Abstract
Objective
Older women and men with rheumatoid arthritis (RA) are at increased risk for fractures, but limited information is available on fracture risk in younger individuals with RA, and whether such risk occurs early following disease onset or only when older. We determined the risk for fractures in both young and older women and men following RA diagnosis.
Methods
We studied a population-based inception cohort with RA from Olmsted County, Minnesota. We identified 822 women and 349 men diagnosed with RA between 1955 and 2007 (308 women and 110 men diagnosed before age 50) and an equal number of paired non-RA subjects, matched by sex and birth year. Incident fractures were collected through review of complete (inpatient and outpatient) medical records available through the linkage system of the Rochester Epidemiology Project.
Results
The hazard ratio (HR) [95% CI] for a non-pathologic fracture occurring from no more than moderate trauma was 1.63 [1.36–1.96] for women and 1.40 [1.02–1.93] for men with RA. Findings were consistent for women and men diagnosed with RA at age ≥ 50 years (HR: 1.43 [1.16–1.77] and 1.34 [0.92–1.94], respectively), or at age < 50 years (HR: 2.34 [1.61–3.42] and 1.74 [0.91–3.30], respectively). However, young women, but not young men, with RA were at increased fracture risk even before age 50 years (HR: 1.95 [1.08–3.51] and 0.82 [0.28–2.45], respectively).
Conclusion
Young men with RA are at increased risk for fractures only when older, whereas young women with RA have an elevated fracture risk even while still young.
Keywords: rheumatoid arthritis, bone fractures, osteoporosis, epidemiology
INTRODUCTION
It is increasingly recognized that bone health is adversely affected in rheumatoid arthritis (RA).(1, 2) Indeed, RA is the only disease specifically included in the World Health Organization’s fracture prediction algorithm, FRAX® (http://shef.ac.uk/FRAX/).(3) However, the risk for fractures in younger individuals with RA is not directly estimated by FRAX®, nor is the risk for fractures at sites other than the hip, spine, wrist and shoulder, considered major osteoporotic fractures. In fact, most studies investigating fracture risk in RA have examined older women, (4–13) with few that included older men.(7, 12) Furthermore, most studies have focused on fractures of the vertebrae or other major osteoporotic fractures even though, collectively, fractures at other skeletal sites account for significant increased morbidity and health care costs.(14)
Despite the fact that the mean age at diagnosis for adult-onset RA is ~55 years (15) and that low bone density has been reported in young women and men with RA, (16–18) there are very few studies on the likelihood of fractures in younger individuals with RA.(19–21) Only one included men, but even this study examined the risk for fractures only at the hip, shoulder, pelvis, and wrist and long-term follow-up was limited.(19)
It is clinically important to know the risk for fractures at any site, in addition to fractures at traditional major osteoporotic sites, for those who develop RA at either younger or older ages. It would also be pertinent to determine whether the risk for fractures occurs early in the course of the disease, or instead, primarily manifests itself later in life, irrespective of disease duration. Therefore, we sought to determine the relative and absolute risk for future fractures in both young and older individuals with RA, using a population-based inception cohort of women and men with an incident diagnosis of RA for whom long-term follow-up was available.
METHODS
Study Subjects
Population-based epidemiologic research is possible in Olmsted County, Minnesota, because comprehensive (inpatient as well as outpatient) medical records for all residents at any local provider are available through a unique medical records linkage system, the Rochester Epidemiology Project (REP).(22, 23) After approval by the Institutional Review Boards (IRB) of Mayo Clinic and Olmsted Medical Center, REP resources were used to identify all residents of Rochester (the central city of Olmsted County) who were ≥ 18 years of age when they fulfilled American College of Rheumatology (ACR; formerly, the American Rheumatism Association) 1987 criteria for RA (24) between January 1, 1955, and December 31, 1994. (25, 26) The cohort was subsequently expanded, using the same methodology, to include all Olmsted County residents fulfilling ACR criteria for RA from January 1, 1980 to December 31, 2007.(27) Potential RA subjects were identified by searching the computerized diagnostic index for any diagnosis of arthritis (excluding degenerative arthritis or osteoarthritis) made for residents during these time frames. The complete medical record for each potential RA subject was then reviewed by trained nurse abstractors using a pretested data collection form to confirm or reject the diagnosis, with RA incidence defined as the date of first fulfillment of 4 of the 7 ACR classification criteria.(25) For each subject identified with incident RA, an individual without RA from the same population was randomly selected, matched for sex, birth year (± 3 years), and residency. Subjects in the non-RA cohort were assigned an index date corresponding to the RA incidence date of their matched pair.
Fracture Ascertainment over Follow-up
After additional approval by the respective IRBs, these subjects were followed until death or last clinical contact through their linked medical records in the community (historical cohort study) and their records were searched by trained nurse abstractors for the occurrence of any fracture. Ascertainment of all clinically evident fractures is believed to be complete.(28) Records at Mayo Clinic, for example, contain the details of every hospitalization and outpatient visit, all emergency room and nursing home care, as well as all radiographic and pathology reports, including autopsies, and all correspondence with each patient.(22) By convention, fractures occurring during daily activities and falls from standing height or less were considered to have resulted from no more than moderate trauma, whereas fractures resulting from motor vehicle accidents and falls from a greater height were deemed from severe trauma. In addition, we are able to distinguish fractures that were due to a specific bone lesion (pathologic fractures), as well as fractures only discovered because of radiographic tests performed in the clinical setting for unrelated causes (incidental fractures). From all fractures identified, we defined a subset of fragility fractures (i.e., all non-pathologic fractures occurring as a result of no more than moderate trauma or identified incidentally), as well as a subset of traditional major osteoporotic fractures (i.e., fragility fractures of the proximal femur [hip], thoracic or lumbar vertebrae [spine], distal forearm [wrist] or proximal humerus [shoulder]).
Statistical Analysis
Each member of the RA and non-RA matched pair was followed from the index date to the earlier of either pair’s date of last follow-up. Fractures that resulted from severe trauma, or that were considered pathological fractures, were excluded from analyses in order to focus on the risk for fractures most likely related to bone fragility. The cumulative incidence rates for fragility fractures were estimated following the RA diagnosis, or index date for the non-RA subjects, using the Kaplan-Meier method, (29) and accounting for the competing risk for death.(30, 31) For women and men separately, unadjusted Cox proportional hazards models were used to assess the impact of RA on first subsequent fragility fracture and, in separate models, first subsequent major osteoporotic fracture. Analyses were then performed by different age-groups at RA diagnosis/index date (age ≥50 years or <50 years). For those in the age <50 years group, we performed additional analyses where follow-up ended either at the earlier date of last known follow-up for a pair or at age 50 years, whichever occurred first. Finally, given changes in RA and osteoporosis management over the time span of our study, we performed exploratory analyses to examine whether calendar year of RA diagnosis influenced fracture risk estimates. Person-years after RA diagnosis (or index date for non-RA subjects) and fracture events were divided into bins for each calendar year. Generalized additive models were used to model the influence of age and calendar year on fracture rates, using smoothing splines to allow for nonlinear associations and assuming fractures follow a Poisson distribution. Models were fit separately for RA and non-RA subjects, and by sex. Predicted fracture rates for calendar year were age-adjusted and the four model results were compared graphically. Analyses were performed using SAS version 9 (SAS Institute, Cary, NC, USA) and R 2.14 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
We identified 1171 Olmsted County residents (822 women and 349 men; 85% from Rochester; 96% white) who had an incident diagnosis of RA between January 1, 1955 and December 31, 2007. Their mean age ± SD at diagnosis was 56 ± 16 years (range: 19–94 years) for RA women and 58 ± 14 years (range: 19–89 years) for RA men. Relevant RA-related characteristics for these women and men are noted in Table 1. By design, the 1171 matched non-RA subjects were similar in age at their index date (56 ± 16 years for women and 58 ± 14 years for men). However, survival was worse in RA than non-RA subjects (at 30 years, 31% vs. 43%, respectively; p <0.001), and total follow-up was consequently less among those with RA. When censored at the earliest follow-up date of either member of a RA and non-RA pair, there was a total of 12,781 person-years of follow-up for each group. The median follow-up for each pair of women was 9 years (range: 4 days to 52 years) and for each pair of men was similarly 9 years (range: 16 days to 44 years).
Table 1.
Characteristics of all Olmsted County, Minnesota, women and men with incident rheumatoid arthritis between 1955 and 2007, and by age at diagnosis (≥50 years and <50 years).
| Women | Men | |||||
|---|---|---|---|---|---|---|
| All | ≥50 years | <50 years | All | ≥50 years | <50 years | |
| N | 822 | 514 | 308 | 349 | 239 | 110 |
| Mean Age (yrs) [at diagnosis] | 56 ± 16 | 66 ± 10 | 40 ± 8 | 58 ± 14 | 65 ± 9 | 41 ± 7 |
| Rheumatoid Factor Positive [ever] | 523 (64%) | 325 (63%) | 198 (64%) | 235 (67%) | 148 (62%) | 87 (79%) |
| Rheumatoid Nodules [ever] | 261 (32%) | 157 (31%) | 104 (34%) | 130 (37%) | 75 (31%) | 55 (50%) |
| Erosions/Destructive Disease [ever] | 447 (54%) | 290 (56%) | 157 (51%) | 176 (50%) | 112 (47%) | 64 (58%) |
| Oral Glucocorticoids [ever] | 542 (66%) | 317 (62%) | 225 (73%) | 252 (72%) | 167 (70%) | 85 (77%) |
Continuous variables are summarized as mean ± SD; categorical variables are summarized as N (%).
The sites of all fractures (Tables 2 and 3) and their proximate causes (Table 4) for both RA and non-RA subjects, stratified by sex and by age are outlined in the Tables. When compared with their non-RA counterparts, women with RA had more fractures at almost all sites (Table 2), while men with RA tended to have more fractures at major osteoporotic sites, as well as at the ribs and pelvis (Table 3). Younger non-RA women and men were more likely to have fractures attributed to severe trauma (Table 4).
Table 2.
Distribution of all fractures and fragility fractures among 822 Olmsted County, Minnesota women following their initial diagnosis of rheumatoid arthritis (RA), by fracture site and proximate cause, compared with an equal number of age-matched non-RA women
| All Women | Women ≥ 50 Years | Women < 50 Years | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fracture site | All Fractures N (%) |
Fragility Fractures* N (%) |
All Fractures N (%) |
Fragility Fractures* N (%) |
All Fractures N (%) |
Fragility Fractures* N (%) |
||||||
| RA | Non-RA | RA | Non-RA | RA | Non-RA | RA | Non-RA | RA | Non-RA | RA | Non-RA | |
| Skull/face | 11 (1%) | 8 (2%) | 8 (1%) | 6 (2%) | 10 (2%) | 5 (1%) | 8 (2%) | 4 (1%) | 1 (0%) | 3 (3%) | 0 (0%) | 2 (3%) |
| Hands/fingers | 25 (3%) | 21 (5%) | 14 (2%) | 9 (3%) | 13 (2%) | 12 (3%) | 8 (2%) | 6 (2%) | 12 (4%) | 9 (8%) | 6 (3%) | 3 (5%) |
| Distal forearm | 46 (6%) | 43 (9%) | 39 (6%) | 31 (9%) | 36 (7%) | 34 (10%) | 31 (7%) | 26 (9%) | 10 (4%) | 9 (8%) | 8 (4%) | 5 (8%) |
| Proximal humerus | 31 (4%) | 17 (4%) | 23 (4%) | 13 (4%) | 23 (4%) | 15 (4%) | 17 (4%) | 12 (4%) | 8 (3%) | 2 (2%) | 6 (3%) | 1 (2%) |
| Other arm | 24 (3%) | 10 (2%) | 15 (2%) | 6 (2%) | 14 (3%) | 6 (2%) | 8 (2%) | 4 (1%) | 10 (4%) | 4 (4%) | 7 (3%) | 2 (3%) |
| Clavicle/scapula/sternum | 16 (2%) | 7 (2%) | 10 (2%) | 4 (1%) | 14 (3%) | 5 (1%) | 8 (2%) | 3 (1%) | 2 (1%) | 2 (2%) | 2 (1%) | 1 (2%) |
| Ribs | 86 (11%) | 49 (11%) | 60 (9%) | 30 (9%) | 50 (9%) | 37 (10%) | 36 (8%) | 26 (9%) | 36 (13%) | 12 (11%) | 24 (11%) | 4 (7%) |
| Thoracic/lumbar vertebrae | 279 (34%) | 158 (34%) | 261 (40%) | 142 (42%) | 185 (35%) | 135 (38%) | 171 (40%) | 123 (44%) | 94 (33%) | 23 (21%) | 90 (41%) | 19 (32%) |
| Cervical vertebrae | 14 (2%) | 4 (1%) | 11 (2%) | 1 (0%) | 8 (1%) | 2 (1%) | 6 (1%) | 0 (0%) | 6 (2%) | 2 (2%) | 5 (2%) | 1 (2%) |
| Pelvis | 50 (6%) | 23 (5%) | 36 (6%) | 19 (6%) | 35 (7%) | 15 (4%) | 26 (6%) | 14 (5%) | 15 (5%) | 8 (7%) | 10 (5%) | 5 (8%) |
| Proximal femur | 68 (8%) | 36 (8%) | 59 (9%) | 31 (9%) | 57 (11%) | 35 (10%) | 49 (11%) | 31 (11%) | 11 (4%) | 1 (1%) | 10 (5%) | 0 (0%) |
| Other leg | 81 (10%) | 43 (9%) | 55 (8%) | 26 (8%) | 47 (9%) | 25 (7%) | 31 (7%) | 18 (6%) | 34 (12%) | 18 (17%) | 24 (11%) | 8 (13%) |
| Feet/toes | 84 (10%) | 44 (10%) | 58 (9%) | 24 (7%) | 42 (8%) | 29 (8%) | 31 (7%) | 15 (5%) | 42 (15%) | 15 (14%) | 27 (12%) | 9 (15%) |
|
| ||||||||||||
| All sites | 815 | 463 | 649 | 342 | 534 | 355 | 430 | 282 | 281 | 108 | 219 | 60 |
excludes severe trauma and pathologic fracture
Table 3.
Distribution of all fractures and fragility fractures among 349 Olmsted County, Minnesota men following their initial diagnosis of rheumatoid arthritis (RA), by fracture site and proximate cause, compared with an equal number of age-matched non-RA men
| All Men | Men ≥ 50 Years | Men < 50 Years | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fracture site | All Fractures N (%) |
Fragility Fractures* N (%) |
All Fractures N (%) |
Fragility Fractures* N (%) |
All Fractures N (%) |
Fragility Fractures* N (%) |
||||||
| RA | Non-RA | RA | Non-RA | RA | Non-RA | RA | Non-RA | RA | Non-RA | RA | Non-RA | |
| Skull/face | 4 (2%) | 2 (1%) | 1 (1%) | 0 (0%) | 2 (1%) | 0 (0%) | 1 (1%) | 0 (0%) | 2 (3%) | 2 (4%) | 0 (0%) | 0 (0%) |
| Hands/fingers | 9 (4%) | 16 (11%) | 2 (1%) | 4 (4%) | 5 (3%) | 10 (11%) | 2 (1%) | 4 (5%) | 4 (7%) | 6 (11%) | 0 (0%) | 0 (0%) |
| Distal forearm | 7 (3%) | 10 (7%) | 2 (1%) | 5 (5%) | 6 (3%) | 4 (4%) | 2 (1%) | 4 (5%) | 1 (2%) | 6 (11%) | 0 (0%) | 1 (4%) |
| Proximal humerus | 9 (4%) | 2 (1%) | 7 (4%) | 2 (2%) | 9 (5%) | 2 (2%) | 7 (5%) | 2 (3%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Other arm | 5 (2%) | 1 (1%) | 3 (2%) | 1 (1%) | 5 (3%) | 1 (1%) | 3 (2%) | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Clavicle/scapula/sternum | 8 (3%) | 7 (5%) | 1 (1%) | 2 (2%) | 4 (2%) | 3 (3%) | 0 (0%) | 2 (3%) | 4 (7%) | 4 (7%) | 1 (2%) | 0 (0%) |
| Ribs | 51 (21%) | 28 (19%) | 38 (21%) | 18 (19%) | 40 (21%) | 21 (23%) | 29 (21%) | 15 (20%) | 11 (18%) | 7 (12%) | 9 (20%) | 3 (13%) |
| Thoracic/lumbar vertebrae | 98 (40%) | 51 (34%) | 91 (50%) | 45 (46%) | 72 (39%) | 32 (34%) | 67 (49%) | 30 (41%) | 26 (43%) | 19 (34%) | 24 (53%) | 15 (65%) |
| Cervical vertebrae | 5 (2%) | 2 (1%) | 2 (1%) | 1 (1%) | 4 (2%) | 2 (2%) | 1 (1%) | 1 (1%) | 1 (2%) | 0 (0%) | 1 (2%) | 0 (0%) |
| Pelvis | 8 (3%) | 2 (1%) | 5 (3%) | 1 (1%) | 6 (3%) | 1 (1%) | 4 (3%) | 1 (1%) | 2 (3%) | 1 (2%) | 1 (2%) | 0 (0%) |
| Proximal femur | 15 (6%) | 5 (3%) | 14 (8%) | 4 (4%) | 12 (6%) | 4 (4%) | 11 (8%) | 4 (5%) | 3 (5%) | 1 (2%) | 3 (7%) | 0 (0%) |
| Other leg | 20 (8%) | 14 (9%) | 12 (7%) | 10 (10%) | 14 (7%) | 8 (9%) | 7 (5%) | 7 (9%) | 6 (10%) | 6 (11%) | 5 (11%) | 3 (13%) |
| Feet/toes | 9 (4%) | 9 (6%) | 4 (2%) | 4 (4%) | 8 (4%) | 5 (5%) | 3 (2%) | 3 (4%) | 1 (2%) | 4 (7%) | 1 (2%) | 1 (4%) |
|
| ||||||||||||
| All sites | 248 | 149 | 182 | 97 | 187 | 93 | 137 | 74 | 61 | 56 | 45 | 23 |
excludes severe trauma and pathologic fracture
Table 4.
Distribution of all fractures by cause among Olmsted County, Minnesota women and men following their new diagnosis of RA made between 1955–2007, and or equivalent index date for matched non-RA subjects, stratified by age at RA diagnosis/index date; only fractures occurring over their follow-up, which ended at the earlier follow-up of the matched pair, are considered.
| Fracture Cause
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Severe Trauma | Falls from Standing | Spontaneous | Pathological | Uncertain | All Causes | |||
|
| ||||||||
| Sex and Age Groups | Subjects | N | N (%)* | N (%)* | N (%)* | N (%)* | N (%)* | N |
| Women ≥ 50 years | RA | 514 | 83 (16%) | 191 (36%) | 190 (36%) | 21 (4%) | 49 (9%) | 534 |
| Non-RA | 514 | 60 (17%) | 132 (37%) | 127 (36%) | 13 (4%) | 23 (6%) | 355 | |
| Women < 50 years | RA | 308 | 62 (22%) | 67 (24%) | 124 (44%) | 0 (0%) | 28 (10%) | 281 |
| Non-RA | 308 | 47 (44%) | 29 (27%) | 23 (21%) | 1 (1%) | 8 (7%) | 108 | |
| Women < 50 years** | RA | 308 | 20 (25%) | 11 (14%) | 33 (42%) | 0 (0%) | 15 (19%) | 79 |
| Non-RA | 308 | 21 (51%) | 12 (29%) | 4 (10%) | 0 (0%) | 4 (10%) | 41 | |
| Men ≥ 50 years | RA | 239 | 45 (24%) | 55 (29%) | 68 (36%) | 5 (3%) | 14 (7%) | 187 |
| Non-RA | 239 | 14 (15%) | 29 (31%) | 31 (33%) | 5 (5%) | 14 (15%) | 93 | |
| Men < 50 years | RA | 110 | 15 (25%) | 12 (20%) | 30 (49%) | 1 (2%) | 3 (5%) | 61 |
| Non-RA | 110 | 32 (57%) | 4 (7%) | 16 (29%) | 1 (2%) | 3 (5%) | 56 | |
| Men < 50 years** | RA | 110 | 8 (50%) | 2 (12%) | 5 (31%) | 1 (6%) | 0 (0%) | 16 |
| Non-RA | 110 | 20 (71%) | 3 (11%) | 4 (14%) | 0 (0%) | 1 (4%) | 28 | |
Percentage (%) of each type of fracture
Follow-up ended at age ≤ 50 years
Fracture Risk for All Women and Men with RA
In 9148 person-years of follow-up for each of the 822 RA and non-RA women, the RA women had a significantly increased risk for a fragility fracture over follow-up compared with their matched non-RA pair (hazard ratio [HR], 1.63, 95% CI: 1.36–1.96) (Table 5). Similarly, in 3633 person-years of follow-up for each of the 349 RA and non-RA men, RA men were also at increased risk for a fragility fracture (HR: 1.40, 95% CI: 1.02–1.93) (Table 5). The cumulative incidence curves for fragility fractures in RA and non-RA women and men, taking into account the competing risk for death, are illustrated in Figure 1. Calendar year of RA diagnosis did not influence our estimates of fracture risk (data not shown). The risk for a first major osteoporotic fracture was also increased for both women and men with RA (HR: 1.78, 95% CI: 1.43–2.21 and 1.65, 95% CI: 1.13–2.42; respectively) (Table 5).
Table 5.
Fracture risk (Hazard Ratio [HR]) for any first fragility fracture or first major osteoporotic fracture (fragility fracture at proximal femur, thoracic/lumbar vertebrae, distal radius, or proximal humerus) among women and men with RA from Olmsted County, Minnesota, stratified by age at diagnosis, and relative to their matched non-RA pair.
| Sex and Age Groups |
Subjects | N | Fragility Fractures | Major Osteoporotic Fractures | ||||
|---|---|---|---|---|---|---|---|---|
| N | Per 1000 P-Y | HR (95% CI) | N | Per 1000 P-Y | HR (95% CI) | |||
| All Women | RA | 822 | 282 | 30.8 | 1.63 (1.36–1.96) | 212 | 23.2 | 1.78 (1.43–2.21) |
| Non-RA | 822 | 193 | 21.1 | – | 129 | 14.1 | – | |
| Age ≥50 | RA | 514 | 201 | 40.0 | 1.43 (1.16–1.77) | 158 | 31.5 | 1.46 (1.15–1.86) |
| Non-RA | 514 | 151 | 30.1 | – | 113 | 22.5 | – | |
| Age < 50 | RA | 308 | 81 | 19.6 | 2.34 (1.61–3.42) | 54 | 13.1 | 4.05 (2.31–7.10) |
| Non-RA | 308 | 42 | 10.2 | – | 16 | 3.9 | – | |
| Age < 50* | RA | 308 | 32 | 16.3 | 1.95 (1.08–3.51) | 14 | 7.1 | 4.80 (1.38–16.73) |
| Non-RA | 308 | 17 | 8.7 | – | 3 | 1.5 | – | |
| All Men | RA | 349 | 88 | 24.2 | 1.40 (1.02–1.93) | 68 | 18.7 | 1.65 (1.13–2.42) |
| Non-RA | 349 | 65 | 17.9 | – | 43 | 11.8 | – | |
| Age ≥ 50 | RA | 239 | 63 | 30.9 | 1.34 (0.92–1.94) | 51 | 25.0 | 1.77 (1.13–2.77) |
| Non-RA | 239 | 50 | 24.5 | – | 31 | 15.2 | – | |
| Age < 50 | RA | 110 | 25 | 15.7 | 1.74 (0.91–3.30) | 17 | 10.7 | 1.49 (0.71–3.12) |
| Non-RA | 110 | 15 | 9.4 | – | 12 | 7.5 | – | |
| Age < 50* | RA | 110 | 6 | 8.1 | 0.82 (0.28–2.45) | 3 | 4.0 | 0.75 (0.17–3.35) |
| Non-RA | 110 | 7 | 9.4 | – | 4 | 5.4 | – | |
Figure 1.
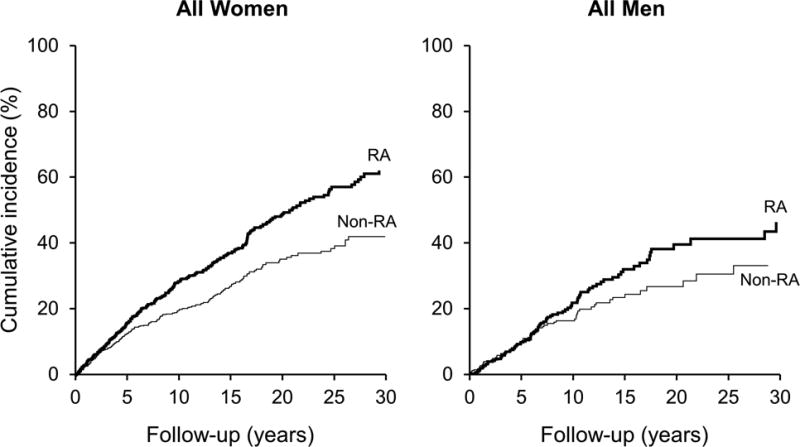
Kaplan-Meier curves for the cumulative incidence of a fragility fracture, accounting for the competing risk of death, in all women with RA and their matched non-RA pair and in all men with RA and their matched non-RA pair, following either RA diagnosis or equivalent index date for non-RA subjects. Cumulative incidence of fragility fractures at 20 years is estimated at 49% vs. 35%, respectively, for RA and non-RA women and 40% vs. 27%, respectively, for RA and non-RA men.
Fracture Risk for Women and Men Diagnosed with RA at Age ≥ 50 years
Altogether, 514 (63%) of the 822 RA women were diagnosed with the condition at age 50 years or later, as were 239 (68%) of the 349 men with RA. Among these 514 RA women and their matched non-RA pair (mean age at diagnosis/index date, 66 ± 10 years), median follow-up was 8 years (range: 4 days-35 years) and total follow-up was 5021 person-years, for each group. Over follow-up, there was a significant 1.4-fold increased risk of any fragility fracture, and a 1.5-fold increase in major osteoporotic fractures, among the older RA women (Table 5). Among the 239 RA men and their matched pair (mean age at diagnosis/index date, 65 ± 9 years; median follow-up, 8 years [range: 16 days–35 years] and total follow-up, 2042 person-years, for each group) only the 1.8-fold increase in major osteoporotic fractures in RA men was statistically significant, not the 1.3-fold increase in any fragility fractures (Table 5). The cumulative incidence curves for fragility fractures and absolute risk estimates for these older women and men are presented in Figure 2.
Figure 2.
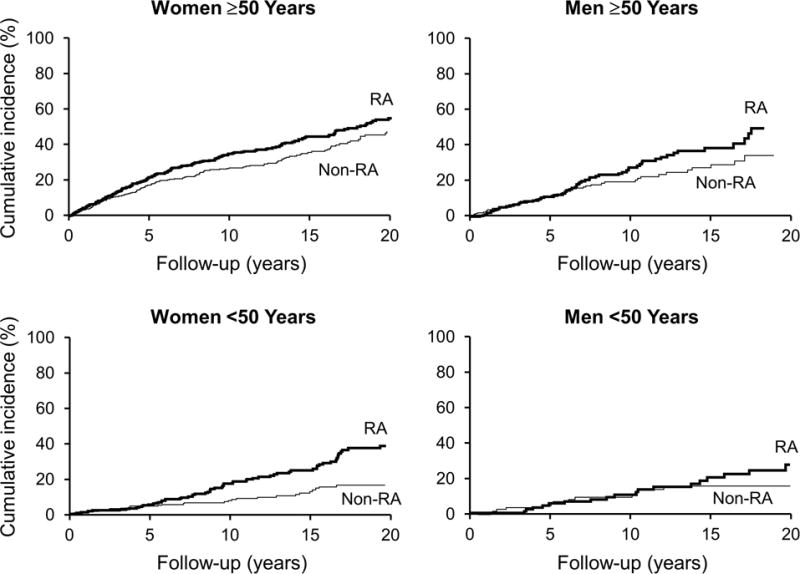
Kaplan-Meier curves for the cumulative incidence of a fragility fracture, accounting for the competing risk of death, following either RA diagnosis or equivalent index date for non-RA subjects each for women and men of different age groups (age ≥ 50 years and age < 50 years at RA diagnosis/index date). Cumulative incidence for a fragility fracture at 20 years in RA and non-RA subjects, respectively, was: 56% vs. 48% for women age ≥ 50 years; 38% vs. 17% for women age < 50 years; 50% vs. 35% for men age ≥ 50 years; and 28% vs. 16% in men age < 50 years.
Fracture Risk for Women and Men Diagnosed with RA at Age < 50 years
Among the 308 younger RA women and their matched pair (mean age at diagnosis/index date, 40 ± 8 years; median follow-up, 11 years [range: 12 days-52 years] and total follow-up, 4127 person-years, for each group), there was a significant 2.3-fold increase in fragility fractures, as well as a 4.0-fold increase in the risk of a major osteoporotic fracture, for RA women (Table 5). In the 110 younger RA men and their matched pair (mean age at diagnosis/index date, 41 ± 7 years; median follow-up, 12 years [range: 6 months–44 years] and total follow-up, 1591 person-years, for each group), there was a 1.7-fold increase in fragility fractures and 1.5-fold increase in major osteoporotic fractures for RA men, but neither reached statistical significance (Table 5). The cumulative incidence curves and absolute risk estimates for fragility fractures for these younger women and men are also illustrated in Figure 2.
Fracture Risk before Age 50 years for Women and Men with RA
Examining the risk for fractures occurring prior to age 50 years, the median follow-up for each pair of women in this sub-analysis was 5 years (maximum follow-up, 27 years). Over the 1963 person-years of follow-up for each group, RA women had a 2.0-fold increase in fragility fractures and a 4.8-fold increase in major osteoporotic fractures, prior to age 50 years, both of which were statistically significant (Table 5). The cumulative incidence of fragility fractures at 10 years was 17.5% vs. 6.7% for RA vs. non-RA women. By contrast, few fractures occurred before the age of 50 among men (median follow-up for each pair, 5 years; maximum follow-up, 31 years). Over the 743 person-years of follow-up in each group, fracture risk was not elevated among the men with RA, as only 6 RA men compared with 7 non-RA men had an incident fragility fracture before age 50 years (Table 5).
DISCUSSION
After age 50 years, both women and men are at risk for fragility fractures,(32) and the extent to which this risk is affected by a diagnosis of RA is clinically relevant. In addition, we were interested in whether women and men diagnosed with RA at younger ages are at risk for fracture early in the course of their disease, or only when they get older. In our population-based cohort of women and men with confirmed new onset RA, where ascertainment of all clinically-evident fractures was complete and long-term follow-up was available, we found that both women and men are at increased risk for fragility fractures, generally, as well as fractures at skeletal sites typically considered osteoporotic. However, men with RA appeared to be at increased risk for fracture only later in the course of their disease, and not before age 50 years. In contrast, the increased fracture risk was observed in both younger and older women with RA and was seen relatively early in the course of their disease. Furthermore, women diagnosed with RA before they were 50 years old were at an increased risk for fragility fractures, and particularly at major osteoporotic sites, even before they reached the age of 50.
Most studies evaluating fracture risk in RA have primarily involved older women,(4–6, 8–11, 13) and few have included younger individuals.(19–21) One large prospective study examining fracture risk in both sexes with RA, only investigated subjects over age 40 years.(7) A recent large study that included both younger women and men with RA examined the risk for fractures only at the hip, shoulder, pelvis or forearm and reported an increased risk for fractures in women, but not in men, under age 50 years.(19) We were able to identify an increased risk not only for major osteoporotic fractures, but also for overall fragility fractures in young women with RA, and even before they reached the age of 50. We also had very long follow-up available, which likely accounts for the differences observed with our study in younger men with RA, where we found their risk for fragility fractures to be elevated only with longer disease duration and not before age 50 years.
Our findings have important clinical implications for younger women diagnosed with RA, who need to be made aware that they are at increased risk for a fragility fracture even while still young. Although their absolute risk is lower than older women with RA, it is still essential to ensure that their modifiable risk factors for fractures are addressed (e.g., smoking cessation, maintaining adequate calcium and vitamin D intake, preventing falls, etc.). The evidence on the efficacy and safety of most osteoporosis drug therapies is limited for younger individuals, especially in women of childbearing potential.
In women with RA, especially if diagnosed when older, their increased fracture risk appears to occur relatively soon after their diagnosis. In contrast, the increased risk for fractures in men with RA appears to occur relatively later following the diagnosis, and primarily at older, not at younger ages. Our different findings in young men compared with young women with RA may be due to the fact that men have greater peak bone mass and larger bone size relative to women, which may in turn confer a protective advantage against fractures in younger years.(33) Although the risk estimate for fractures were similar between younger and older men with RA, the relatively fewer men studied limited our statistical power for analyses of subgroups. Nevertheless, our results suggest that both young and older men with RA have an increased risk for fracture, primarily as they advance in age, and particularly at major osteoporotic sites.
Fracture risk associated with RA is multifactorial.(2) The use of glucocorticoids, often used to control the disease, is considered an important risk for fracture.(34,35) Additional risk factors unique to RA include RA-related inflammation, characterized by high levels of pro-inflammatory cytokines, as well as immune system dysregulation, that is now increasingly recognized to adversely affect bone metabolism.(36–38) Furthermore, decreases in physical activity related to joint pain or damage may not only further aggravate bone loss (2,39,40) but also contribute to an increased risk for falls, (41) and thereby fractures. Such risk factors were not explored in the current study, as it would require a different study design, especially since the majority of our RA population was exposed to steroids. The main focus of our present work was to determine whether there was a risk for fragility fractures in younger women and men with RA, which has not been previously established, and whether any risk observed was soon after diagnosis, or only when older. Further exploration is needed to determine the extent to which any risk factors differentially influence the likelihood for fractures not only among women vs. men but also among those with RA who are younger vs. older. Work is particularly needed in determining who, among the younger women with RA, are at greatest risk for early fracture so appropriate management may be effectively individualized.
It should be noted that our estimates of fracture risk in RA may not be the same for other races, as our population was largely white and non-Hispanic, reflecting the population demographics of Olmsted County. However, hip fracture rates in Olmsted County are reflective of hip fractures rates in US whites generally,(42) so our findings on fragility fracture risk are likely generalizable to US whites. Although we were able to study a relatively large number of both women and men with RA, our numbers of men were still smaller, reflecting the lower incidence of RA in men, and which likely limited our statistical power in subgroup analyses. Nevertheless, the risk for fragility fractures and major osteoporotic fractures were significantly increased in all men with RA, and the estimates of fracture risk observed in younger and older men, separately, were consistent with these overall results. A unique advantage of our study is that we were able to study RA subjects from the time of their initial diagnosis, which allowed us to evaluate the risk for fractures in older subjects with new onset RA, not those with a combination of new and longstanding disease. Furthermore, all RA subjects were identified from medical records, not self-report, and were confirmed through comprehensive chart review to meet 1987 ACR classification criteria for RA. Similarly, all fractures in our study were documented in contemporary medical records and confirmed by trained nurse abstractors. Moreover, an ascertainment of trauma fracture etiology was possible. While those with RA may be subject to increased radiographic imaging that could lead to an increase in detection of asymptomatic fractures, relative to their matched non-RA pair, when we excluded fractures that were identified incidentally, our overall findings remained the same. In our exploratory analyses, calendar year of RA diagnosis did not influence our estimates of fracture risk.
In summary, men with RA are at risk for fragility fractures when they are older, particularly at major osteoporotic sites, while women with RA are at increased risk for fragility fractures at any age after diagnosis. Specifically, we found that young women with RA are at increased risk for fragility fractures occurring even before they reach the age of 50 years. While minimizing known risks for bone loss and fractures is important for all with RA, this is especially important to emphasize in young women with RA who may not appreciate their early risk for fragility fractures.
Acknowledgments
The authors thank Leona Bellrichard, R.N., Marcia Erickson, R.N., Wendy Gay, R.N., Julie Gingras, R.N., Joan LaPlante, R.N., Constance Neuman, R.N., Cynthia Nosek, R.N., and Diane Wilke, R.N. for their work in data abstraction.
This work was supported by research grants PO1 AG04875-24 and R01 AR46849 and made possible by the Rochester Epidemiology Project (R01 AG034676 from the National Institute on Aging) from the National Institutes of Health, U.S. Public Health Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
DISCLOSURES
All authors have no conflicts of interest with respect to this work. Dr. Amin served on a scientific advisory board for Merck & Co.
Contributor Information
Shreyasee Amin, Email: amin.shreyasee@mayo.edu.
Sherine E. Gabriel, Email: gabriel@mayo.edu.
Sara J. Achenbach, Email: achenbach@mayo.edu.
Elizabeth J. Atkinson, Email: atkinson@mayo.edu.
L. Joseph Melton, III, Email: melton.j@mayo.edu.
References
- 1.Sinigaglia L, Varenna M, Girasole G, Bianchi G. Epidemiology of osteoporosis in rheumatic diseases. Rheumatic Diseases Clinics of North America. 2006;32(4):631–658. doi: 10.1016/j.rdc.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Amin S. Bone loss and fractures in rheumatoid arthritis. In: Maricic MJ, Gluck O, editors. Bone Disease in Rheumatology. Lippincott, Williams & Wilkins; Philadelphia: 2005. pp. 73–78. [Google Scholar]
- 3.Kanis JA, Borgstrom F, De Laet C, Johansson H, Johnell O, Jonsson B, Oden A, Zethraeus N, Pfleger B, Khaltaev N. Assessment of fracture risk. Osteoporosis International. 2005;16(6):581–589. doi: 10.1007/s00198-004-1780-5. [DOI] [PubMed] [Google Scholar]
- 4.Wright NC, Lisse JR, Walitt BT, Eaton CB, Chen Z, Women’s Health Initiative I. Arthritis increases the risk for fractures–results from the Women’s Health Initiative. Journal of Rheumatology. 2011;38(8):1680–1688. doi: 10.3899/jrheum.101196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vis M, Haavardsholm EA, Boyesen P, Haugeberg G, Uhlig T, Hoff M, Woolf A, Dijkmans B, Lems W, Kvien TK. High incidence of vertebral and non-vertebral fractures in the OSTRA cohort study: a 5-year follow-up study in postmenopausal women with rheumatoid arthritis. Osteoporosis International. 2011;22(9):2413–2419. doi: 10.1007/s00198-010-1517-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furuya T, Kotake S, Inoue E, Nanke Y, Yago T, Kobashigawa T, Ichikawa N, Tanaka E, Momohara S, Nakajima A, Hara M, Tomatsu T, Yamanaka H, Kamatani N. Risk factors associated with incident clinical vertebral and nonvertebral fractures in Japanese women with rheumatoid arthritis: a prospective 54-month observational study. Journal of Rheumatology. 2007;34(2):303–310. [PubMed] [Google Scholar]
- 7.van Staa TP, Geusens P, Bijlsma JWJ, Leufkens HGM, Cooper C. Clinical assessment of the long-term risk of fracture in patients with rheumatoid arthritis. Arthritis & Rheumatism. 2006;54(10):3104–3112. doi: 10.1002/art.22117. [DOI] [PubMed] [Google Scholar]
- 8.Arai K, Hanyu T, Sugitani H, Murai T, Fujisawa J, Nakazono K, Kondo N, Endo N. Risk factors for vertebral fracture in menopausal or postmenopausal Japanese women with rheumatoid arthritis: a cross-sectional and longitudinal study. Journal of Bone & Mineral Metabolism. 2006;24(2):118–124. doi: 10.1007/s00774-005-0657-9. [DOI] [PubMed] [Google Scholar]
- 9.Orstavik RE, Haugeberg G, Uhlig T, Mowinckel P, Falch JA, Halse JI, Kvien TK. Self reported non-vertebral fractures in rheumatoid arthritis and population based controls: incidence and relationship with bone mineral density and clinical variables. Annals of the Rheumatic Diseases. 2004;63(2):177–182. doi: 10.1136/ard.2003.005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orstavik RE, Haugeberg G, Mowinckel P, Hoiseth A, Uhlig T, Falch JA, Halse JI, McCloskey E, Kvien TK. Vertebral deformities in rheumatoid arthritis: a comparison with population-based controls. Archives of Internal Medicine. 2004;164(4):420–425. doi: 10.1001/archinte.164.4.420. [DOI] [PubMed] [Google Scholar]
- 11.Peel NF, Moore DJ, Barrington NA, Bax DE, Eastell R. Risk of vertebral fracture and relationship to bone mineral density in steroid treated rheumatoid arthritis. Annals of the Rheumatic Diseases. 1995;54(10):801–806. doi: 10.1136/ard.54.10.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper C, Coupland C, Mitchell M. Rheumatoid arthritis, corticosteroid therapy and hip fracture. Annals of the Rheumatic Diseases. 1995;54(1):49–52. doi: 10.1136/ard.54.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spector TD, Hall GM, McCloskey EV, Kanis JA. Risk of vertebral fracture in women with rheumatoid arthritis. BMJ. 1993;306(6877):558. doi: 10.1136/bmj.306.6877.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delmas PD, Marin F, Marcus R, Misurski DA, Mitlak BH. Beyond hip: importance of other nonspinal fractures. American Journal of Medicine. 2007;120(5):381–387. doi: 10.1016/j.amjmed.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Myasoedova E, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. Is the incidence of rheumatoid arthritis rising?: results from Olmsted County, Minnesota, 1955–2007. Arthritis & Rheumatism. 2010;62(6):1576–1582. doi: 10.1002/art.27425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tengstrand B, Hafstrom I. Bone mineral density in men with rheumatoid arthritis is associated with erosive disease and sulfasalazine treatment but not with sex hormones. Journal of Rheumatology. 2002;29(11):2299–2305. [PubMed] [Google Scholar]
- 17.Shenstone BD, Mahmoud A, Woodward R, Elvins D, Palmer R, Ring F, Bhalla AK. Bone mineral density in nonsteroid treated early rheumatoid arthritis. Annals of the Rheumatic Diseases. 1994;53(10):681–684. doi: 10.1136/ard.53.10.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Compston JE, Crawley EO, Evans C, O’Sullivan MM. Spinal trabecular bone mineral content in patients with non-steroid treated rheumatoid arthritis. Annals of the Rheumatic Diseases. 1988;47(8):660–664. doi: 10.1136/ard.47.8.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SY, Schneeweiss S, Liu J, Daniel GW, Chang C-L, Garneau K, Solomon DH. Risk of osteoporotic fracture in a large population-based cohort of patients with rheumatoid arthritis. Arthritis Research & Therapy. 2010;12(4):R154. doi: 10.1186/ar3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El Maghraoui A, Rezqi A, Mounach A, Achemlal L, Bezza A, Ghozlani I. Prevalence and risk factors of vertebral fractures in women with rheumatoid arthritis using vertebral fracture assessment. Rheumatology. 2010;49(7):1303–1310. doi: 10.1093/rheumatology/keq084. [DOI] [PubMed] [Google Scholar]
- 21.Hooyman JR, Melton LJ, 3rd, Nelson AM, O’Fallon WM, Riggs BL. Fractures after rheumatoid arthritis. A population-based study. Arthritis & Rheumatism. 1984;27(12):1353–1361. doi: 10.1002/art.1780271205. [DOI] [PubMed] [Google Scholar]
- 22.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clinic Proceedings. 1996;71(3):266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 23.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. American Journal of Epidemiology. 2011;173(9):1059–1068. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis & Rheumatism. 1988;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 25.Gabriel SE, Crowson CS, O’Fallon WM. The epidemiology of rheumatoid arthritis in Rochester, Minnesota, 1955–1985. Arthritis & Rheumatism. 1999;42(3):415–420. doi: 10.1002/1529-0131(199904)42:3<415::AID-ANR4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 26.Doran MF, Pond GR, Crowson CS, O’Fallon WM, Gabriel SE. Trends in incidence and mortality in rheumatoid arthritis in Rochester, Minnesota, over a forty-year period. Arthritis & Rheumatism. 2002;46(3):625–631. doi: 10.1002/art.509. [DOI] [PubMed] [Google Scholar]
- 27.Crowson CS, Liang KP, Therneau TM, Kremers HM, Gabriel SE. Could accelerated aging explain the excess mortality in patients with seropositive rheumatoid arthritis? Arthritis & Rheumatism. 2010;62(2):378–382. doi: 10.1002/art.27194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melton LJ, 3rd, Crowson CS, O’Fallon WM. Fracture incidence in Olmsted County, Minnesota: comparison of urban with rural rates and changes in urban rates over time. Osteoporosis International. 1999;9(1):29–37. doi: 10.1007/s001980050113. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan EL, Meier P. Non-parametic estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 30.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Statistics in Medicine. 1999;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 31.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Annals of Statistics. 1988;16(3):1141–1154. [Google Scholar]
- 32.Melton LJ, 3rd, Chrischilles EA, Cooper C, Lane AW, Riggs BL. Perspective. How many women have osteoporosis? Journal of Bone & Mineral Research. 1992;7(9):1005–1010. doi: 10.1002/jbmr.5650070902. [DOI] [PubMed] [Google Scholar]
- 33.Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocrine Reviews. 2008;29(4):441–464. doi: 10.1210/er.2008-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Everdingen AA, Jacobs JWG, Siewertsz Van Reesema DR, Bijlsma JWJ. Low-dose prednisone therapy for patients with early active rheumatoid arthritis: clinical efficacy, disease-modifying properties, and side effects: a randomized, double-blind, placebo-controlled clinical trial. Annals of Internal Medicine. 2002;136(1):1–12. doi: 10.7326/0003-4819-136-1-200201010-00006. [Summary for patients in Ann Intern Med. 2002 Jan 1;136(1):I-26; PMID: 11777372] [DOI] [PubMed] [Google Scholar]
- 35.de Nijs RN, Jacobs JW, Bijlsma JW, Lems WF, Laan RF, Houben HH, ter Borg EJ, Huisman AM, Bruyn GA, van Oijen PL, Westgeest AA, Algra A, Hofman DM, Osteoporosis Working Group DSfR Prevalence of vertebral deformities and symptomatic vertebral fractures in corticosteroid treated patients with rheumatoid arthritis. Rheumatology. 2001;40(12):1375–1383. doi: 10.1093/rheumatology/40.12.1375. [DOI] [PubMed] [Google Scholar]
- 36.Takayanagi H. Osteoimmunology and the effects of the immune system on bone. Nature Reviews Rheumatology. 2009;5(12):667–676. doi: 10.1038/nrrheum.2009.217. [DOI] [PubMed] [Google Scholar]
- 37.Sato K, Takayanagi H. Osteoclasts, rheumatoid arthritis, and osteoimmunology. Current Opinion in Rheumatology. 2006;18(4):419–426. doi: 10.1097/01.bor.0000231912.24740.a5. [DOI] [PubMed] [Google Scholar]
- 38.Clowes JA, Riggs BL, Khosla S. The role of the immune system in the pathophysiology of osteoporosis. Immunological Reviews. 2005;208:207–227. doi: 10.1111/j.0105-2896.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- 39.Laan RF, Buijs WC, Verbeek AL, Draad MP, Corstens FH, van de Putte LB, van Riel PL. Bone mineral density in patients with recent onset rheumatoid arthritis: influence of disease activity and functional capacity. Annals of the Rheumatic Diseases. 1993;52(1):21–26. doi: 10.1136/ard.52.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sambrook PN, Eisman JA, Champion GD, Yeates MG, Pocock NA, Eberl S. Determinants of axial bone loss in rheumatoid arthritis. Arthritis & Rheumatism. 1987;30(7):721–728. doi: 10.1002/art.1780300701. [DOI] [PubMed] [Google Scholar]
- 41.Kaz Kaz H, Johnson D, Kerry S, Chinappen U, Tweed K, Patel S. Fall-related risk factors and osteoporosis in women with rheumatoid arthritis. Rheumatology. 2004;43(10):1267–1271. doi: 10.1093/rheumatology/keh304. [DOI] [PubMed] [Google Scholar]
- 42.Melton LJ, 3rd, Kearns AE, Atkinson EJ, Bolander ME, Achenbach SJ, Huddleston JM, Therneau TM, Leibson CL. Secular trends in hip fracture incidence and recurrence. Osteoporosis International. 2009;20(5):687–694. doi: 10.1007/s00198-008-0742-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


