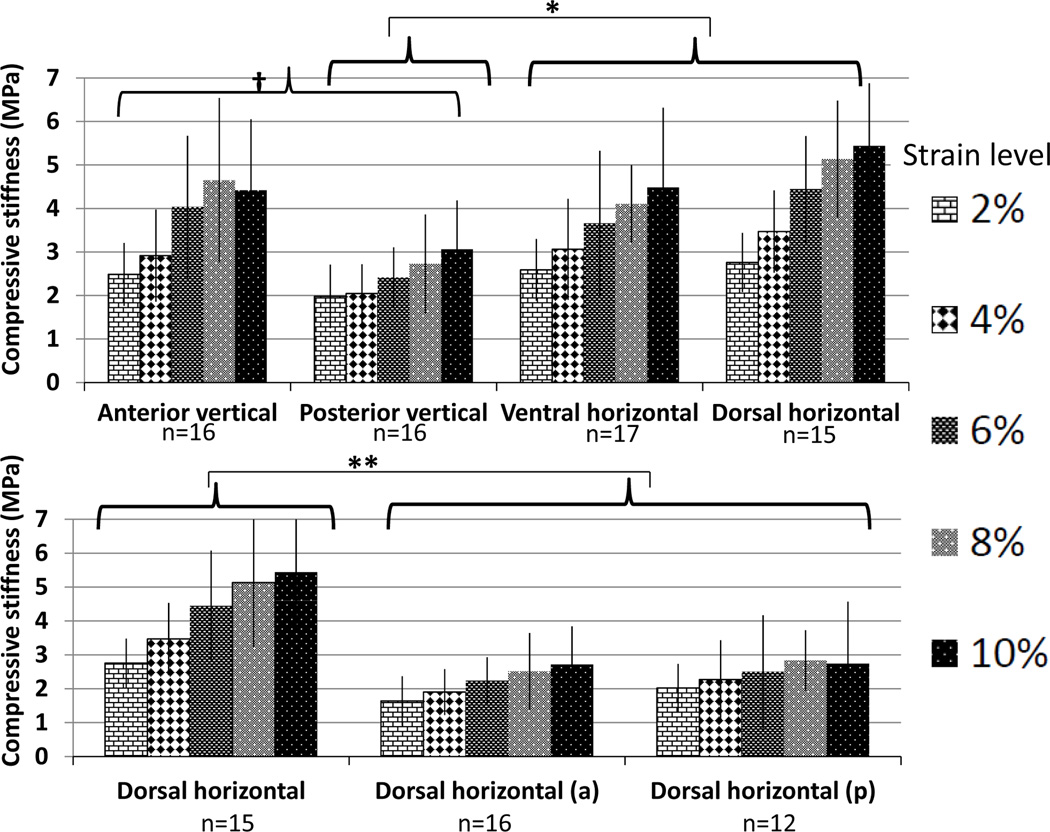
Compression results are presented in Figures 4 and 5 and Tables 1 and 2. Detailed descriptions of the results can be found in the supplementary materials. Preloading at 1.5 vs. 2.5N had no statistically significant effect on mechanical properties, so values were averaged. The septum displayed non-linear compression behavior, with stiffness increasing with increased strain as well as increased strain rate (Figure 4). Consequently, stiffness and relaxation stress had to be analyzed separately for each strain level. Results were similar at all strain levels, but statistical significance was usually present only for the higher strain levels The posterior vertical samples were less stiff than the other regions for almost all comparisons. The dorsal horizontal region tended to be stiffer than the ventral horizontal. Stress absorption was generally less in slowly loaded samples, and the anterior vertical site absorbed less stress than the horizontal sites. Ultimate strain (about 30%) was the same in all samples, but the anterior vertical location had higher ultimate stress values.
Figure 4.
Mean compressive stiffness (Young’s elastic modulus) of the nasal septum. Error bars represent standard deviation. Increased strain level and rate both resulted in increased stiffness. Locations are illustrated in Figure 2. Top: Samples loaded at 1%/sec. The posterior vertical samples were less stiff than the anterior vertical and dorsal horizontal samples at all strains, and higher than the ventral horizontal samples at 6, 8 and 10% strain. Bottom: The dorsal horizontal samples loaded at 1%/sec were stiffer than nearby samples loaded horizontally at 0.1%/sec. * p<0.05; **p<0.01
Figure 5.
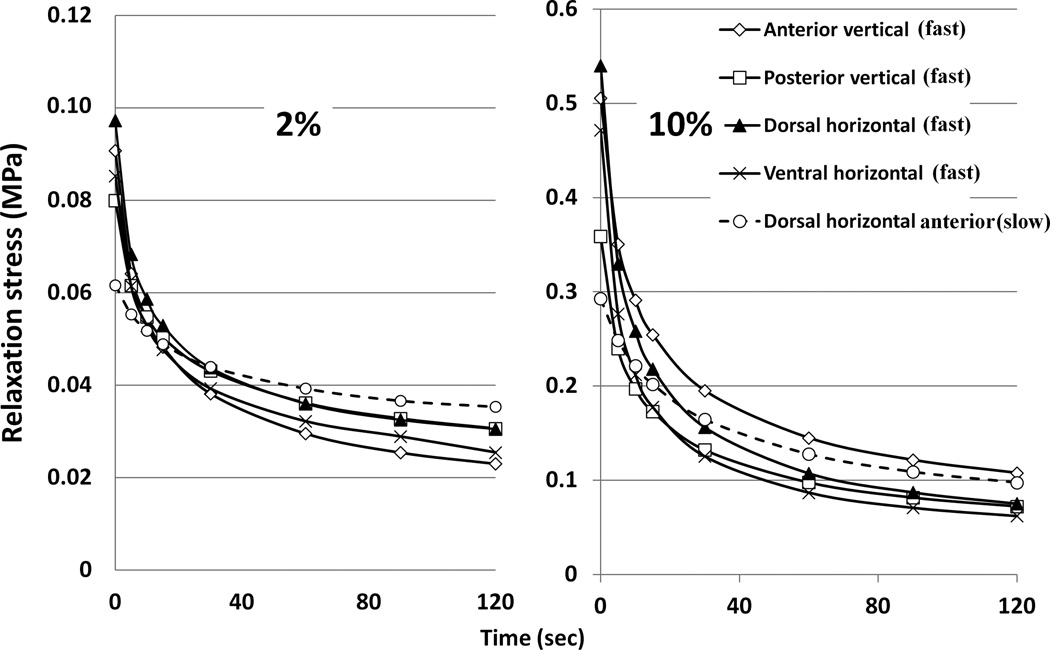
Drop in compression relaxation stress for nasal septum samples loaded at 2 and 10% strain. Intermediate strain points followed similar patterns and are omitted for clarity. One slowly loaded (0.1%/sec) location (DH (a), dashed line) is contrasted with the four locations loaded at 1%/sec. Greater drops in relaxation stress were consistently seen in the samples loaded at the faster rate. Although the stress in the slowly loaded locations was very low at time 0, the residual stress at 120 sec was as high (10% strain) or higher (2% strain) than that for the faster loaded locations.
Table 1.
Compression relaxation stress at 0 sec, and percent loss at 10, 30 and 120 sec after holding strain at 2, 6 and 10%. Results were similar for the intermediate strain levels. See Figure 2 for locations and abbreviations.
| 2% | 6% | 10% | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Relaxation stress at 0 sec (MPa) |
% loss at 10 sec |
% loss at 30 sec |
% loss at 120 sec |
Relaxation stress at 0 sec (MPa) |
% loss at 10 sec |
% loss at 30 sec |
% loss at 120 sec |
Relaxation stress at 0 sec (MPa) |
% loss at 10 sec |
% loss at 30 sec |
% loss at 120 sec |
|
| AV (1%/sec) |
0.08±0.02 | 30.8±7.6 | 42.8±9.2 | 54.0±13.7† | 0.26±0.09 | 38.9±7.2 | 49.9±10.1 | 71.0±9.6 | 0.50±0.20 | 42.2±9.7 | 50.7±10.0 | 77.7±9.2 |
| PV (1%/sec) |
0.08±0.02 | 31.0±12.4 | 45.9±13.8 | 61.7±14.8 | 0.19±0.05 | 41.8±10.5 | 59.2±10.6 | 75.2±10.0 | 0.36±0.12 | 45.9±9.3 | 64.3±13.3 | 80.7±8.9 |
| DH (1%/sec) |
0.10±0.03 | 35.7±9.5 | 49.7±11.2 | 62.9±17.5* | 0.28±0.09* | 47.1±12.4 | 66.6±13.4 | 81.8±10.1*† | 0.55±0.17 | 50.8±13.0 | 69.3±12.3 | 84.1±10.2*† |
| VH (1%/sec) |
0.08±0.01 | 38.9±11.1 | 45.8±10.6 | 71.0±13.2† | 0.24±0.08 | 49.6±10.4 | 67.5±9.5 | 81.5±8.2† | 0.50±0.14 | 54.9±12.1 | 74.5±13.9 | 87.2±8.1† |
| DH (a) (0.1%/sec) |
0.06±0.02 | 16.2±8.3 | 29.1±9.5 | 43.4±17.1* | 0.16±0.04* | 24.3±10.3 | 41.9±12.2 | 61.9±13.5* | 0.29±0.12 | 23.9±8.2 | 43.3±11.3 | 66.4±11.1* |
| DH (p) (0.1%/sec) |
0.07±0.02 | 19.5±6.9 | 33.9±8.6 | 51.6±14.7* | 0.15±0.06* | 27.3±10.1 | 46.3±11.2 | 67.2±11.6* | 0.24±0.11 | 24.0±6.8 | 44.5±9.7 | 68.0±8.9* |
Dorsal horizontal samples loaded at 1%/sec (DH) showed a greater drop in relaxation stress compared to those loaded at 0.1%/sec (DH (a) and DH (p)) (p<0.05, repeated measures ANOVA).
Horizontal samples (DH and VH) showed a greater drop in relaxation stress compared to the anterior vertical samples (AV) (p<0.03, repeated measures ANOVA) for all strain levels (except 2% dorsal horizontal)
Table 2.
Ultimate compressive stress and strain of the septum samples
| 1%/sec loading rate | 0.1%/sec loading rate | |||||
|---|---|---|---|---|---|---|
| Sample location and loading direction |
Anterior vertical n=16 |
Posterior vertical n=17 |
Ventral horizontal n=16 |
Dorsal horizontal n=14 |
Dorsal horizontal (a) n=13 |
Dorsal horizontal (p) n=8 |
| Ultimate stress (MPa) | 3.3±1.4* | 1.6±0.4 | 2.2±0.8 | 1.9±0.6 | 1.5±0.6 | 1.4±0.3 |
| Ultimate strain (%) | 33.9±9.4 | 32.5±6.6 | 29.3±11.4 | 25.3±4.4 | 33.8±5.5 | 32.9±10.9 |
The anterior vertical samples had higher ultimate stress than all other locations (p<0.01, repeated measures ANOVA).
Tension
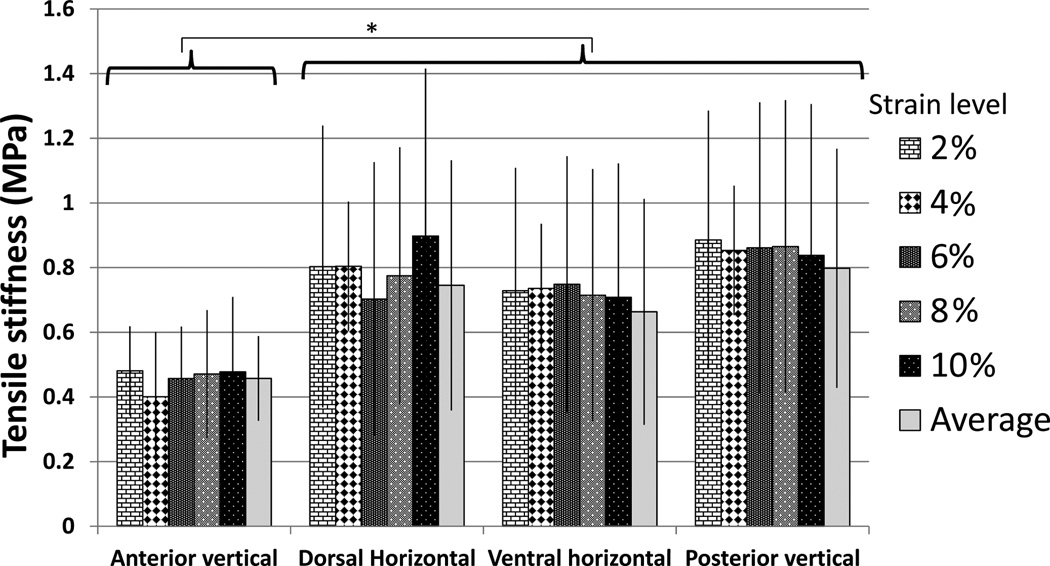
Tension results are presented in Figures 6 and 7, Tables 3 and 4, and supplementary materials. Because preload affected tensile stiffness, only the 1.5N preloaded specimens were analyzed. Another contrast with the compression is that the stiffness was not related to strain level (Figure 6). Therefore, average tensile stiffness could be calculated, simplifying statistical treatment. The anterior vertical (AV) samples had lower tensile stiffness than the other regions, but there were no regional differences in stress absorption. Ultimate strain was over 100% and did not differ regionally, but tensile strength was higher in the dorsal horizontal than ventral horizonal samples.
Figure 6.
Mean tensile stiffness (Young’s elastic modulus) of the nasal septum, error bars represent standard deviation. Note the difference in scale from Figure 4. Tensile stiffness was relatively constant regardless of magnitude of strain. * The anterior vertical samples were less stiff than the other locations, p<0.01.
Figure 7.
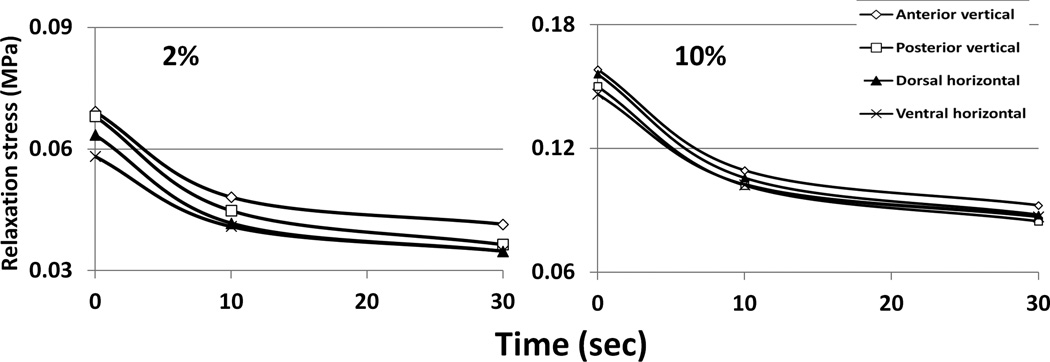
Drop in tensile relaxation stress for septal samples loaded at 2% and 10% strain. Intermediate strain points followed similar patterns and are not shown for clarity.
Table 3.
Tensile relaxation stress at 0 sec, and percent loss at 10 and 30 sec after holding strain at 2, 6 and 10%. Results were similar for the intermediate strain levels.
| 2% | 6% | 10% | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Relaxation stress at 0 sec (MPa) |
% loss at 10 sec |
% loss at 30 sec |
Relaxation stress at 0 sec (MPa) |
% loss at 10 sec |
% loss at 30 sec |
Relaxation stress at 0 sec (MPa) |
% loss at 10 sec |
% loss at 30 sec |
|
| Anterior vertical |
0.05±0.02 | 35.1±7.2 | 45.4±9.4 | 0.08±0.04 | 37.5±8.0 | 46.4±10.9 | 0.11±0.06 | 39.1±9.2 | 50.1±10.8 |
| Posterior vertical |
0.06±0.02 | 35.8±8.1 | 47.0±10.1 | 0.10±0.03 | 36.4±7.8 | 48.2±9.2 | 0.14±0.06 | 35.1±8.6 | 47.4±11.2 |
| Dorsal horizontal |
0.06±0.02 | 34.5±8.3 | 44.7±9.9 | 0.09±0.04 | 34.6±6.6 | 44.1±9.6 | 0.15±0.05 | 33.9±5.7 | 45.4±6.8 |
| Ventral horizontal |
0.06±0.02 | 33.4±5.8 | 44.5±7.4 | 0.09±0.04 | 32.2±10.7 | 40.4±13.5 | 0.13±0.05 | 34.4±6.2 | 46.0±7.1 |
Table 4.
Ultimate tensile stress and strain of the septum samples
| Sample location and loading direction |
Anterior vertical (n=14) |
Posterior vertical (n=18) |
Dorsal horizontal (n=15) |
Ventral horizontal (n=13) |
|---|---|---|---|---|
| Ultimate stress (MPa) | 2.0±0.7* | 1.1±0.4 | 1. 4±0.5† | 1.1±0.4 |
| Ultimate strain (%) | 157.2±72.5 | 105.8±42.0 | 126.4±56.4 | 122.7±60.0 |
Anterior vertical samples were significantly stronger than all other locations (p<0.01)
Dorsal horizontal samples were significantly stronger than ventral horizontal samples (p=0.01)
Discussion
Technical considerations and limitations
Methods for testing the nasal septum are not standardized. Rectangular specimens of widely varying proportions have been used (Flam, 1974;Grellmann et al., 2006; Richmon et al., 2005, 2006; Wong et al., 2001; Xia et al., 2012), despite the preferability of cylinders to avoid stress concentration (Keaveny et al., 1993), natural geometry of the septum dictated rectangularity. The present study aimed to test the septum as a functional anatomical structure. Therefore, specimens were not machined, leading to minor thickness variations; relatively large specimens were used; and the septum was tested with the perichondrium in place. Other workers have removed the perichondrium and machined the cartilage to produce a more uniform but less lifelike structure (Richmon et al., 2006; Wong et al., 2001; Xia et al., 2012). The perichondrium contributes to the tensile properties of the septum (Kim et al., 2005) and might also affect the compressive values by confining the cartilage. Given these considerations, our numerical values for septal properties should be compared to other studies only with great caution.
The use of linear regression to calculate stiffness was a simplification. Septal samples are viscoelastic and mild deviation from linearity was present.. However, the linear regression coefficients were high (r2 >0.95), so the discrepancy between the calculated and actual stiffness was minimal.
The compressive and tensile properties reported here are not fully comparable. First, the tensile tests were performed at 10%/sec, whereas the compressive tests were performed at 0.1 and 1%/sec. Higher strain rates result in higher stiffness in cartilage (DiSilvestro et al., 2001; Flam, 1974; Langelier and Buschmann, 2003; Li and Herzog, 2004). Thus the tensile stiffness values were overestimated. Second, tension samples were stored at 4°C whereas compression samples were frozen. Freezing decreases the mechanical properties of cartilage (around 30%, calculated from Langelier and Buschmann, 2003). Thus, the compressive values were underestimated. Even with tensile values overestimated and compressive values underestimated, compressive stiffness (Figure 4) was much greater than tensile stiffness (Figure 6), supporting the fact that cartilage is adapted to compressive rather than tensile loads. Despite the probable underestimation of compressive properties, our results of 4–5 MPa are comparable to those for porcine septal cartilage in bending (Gaon et al., 2003; Wong et al., 2001) and unconfined compression (Zemek et al., 2012). Our finding of lower compressive moduli in the posterior region of the porcine septum also agrees with previous findings (Zemek et al. 2012). On the other hand, despite overestimation, tensile stiffness (0.5–0.9 MPa, Figure 6) are lower than human values of over 3 MPa (Grellmann et al., 2006; Richmon et al., 2005; Westreich et al. 2007). This is particularly notable because of our inclusion of the perichondrium, a structure that should have increased the tensile properties.
Because the strain levels of 2%–10% are greater than during normal function (up to 1%, Al Dayeh et al. 2009), we recalculated stiffness values at 0.5 and 1% using data from 2% and 10% loading cycles; the stiffness at 0.5% and 1% strain was very similar to stiffness at the 2% endpoint (p>0.4, RM-ANOVA). Thus, it is likely that the stress-strain relation is fairly linear for strain<2%. Loading order also could have affected the results, especially if higher strain levels (i.e. 10%) came early and damaged the cartilage. Strains higher than 5% may weaken cartilage (Morel and Quinn, 2004). However, post-hoc comparison of samples loaded first at 2–4% versus those loaded first at 8–10%, showed no difference (p>0.3, t-test), indicating that non-failure testing did not damage the tissue.
Overall comparison of compressive and tensile properties
Although the difference in protocols prevents quantitative conclusions, patterns are clear. The septum was stiffer under compression than under tension (Figures 4, 6). In addition, the drop in relaxation stress was more dramatic in compression compared to tension, especially at higher strain levels (Tables 1, 3). Further, tensile stress relaxation appears to reach an asymptote by 30 sec, whereas compression relaxation was still dropping at 120 sec (Figures 5, 7).
Although the septum was weaker under tension than compression, it was strikingly more deformable. It is likely that the perichondrium contributed substantially to these high tensile ultimate strains, as the collagen fibers were first aligned and then stretched. Ultimate tensile strain in studies with the perichondrium removed is typically less (about 40% in bovine septum, Flam, 1974).
Loading rate and strain level
Under compression the septum was consistently stiffer at higher strain and faster loading. These results are in accordance with previous studies (DiSilvestro et al., 2001; Langelier and Buschmann, 2003; Li and Herzog, 2004). Such nonlinearity to strain level was not present in tensile tests. The difference can be attributed to the fact that cartilage compressive properties are dominated by water and proteoglycans. As the strain amplitude increases, the load is increasingly resisted by the fluid component of cartilage. In addition, higher strain rates may increase drag forces, allowing less time for fluid to flow through the porous proteoglycans, elevating fluid pressure and causing “self stiffening” (Langelier and Buschmann, 2003; Li and Herzog, 2004). In contrast, tensile properties are dictated by resistance of collagen fibers to stretch (Huang et al., 2001; Mow and Wang, 1999; Quinn et al., 2001), a phenomenon that is not affected by strain amplitude once the elastic portion of the stress-strain curve is reached.
Strut or stress dampener for dynamic loads?
The strut model envisions that the septum is stiff in order to resist masticatory loads, an idea strongly advocated for humans by Moss and colleagues (1968). The septum does not seem well adapted for this role. The compressive stiffness values recorded, while typical for cartilage (Mow and Wang, 1999), are much lower than the elastic moduli of bone (20,000 MPa, Carter and Beaupre, 2001). Thus the nasal septum is probably not stiff enough to assist the bone in resisting masticatory loads. However, the septum displayed notable compressive stress relaxation and so is better suited to absorb impact loads, such as those of mastication. These findings, together with the high deformation of the septum during mastication (Al Dayeh et al., 2009), suggest that the septum, together with sutures (Jaslow and Biewener, 1995), helps to dampen the loads generated during mastication. Septal strain has not been measured or modeled during mastication in other species, but in its superior continuation, the bony interorbital septum, Ross (2001) reported low and variable strains in a variety of primates. This suggests that masticatory stresses, if borne by the septum, are not transmitted efficiently beyond the nose and that this general conclusion is true for primates (including humans) as well as pigs.
Trauma also produces impact loading, typically more extreme than functional behavior. Stress dampening is relevant here as well, although a modeling study of the human septum suggests that the bone-cartilage junctions would be most important in providing a “crumple zone” to protect the rest of the skull from injury (Lee et al., 2010).
Adaptation to habitual loading regimes
Even if not important in resisting masticatory loads, the septum may show adaptations that reflect its predominant deformation pattern (Figure 1). There is little previous literature on directional variation in septal mechanical properties. Bovine (Xia et al., 2012) and human (Richmon et al., 2006) septa have lower moduli in the coronal plane (which we did not test) than in the sagittal plane, with only negligible differences between vertical and horizontal loading in the sagittal plane. We also did not see consistent vertical vs. horizontal differences. Regional differences are not found in the relatively small human septum (Richmon et al., 2005), but are prominent in the large porcine septum (Wong et al., 2001; Zemek et al., 2012).
If the snout is dorsally flexed (Rafferty and Herring, 1999; Rafferty et al., 2003), then regional adaptation to horizontal dorsal compression and ventral tension is expected. Evidence for dorsal flexion was weak. There was a tendency for increased compressive stiffness dorsally, but the ventral location showed no tendency for greater tensile stiffness. On the other hand, if the septum is anteroposteriorly compressed (Al Dayeh et al., 2009), adaptation to horizontal compression would be expected. Evidence for this role was inconsistent. The difficulty here is that the anterior and posterior vertical locations differed whereas the horizontal strut model predicts them to be the same. The anterior vertical location, clearly unique, displayed higher compressive stiffness and lower tensile stiffness. These findings suggest specialization of the anterior part of the cartilage beyond simple sagittal plane deformation. Histologically, this part is relatively thick, and it is firmly attached to the mobile snout disc and to the premaxillary and nasal bones, rendering it subject to non-masticatory loads, such as digging (Al Dayeh et al., 2009). Taken together, these findings indicate that the anterior location is adapted to withstand increased physiologic loading in a variety of directions.
Growth and static support
If the septal cartilage plays an active role in midfacial growth, as thought by many clinicians to be true for humans (Delaire and Precious, 1987), then its growth pressure should be sufficient to separate the facial sutures, and it should be stiff enough to withstand the recoil pressure of the sutures. The fact that the septum is an efficient stress absorber works against this scenario. However, a trend toward decreased stress absorption and increased residual stress with lower strain rates was observed. Thus, the residual stress might be sufficient to separate the sutures, depending on sutural resistance. The most relevant sutural data are for the nasofrontal suture in pigs (Popowics and Herring, 2007); reported stiffness was an order of magnitude higher than the septal stiffness reported here, thus undermining the postulated growth role. Stress absorption in the suture was less (approximately 40%, compared to 70% in the cartilage) which also argues against a growth role. However, the values reported for the suture are not comparable to those for the septum. Suture samples included parts of the nasal and frontal bones. Because the suture is more flexible than surrounding bones, most of the strain would have occurred at the suture; however, the total sample length was used for calculations. If only the suture gap (around 340 µm, Popowics and Herring, 2007) is used in the calculation, stiffness is an order of magnitude lower, and stiffness values become comparable to those of the septum. Thus, the septum may be stiff enough to withstand the resistance of the suture, especially given the fact that residual stress decays slowly, and not to zero. A growth role remains possible.
Similar to the postulated growth role of the septum is the notion that it contributes to the static support of the nasal skeleton against the weight of the overlying skull and soft tissue. A small experimental study of statically loaded human skulls showed that the septum partially braces the nasal bones (Clark and Wallace, 1970). Removal or damage of the septum results in partial collapse of the nasal bones in animals (Moss et al., 1968; Stenström and Thilander, 1970). In humans a saddle-nose effect results because the external nose is supported only by cartilage, and the septum is stiffer than the lateral cartilages (Westreich et al., 2007).
Conclusion
The compressive and tensile mechanical properties of the nasal septum were investigated in order to shed light on the its role in facial integrity and growth. The septum is stiffer and stronger under compression than under tension and shows regional variations that suggest specialization of the anterior region. The results suggest that the septum acts as a stress dampener helping to dissipate dynamic loads. The septum serves in static support of the nose and may be mechanically capable, under slow loading rates, of separating facial sutures, thus playing an active role in midfacial growth.
Supplementary Material
Acknowledgments
The authors would like to thank Adam Veitschegger and Alice Nunes for their help with the experiments. We also would like to thank Tracy Popowics for her help in developing the testing protocol, Hanson Fong for advice, and two anonymous reviewers for their helpful recommendations. This study was funded by NIDCR grant (PHS) DE 08513.
This project was supported by PHS award DE08513 from NIDCR
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al Dayeh AA, Rafferty KL, Egbert M, Herring SW. Deformation of nasal septal cartilage during mastication. Journal of Morphology. 2009;270:1209–1218. doi: 10.1002/jmor.10750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Dayeh AA, Rafferty KL, Egbert M, Herring SW. Real-time monitoring of the growth of the nasal septal cartilage and nasoforntal suture in minipigs. American Journal of Orthodontics and Dentofacial Orthopedics. 2013;143:773–783. doi: 10.1016/j.ajodo.2013.01.012. [DOI] [PubMed] [Google Scholar]
- Badoux DM. Framed structures in the mammalian skull. Acta Morphologica Neerlando-Scandinavica. 1966;6:239–250. [PubMed] [Google Scholar]
- Badoux DM. Cremona Diagrams of Framed Structures in Skull of Canis Familiaris and Sus Scrofa Scrofa. Proceedings of the Koninklijke Nederlandse Akademie Van Wetenschappen Series C-Biological and Medical Sciences. 1968;71:229–244. [PubMed] [Google Scholar]
- Chao KKH, Ho KHK, Wong BJF. Measurement of the elastic modulus of rabbit nasal septal cartilage during Nd : YAG (lambda=1.32 mu m) laser irradiation. Lasers in Surgery and Medicine. 2003;32:377–383. doi: 10.1002/lsm.10176. [DOI] [PubMed] [Google Scholar]
- Clark G, Wallace C. Analysis of nasal support. Archives of Otolaryngology. 1970;92:118–123. doi: 10.1001/archotol.1970.04310020016005. [DOI] [PubMed] [Google Scholar]
- Delaire J, Precious D. Interaction of the development of the nasal septum, the nasal pyramid and the face. International Journal of Pediatric Otorhinolaryngology. 1987;12:311–326. doi: 10.1016/s0165-5876(87)80007-1. [DOI] [PubMed] [Google Scholar]
- DiSilvestro MR, Zhu Q, Suh J-KF. Biphasic Poroviscoelastic Simulation of the Unconfined Compression of Articular Cartilage: II---Effect of Variable Strain Rates. Journal of Biomechanical Engineering. 2001;123:198–200. doi: 10.1115/1.1351887. [DOI] [PubMed] [Google Scholar]
- Flam E. The tensile and flexural properties of bovine nasal cartilage. Journal of Biomedical Materials Research. 1974;8:277–282. doi: 10.1002/jbm.820080509. [DOI] [PubMed] [Google Scholar]
- Gaon MD, Ho KHK, Wong BJF. Measurement of the elastic modulus of porcine septal cartilage specimens following Nd : YAG laser treatment. Lasers in Medical Science. 2003;18:148–153. doi: 10.1007/s10103-003-0275-5. [DOI] [PubMed] [Google Scholar]
- Grellmann W, Berghaus A, Haberland EJ, Jamali Y, Holweg K, Reincke K, Bierogel C. Determination of strength and deformation behavior of human cartilage for the definition of significant parameters. Journal of Biomedical Materials Research Part A. 2006;78A:168–174. doi: 10.1002/jbm.a.30625. [DOI] [PubMed] [Google Scholar]
- Huang CY, Mow VC, Ateshian GA. The role of flow-independent viscoelasticity in the biphasic tensile and compressive responses of articular cartilage. Journal of Biomechanical Engineering-Transactions of the Asme. 2001;123:410–417. doi: 10.1115/1.1392316. [DOI] [PubMed] [Google Scholar]
- Jaslow CR, Biewener AA. Strain patterns in the horncores, cranial bones and sutures of goats (Capra hircus) during impact loading. Journal of Zoology. 1995;235:193–210. [Google Scholar]
- Keaveny TM, Brochers RE, Gibson LJ, Hayes WC. Trabecular bone modulus and strength can depend on specimen geometry. Journal of Biomechanics. 1993;26:991–1000. doi: 10.1016/0021-9290(93)90059-n. [DOI] [PubMed] [Google Scholar]
- Kim DW, Egan KK, O'Grady K, Toriumi DM. Biomechanical strength of human nasal septal lining: comparison of the constituent layers. Laryngoscope. 2005;115:1451–1453. doi: 10.1097/01.mlg.0000168579.94187.f6. [DOI] [PubMed] [Google Scholar]
- Kvinnsland S. Partial resection of cartilaginous nasal-septum in rats - Its influence on growth. Angle Orthodontist. 1974;44:135–140. doi: 10.1043/0003-3219(1974)044<0135:PROTCN>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Langelier E, Buschmann MD. Increasing strain and strain rate strengthen transient stiffness but weaken the response to subsequent compression for articular cartilage in unconfined compression. Journal of Biomechanics. 2003;36:853–859. doi: 10.1016/s0021-9290(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Liong K, Lee HP. Deformation of nasal septum during nasal trauma. The Laryngoscope. 2010;120:1931–1939. doi: 10.1002/lary.21072. [DOI] [PubMed] [Google Scholar]
- Li LP, Herzog W. Strain-rate dependence of cartilage stiffness in unconfined compression: the role of fibril reinforcement versus tissue volume change in fluid pressurization. Journal of Biomechanics. 2004;37:375–382. doi: 10.1016/s0021-9290(03)00263-x. [DOI] [PubMed] [Google Scholar]
- Martin BD, Sharkey N. Skeletal Tissue Mechanics. New York: Springer; 1998. [Google Scholar]
- McLaughlin E, Zhang Y, Pashley D, Borke J, Yu J. The load-displacement characteristics of neonatal rat cranial sutures. Cleft Palate-Craniofacial Journal. 2000;37:590–595. doi: 10.1597/1545-1569_2000_037_0590_tldcon_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- Morel V, Quinn TM. Cartilage injury by ramp compression near the gel diffusion rate. Journal of Orthopaedic Research. 2004;22:145–151. doi: 10.1016/S0736-0266(03)00164-5. [DOI] [PubMed] [Google Scholar]
- Moss ML, Bromberg BE, Song IG, Eisenman G. The passive role of nasal septal cartilage in mid-facial growth. Plastic and Reconstructive Surgery. 1968;41:536–542. doi: 10.1097/00006534-196806000-00004. [DOI] [PubMed] [Google Scholar]
- Mow VC, Wang CCB. Some bioengineering considerations for tissue engineering of articular cartilage. Clinical Orthopaedics and Related Research. 1999;367:S204–S223. doi: 10.1097/00003086-199910001-00021. [DOI] [PubMed] [Google Scholar]
- Naumann A, Dennis JE, Awadallah A, Carrino DA, Mansour JM, Kastenbauer E, Caplan AI. Immunochemical and mechanical characterization of cartilage subtypes in rabbit; Journal of Histochemistry &. Cytochemistry. 2002;50:1049–1058. doi: 10.1177/002215540205000807. [DOI] [PubMed] [Google Scholar]
- Popowics TE, Herring SW. Load transmission in the nasofrontal suture of the pig, Sus scrofa. Journal of Biomechanics. 2007;40:837–844. doi: 10.1016/j.jbiomech.2006.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn TM, Allen RG, Schalet BJ, Perumbuli P, Hunziker EB. Matrix and cell injury due to sub-impact loading of adult bovine articular cartilage explants: effects of strain rate and peak stress. Journal of Orthopaedic Research. 2001;19:242–249. doi: 10.1016/S0736-0266(00)00025-5. [DOI] [PubMed] [Google Scholar]
- Race A, Broom ND, Robertson P. Effect of loading rate and hydration on the mechanical properties of the disc. Spine. 2000;25:662–669. doi: 10.1097/00007632-200003150-00003. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan P, Mao JJ. Nanomechanical properties of facial sutures and sutural mineralization front. Journal of Dental Research. 2004;83:470–475. doi: 10.1177/154405910408300607. [DOI] [PubMed] [Google Scholar]
- Rafferty KL, Herring SW. Craniofacial sutures: Morphology, growth, and in vivo masticatory strains. Journal of Morphology. 1999;242:167–179. doi: 10.1002/(SICI)1097-4687(199911)242:2<167::AID-JMOR8>3.0.CO;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafferty KL, Herring SW, Marshall CD. Biomechanics of the rostrum and the role of facial sutures. Journal of Morphology. 2003;257:33–44. doi: 10.1002/jmor.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmon JD, Sage A, Wong VW, Chen AC, Sah RL, Watston D. Compressive biomechanical properties of human nasal septal cartilage. American Journal of Rhinology. 2006;20:496–501. doi: 10.2500/ajr.2006.20.2932. [DOI] [PubMed] [Google Scholar]
- Richmon JD, Sage AB, Shelton E, Schumacher BL, Sah RL, Watson D. Effect of growth factors on cell proliferation, matrix deposition, and morphology of human nasal septal chondrocytes cultured in monolayer. Laryngoscope. 2005;115:1553–1560. doi: 10.1097/01.MLG.0000175541.31131.A5. [DOI] [PubMed] [Google Scholar]
- Richmon JD, Sage AB, Wong VW, Chen AC, Pan C, Sah RL, Watson D. Tensile biomechanical properties of human nasal septal cartilage. American Journal of Rhinology. 2005;19:617–622. [PubMed] [Google Scholar]
- Ross CF. In vivo function of the craniofacial haft: the interorbital "pillar". American Journal of Physical Anthropology. 2001;116:108–139. doi: 10.1002/ajpa.1106. [DOI] [PubMed] [Google Scholar]
- Rotter N, Tobias G, Lebl M, Roy AK, Hansen MC, Vacanti CA, Bonassar LJ. Age-related changes in the composition and mechanical properties of human nasal cartilage. Archives of Biochemistry and Biophysics. 2002;403:132–140. doi: 10.1016/S0003-9861(02)00263-1. [DOI] [PubMed] [Google Scholar]
- Roy R, Kohles SS, Zaporojan V, Peretti GM, Randolph MA, Xu JW, Bonassar LJ. Analysis of bending behavior of native and engineered auricular and costal cartilage. Journal of Biomedical Materials Research Part A. 2004;68A:597–602. doi: 10.1002/jbm.a.10068. [DOI] [PubMed] [Google Scholar]
- Sarnat BG, Wexler MR. Growth of Face and Jaws after Resection of Septal Cartilage in Rabbit. American Journal of Anatomy. 1966;118 doi: 10.1002/aja.1001180306. 755-&. [DOI] [PubMed] [Google Scholar]
- Scott JH. The cartilage of the nasal septum. British Dental Journal. 1953;95:37–40. [Google Scholar]
- Stenström SJ, Thilander BL. Effects of nasal septal cartilage resections on young guinea pigs. Plastic and Reconstructive Surgery. 1970;45:160–170. doi: 10.1097/00006534-197002000-00010. [DOI] [PubMed] [Google Scholar]
- Tanaka E, Miyawaki Y, del Pozo R, Tanne K. Changes in the biomechanical properties of the rat interparietal suture incident to continuous tensile force application. Archives of Oral Biology. 2000;45:1059–1064. doi: 10.1016/s0003-9969(00)00082-0. [DOI] [PubMed] [Google Scholar]
- Westreich RW, Courtland HW, Nasser P, Jepsen K, Lawson W. Defining nasal cartilage elasticity - Biomechanical testing of the tripod theory based on a cantilevered model. Archives of Facial Plastic Surgery. 2007;9:264–270. doi: 10.1001/archfaci.9.4.264. [DOI] [PubMed] [Google Scholar]
- Wong BJF, Chao KKH, Kim HK, Chu EA, Dao X, Gaon M, Sun CH, Nelson JS. The porcine and lagomorph septal cartilages: Models for tissue engineering and morphologic cartilage research. American Journal of Rhinology. 2001;15:109–116. doi: 10.2500/105065801781543790. [DOI] [PubMed] [Google Scholar]
- Xia Y, Zheng S, Szarko M, Lee J. Anisotropic properties of bovine nasal cartilage. Microscopy Research and Technique. 2012;75:300–306. doi: 10.1002/jemt.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemek AJ, Protsenko DE, Wong BJF. Mechanical properties of porcine cartilage after uniform RF heating. Lasers in Surgery and Medicine. 2012;44:572–579. doi: 10.1002/lsm.22057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.