…a strong association exists between elevated cytokines/chemokines in stenotic kidneys and renal functional recovery after revascularization in an experimental swine model of ARAS (…)
Keywords: cytokines, hemodynamics, inflammation, renal hypertension, renovascular hypertension
Abstract
Background
Renal parenchymal inflammation is a critical determinant of kidney injury in renal artery stenosis (RAS) but is difficult to assess in the single kidney without tissue samples. Whether renal vein (RV) levels of inflammatory markers reflect active parenchymal inflammation remains unknown. We evaluated the relationship between net RV cytokine release and tissue inflammation in the post-stenotic kidney.
Methods
Pigs were studied after 10 weeks of RAS treated 4 weeks earlier with intra-renal vehicle or anti-inflammatory mesenchymal stem cells (MSCs) or normal control. Single-kidney renal blood flow was measured by fast computerized tomography. RV and inferior vena cava levels of tumor necrosis factor (TNF)-α, interferon (IF)-γ, monocyte chemoattractant protein (MCP-1) and interleukin (IL)-10 were measured by enzyme-linked immunosorbent assay, and their net release calculated. Renal expression of the same cytokines was correlated with their net release.
Results
Net release of TNF-α, IF-γ and MCP-1 was higher in RAS compared with normal and to the contralateral kidney (all P < 0.05), decreased in MSC-treated pigs as was their tissue expression. Contrarily, the release of the anti-inflammatory IL-10 was lower in RAS and normalized in RAS + MSC. The net release of TNF-α, MCP-1 and IL-10 directly correlated with their tissue expression. The ratio of inflammatory-to-reparative macrophages directly correlated with the release of MCP-1, but inversely with the release of IL-10. In vitro cultured MSCs also induced a shift in the macrophage phenotype from inflammatory (M1) to reparative (M2).
Conclusions
Our findings demonstrate that the release of inflammatory markers from the affected kidney provides an index of renal tissue inflammation in experimental RAS.
INTRODUCTION
Renal artery stenosis (RAS) is a leading cause of secondary hypertension among the elderly population [1] and may accelerate cardiovascular disease and progression to chronic renal failure [2]. Over the last couple of decades, several lines of investigation have identified multiple mechanisms responsible for producing parenchymal tissue injury in RAS. Activation of renin–angiotensin system, oxidative stress and fibrosis have all been touted as major players in amplifying functional and structural damage in the stenotic kidney [3].
In addition, inflammation represents an important pathway that mediates deleterious processes in the stenotic kidney [4]. Experimental studies have shown that macrophage and T cell infiltration can exacerbate kidney damage by secreting pro-inflammatory and pro-fibrotic cytokines, leading to fibrosis and microvascular damage [5, 6]. We have previously shown that the post-stenotic human kidney releases inflammatory cytokines that portend renal injury [7] and that the elevated release of these cytokines from the stenotic swine kidneys is associated with attenuated renal functional recovery after revascularization [8]. Furthermore, we have recently demonstrated that the progressive influx of CD68+ macrophages in the human post-stenotic kidneys correlated with disease severity [9].
Macrophages have recently gained considerable attention due to their ability to orchestrate the entire inflammatory response. Although ‘classically’ activated macrophages (M1) initially accumulate in the renal parenchyma after an ischemic insult, ‘alternatively’ activated (M2) macrophages appear later in the healing process and exert anti-inflammatory and reparative properties [10]. Therefore, switching from a pro-inflammatory (M1) to a trophic (M2) macrophage phenotype may confer protection and attenuate the progression to fibrosis in the hypo-perfused kidney.
Detecting and monitoring intra-renal inflammatory activity often necessitates biopsy [9]. However, whether levels of inflammatory markers detected in venous blood draining the stenotic kidney reflects their renal parenchymal expression is unknown. This study tested the hypothesis that renal cytokine release reflects tissue inflammation in the post-stenotic swine kidney. We have recently shown in swine atherosclerotic RAS that adipose tissue-derived mesenchymal stem cells (MSCs) exert potent anti-inflammatory effects [11] and decrease T cells and CD163+ macrophages infiltration in the post-stenotic kidney, as well as the expression of pro-inflammatory cytokines [12]. Therefore, in order to modulate cytokine release, we took advantage of the anti-inflammatory properties of MSCs, delivered into the renal artery in a subgroup of pigs.
MATERIALS AND METHODS
All experiments were approved by the institutional Animal Care and Use Committee. Eighteen domestic female pigs were studied after 10 weeks of observation. At baseline, animals were anesthetized (intramuscular telazol 5 mg/kg and xylazine 2 mg/kg), intubated and mechanically ventilated. Anesthesia was maintained with intravenously (ketamine 0.2 mg/kg/min and xylazine 0.03 mg/kg/min). RAS was induced in 12 animals by placing in the main renal artery a local-irritant coil, which leads to the gradual development of unilateral RAS over a 7–10-day period, as reported previously [13]. Normal animals underwent a sham procedure. Fat tissue was collected during the procedure for the subsequent isolation of MSCs.
Six weeks later, we evaluated the degree of stenosis using angiography and performed a sham procedure in six normal and six RAS pigs, whereas the other six were treated with a single intra-renal infusion of autologous adipose tissue-derived MSCs (10 × 106 cells/mL suspended in 10 mL). Cells were pre-labeled with Chloromethylbenzamido-DiI and infused slowly over 5 min into a 5F catheter engaged proximal to the stenosis.
Four weeks later, we evaluated the degree of stenosis using angiography. Then, stenotic kidney renal hemodynamics and function were assessed using multi-detector computerized tomography (MDCT) [14]. Briefly, renal regional perfusion, renal blood flow (RBF) and glomerular filtration rate (GFR) were measured from tissue time-attenuation curves obtained in the regions of interest selected from the aorta, renal cortex and medulla [15]. MDCT images were analyzed with the Analyze™ software package (Biomedical Imaging Resource, Mayo Clinic, MN, USA).
One week later, animals were euthanized with intravenous sodium pentobarbital (100 mg/kg, Fatal Plus, Vortech Pharmaceuticals, Dearborn, MI, USA) and the kidneys removed, dissected and placed in liquid nitrogen or preserved in formalin for in vitro experiments. Cytokine levels were measured in the systemic veins, renal veins (RVs) and urine, and their expression (western blot) and localization (staining) in renal tissue.
Inflammatory cytokines
Under fluoroscopic guidance, catheters were advanced into the inferior vena cava (IVC) and stenotic kidney RV to collect samples. All blood samples were centrifuged and plasma aliquots stored at −80°C until it was assayed. RV and IVC (below the RV) samples were collected and plasma levels of tumor necrosis factor (TNF)-α, interferon (IF)-γ, monocyte chemoattractant protein (MCP)-1 and interleukin (IL)-10 were measured by enzyme-linked immunosorbent assay (Invitrogen Cat# KSC3011 and Cat# KSC0101; Kingfisher Biotech Cat# VS0081S-002 and VS0259s-002). Then, we estimated cytokine gradient (RV-IVC) and net renal release (gradient × RBF) of each measured analyte, as described previously [7, 8]. In addition, we compared the difference between right (stenotic) and left (contralateral) cytokine release in RAS and RAS + MSC pigs, calculated using the following formula: release = RV × RBF.
Urine samples were collected through supra-pubic catheter and levels of the same markers measured (ALPCO, Cat# 45-TNFHUU-E01 and 61-IFGPO-E01). Finally, the renal protein expression of TNF-α (Santa Cruz, 1:200), IF-γ (Santa Cruz, 1:200), MCP-1 (MyBioSource, 1:7500) and IL-10 (Santa Cruz, 1:200) was assessed by western blot [13, 16]. Cellular localization of the same inflammatory cytokines was assessed by double immunofluorescence staining with macrophage (CD68) and tubular (cytokeratin) markers.
Inflammatory cells
Standard immunostaining with antibodies against CD68+/inducible nitric oxide synthase (iNOS)+ (M1) (abcam cat#: ab15323, 1:100) and CD68+/Arginase-1+ (M2) (abcam cat#: HPA004114, 1:100) macrophages and CD3+ (abcam, cat# ab16669) T-cells was performed in 5-µm sections. The number of M1 and M2 macrophages was quantified in 15–20 fields, and the results from all fields averaged. Furthermore, immunofluorescence staining with antibodies against M2a (CD163+/CD206+/Fizz1+), M2b (CD163+/86+) and M2c (CD163+/CD206+/Fizz1−) macrophages was used to count cells per field.
Renal morphology
Renal scarring and tubular injury were assessed and quantified in kidney sections stained with Masson trichrome and H&E staining, as shown previously [16].
Labeled MSCs were counted manually in frozen kidney sections (5 μm) from the stenotic kidney under fluorescence microscopy. The number of cells/mm2 was averaged and multiplied by the renal section thickness, and then by renal volume obtained by MDCT, to calculate the MSC retention rate, as described previously [12, 17].
MSC effects on macrophages
Human monocytes were cultured for 18 h in RPMI 1640 media supplemented with macrophage colony-stimulating factor, lipopolysaccharide and IF-γ to induce M1 polarization [18]. M1-polarized cells were cultured alone or co-cultured with porcine MSCs and expression of iNOS, TNF-α, IL-10 and arginase-1 (all 1:200, Santa Cruz, CA, USA) was evaluated using western blotting.
Statistical analysis
Statistical analysis was performed using JMP software package version 8.0 (SAS Institute Inc., Cary, NC, USA). Normally distributed data were expressed as the mean ± SD and comparisons within and among the groups were performed using analysis of variance (ANOVA)/Student's two-tailed t-test. For data that did not show a Gaussian distribution, non-parametric (Kruskal–Wallis/ Wilcoxon) were used. A P-value <0.05 was considered significant. Regressions were calculated by the least-squares fit (for detailed Methods and Results, see Supplementary data).
RESULTS
Body weight was similar in all pigs (P = 0.75, ANOVA). Ten weeks after induction of RAS, both sham and MSC-treated RAS pigs achieved hemodynamically significant stenoses (P = 0.009 and P = 0.001 versus normal), and blood pressure was significantly and similarly elevated compared with normal (both P < 0.05 versus normal). Serum creatinine and plasma renin activity levels were similar among the groups (Table 1).
Table 1.
Systemic characteristics and single-kidney hemodynamics (mean ± SD) 10 weeks after baseline in normal, untreated RAS and RAS pigs treated with MSCs
| Normal | RAS | RAS + MSC | |
|---|---|---|---|
| Body weight (kg) | 50.4 ± 1.1 | 52.2 ± 5.8 | 50.8 ± 3.1 |
| Degree of stenosis (%) | 0 | 77.0 ± 25.7* | 74.8 ± 20.3* |
| Systolic blood pressure (mmHg) | 118.7 ± 21.1 | 141.4 ± 24.9* | 143.0 ± 25.2* |
| Diastolic blood pressure (mmHg) | 88.7 ± 19.0 | 111.0 ± 15.6* | 115.8 ± 26.4* |
| Mean arterial pressure (mmHg) | 98.7 ± 19.5 | 121.1 ± 18.5* | 124.9 ± 25.9* |
| Serum creatinine (mg/dL) | 1.3 ± 0.2 | 1.6 ± 0.3*,** | 1.3 ± 10.3 |
| PRA (ng/mL/h) | 0.10 ± 0.01 | 0.27 ± 0.22 | 0.18 ± 0.13 |
| Cortical volume (cc) | 103.9 ± 4.0 | 58.1 ± 21.6*,** | 81.5 ± 20.8* |
| Cortical perfusion (mL/min/cc tissue) | 5.4 ± 0.3 | 2.7 ± 0.7*,** | 4.3 ± 0.8* |
| RBF (mL/min) | 650.4 ± 92.7 | 299.1 ± 78.6*,** | 497.3 ± 124.3* |
| GFR (mL/min) | 97.0 ± 8.1 | 49.7 ± 7.1*,** | 80.6 ± 20.1 |
PRA, plasma renin activity; RBF, renal blood flow; GFR, glomerular filtration rate.
*P≤0.05 versus normal.
**P < 0.05 versus RAS + MSC.
Renal hemodynamics and function
Cortical volume, perfusion and RBF were reduced in RAS compared with normal but improved (although not normalized) in RAS + MSC pigs. However, treatment with MSC restored GFR to normal levels (Table 1, P = 0.012 versus RAS, P = 0.17 versus normal).
Systemic, RV and urine levels of inflammatory markers
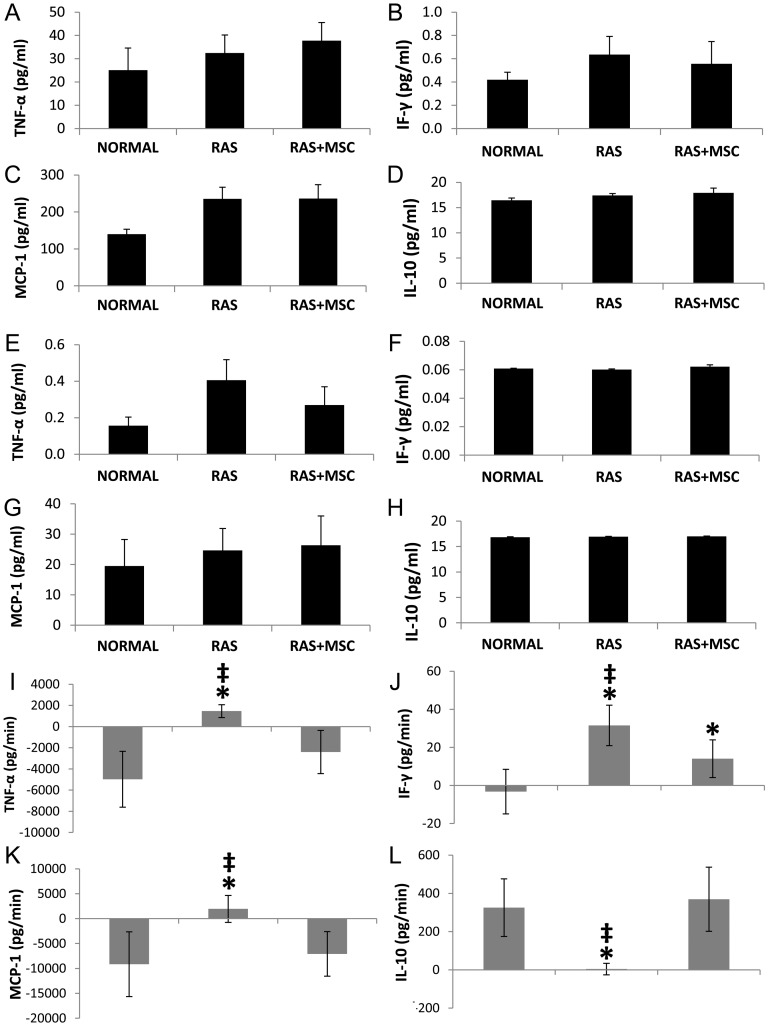
IVC (Figure 1A–D) and urine (Figure 1E–H) levels of TNF-α, IF-γ, MCP-1 and IL-10 were similar among the groups, although circulating MCP-1 levels tended to be higher in RAS compared with normal (P = 0.08). The net release of TNF-α, IF-γ and MCP-1 from the RV was higher in the RAS kidney compared with normal and decreased in MSC-treated pigs (Figure 1I–K). Conversely, IL-10 release was lower in RAS compared with normal and returned to normal levels in RAS + MSC animals (Figure 1L). Furthermore, the difference between the release of IF-γ, TNF-α and MCP-1 in the stenotic and contralateral kidneys was positive in RAS and negative in RAS + MSC (Supplementary Figure 2SA–C, P < 0.05 all), whereas the difference in the release of IL-10 showed the opposite pattern (Supplementary Figure 2SD, P < 0.001).
FIGURE 1:
IVC levels of TNF-α, IF-γ, MCP-1 and IL-10 in normal, RAS and RAS + MSC pigs (A–D). Urine levels of the same cytokines did not differ among the groups (E–H). Net release of TNF-α, IF-γ, MCP-1 and IL-10 in normal, RAS and RAS + MSC pigs (I–L). *P≤0.05 versus normal, ‡P < 0.05 versus RAS + MSC.
Tissue expression and localization of inflammatory markers
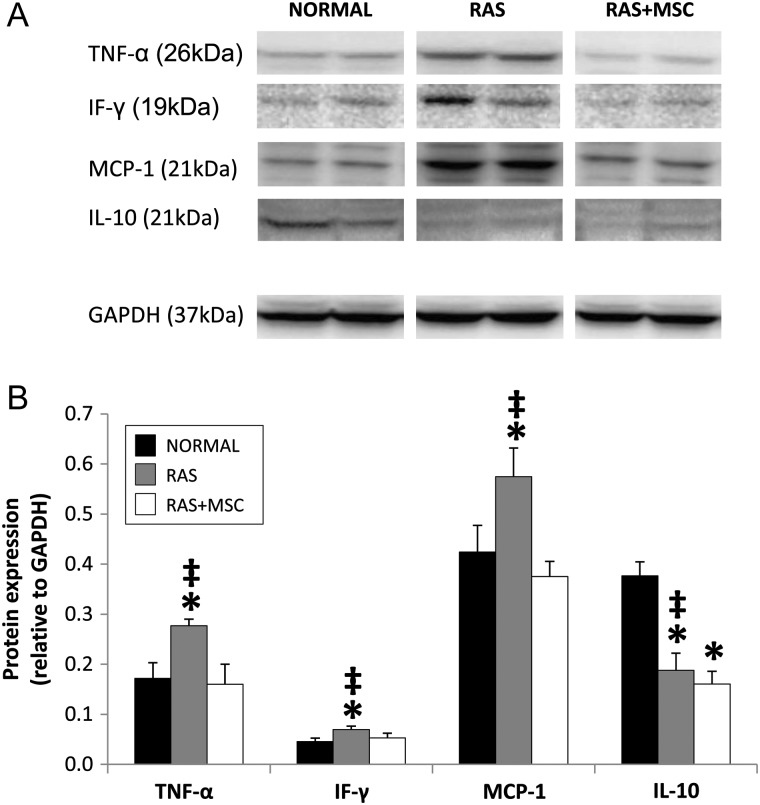
The renal expression of TNF-α, IF-γ and MCP-1 was higher in RAS compared with normal and decreased in MSC-treated pigs, whereas the expression of IL-10 was decreased in both RAS and RAS + MSC (Figure 2A and B). IF-γ and IL-10 were commonly observed in renal tubules and co-stained with cytokeratin (Supplementary Figure 3SB and D), whereas TNF-α and MCP-1 were expressed mostly in the interstitium and co-stained with CD68+ macrophages (Supplementary Figure 4SA and C). A fraction of IL-10 was also expressed at the interstitium and co-stained with CD68 (Supplementary Figures 3SD and 4SD).
FIGURE 2:
Representative immunoblots (A) and renal protein expression (B) of TNF-α, IF-γ, MCP-1 and IL-10 in the study groups. *P≤0.05 versus normal, ‡P < 0.05 versus RAS + MSC.
Inflammatory cells
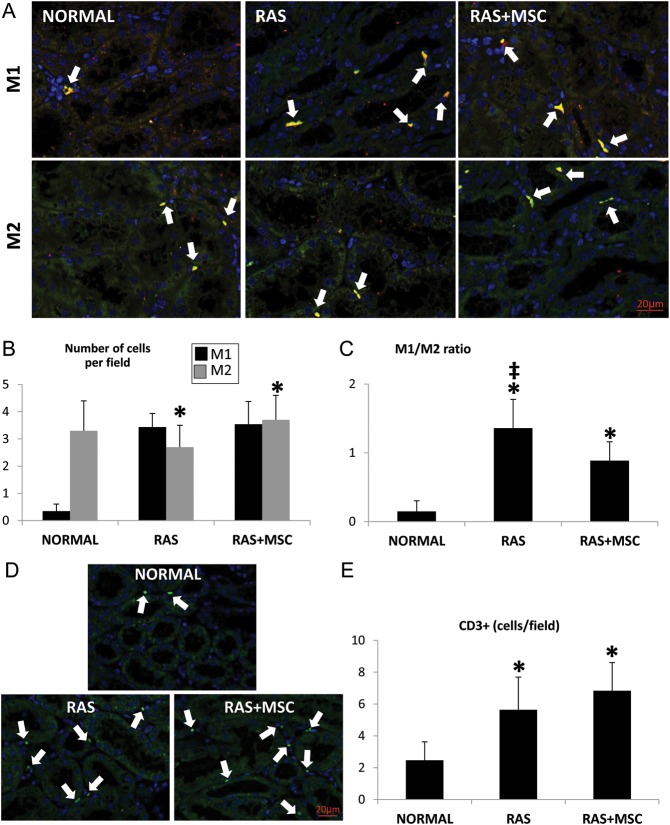
The number of M1 macrophages was similarly elevated in both RAS groups compared with normal, whereas the number of M2 macrophages tended to be higher in RAS + MSC compared with RAS (P = 0.08, Figure 3A and B). However, the M1/M2 ratio was higher in the RAS and decreased in animals treated with MSC (Figure 3C). The number of M2a and M2b cells did not differ among the groups (Supplementary Figure 5SB–C), whereas RAS + MSC showed higher numbers of M2c macrophages compared with normal and RAS (Supplementary Figure 5SD, P < 0.05 both).
FIGURE 3:
Representative fluorescence staining (40×) for M1: anti-macrophage CD68 (green)/iNOS (red) and M2: CD68 (green)/Arginase-1 (red) macrophages (A) and their quantification (B). (C) Quantification of the M1/M2 ratio. Representative fluorescence staining (40×) for CD3+ T cells (D, green) and its quantification (E). *P≤0.05 versus normal, ‡P < 0.05 versus RAS + MSC.
Finally, the number of CD3+ T cells was similarly elevated in both RAS groups compared with normal (Figure 3D–E, P < 0.05 versus normal).
Renal morphology
Four weeks after intra-renal delivery, MSC showed the retention rate of around 12%, were commonly observed in the interstitium (Supplementary Figure 1SA) and tubular structures (Supplementary Figure 1SB) and few in perivascular regions.
Tubulo-interstitial fibrosis (Supplementary Figure 6SA) and tubular injury (Supplementary Figure 7SA) were greater in RAS compared with normal, yet restored in MSC-treated pigs.
Correlations between net release and tissue expressionof inflammatory markers
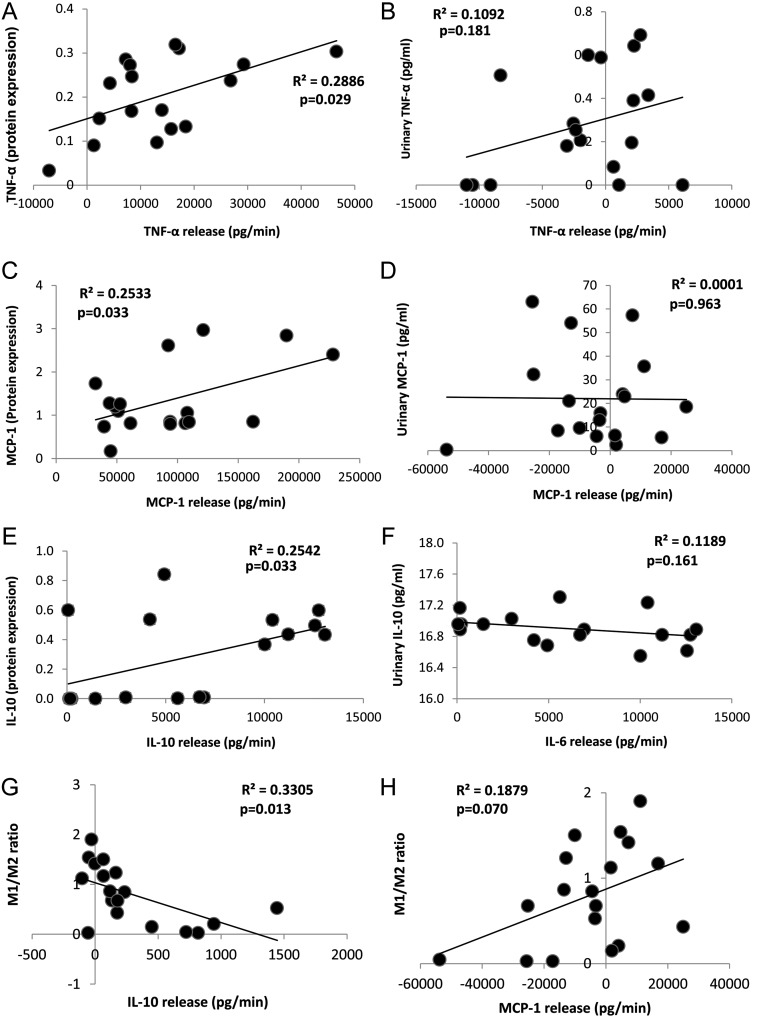
Stenotic-kidney release of TNF-α, MCP-1 and IL-10 directly correlated with their tissue protein expression (Figure 4A, C and E), but not with their urinary levels (Figure 4B, D and F). The net release of IF-γ did not correlate with either its tissue expression (R2 = 0.106, P = 0.188) or urinary levels (R2 = 0.001, P = 0.892). No significant correlations were found for any cytokine between systemic concentrations alone and tissue expression.
FIGURE 4:
A direct correlation was found between net renal release of TNF-α, IF-γ and MCP-1 and their tissue expression (A, C and E, left), but not with their urinary levels (B, D and F, right). Correlations between net release of IL-10 (H) and MCP-1 (G) and the M1/M2 macrophage ratio.
Correlations between cytokine net release and tissue inflammation or damage
The M1/M2 ratio inversely correlated with the release of IL-10 (Figure 4H, P = 0.01) and tended to correlate directly with the release of MCP-1 (Figure 4G, P = 0.07). No correlations were found between the number and the net release of any inflammatory marker and the number of either M1 or M2 macrophages or T cells (data not shown).
The net release of TNF-α and MCP-1 (but not IF-γ) correlated directly (Supplementary Figures 6SB, D and E and Supplementary Figure 7SB, D and E), whereas the release of IL-10 correlated inversely with both tubulo-interstitial fibrosis and tubular damage (Supplementary Figures 6SD and 7SD).
MSC effects on macrophages
Compared with quiescent monocytes, M1-polarized macrophages overexpressed iNOS and TNF-α, whereas arginase-1 and IL-10 expression was down-regulated. Co-culture with MSC increased the expression of these proteins (although IL-10 remained suppressed), indicating that MSC directly induced a phenotypic switch of M1 to M2 macrophages and decreased TNF-α expression (Supplementary Figure 8SA and B).
DISCUSSION
This study demonstrates that the release of inflammatory markers from the affected kidney, but not their concentrations alone, is a useful index of renal tissue inflammation in experimental RAS and can track its modulation by an anti-inflammatory intervention. Moreover, our findings suggest that the release of inflammatory cytokines detected in the RV might reflect the relative activities of macrophage subpopulations with the inflammatory phenotype. These observations may contribute towards the development of refined management strategies to improve renal outcomes in patients with RAS.
The prevalence of RAS increases with age, and it affects almost 7% of individuals older than 65 years of age [1]. Furthermore, patients with RAS have an increased incidence of adverse cardiovascular events and the potential for progression to end-stage renal disease [2]. Therefore, understanding and early detection of the mechanisms underlying disease progression in RAS is critical and would be useful for the design of therapeutic approaches.
Inflammation characterized by Th-1 lymphocyte activation and macrophage infiltration amplifies renal parenchymal damage in experimental and clinical RAS [19, 20]. Significant RAS is characterized by macrophage accumulation in the post-stenotic kidney [9], and the inhibition of MCP-1 confers renoprotective effects [5]. Therefore, these observations provide the impetus for the early assessment of renal parenchymal inflammation to reduce progression to chronic renal failure. However, the direct assessment of renal inflammatory activity in situ often requires tissue obtained using renal biopsy, a procedure associated with potential risk [21]. Hence, identifying clinically feasible markers to monitor renal parenchymal inflammatory burden could provide valuable information regarding the patient's risk for progression to renal dysfunction and response to therapy.
RV sampling has been used in RAS patients to evaluate renin production, an index of renal ischemia [22]. Its major advantage over peripheral vein measurements is the potential to assess single-kidney contributions in asymmetric kidney disease and to localize the site of production to the kidney. Comparing RV with systemic measurements (such as the IVC) affords estimating the gradient across the kidney [23], and factoring-in single-kidney RBF allows the calculation of net venous release of cytokines from each kidney [7, 8], excluding their metabolism or urinary excretion. The potential value of this approach is underscored by our recent demonstration that the post-stenotic human kidney releases inflammatory cytokines that parallel renal hypoperfusion and tissue ischemia [7]. Furthermore, the net release of inflammatory markers, but not their systemic levels, constitutes an important determinant of renal functional outcomes after revascularization [8]. Indeed, a previous study in RAS patients undergoing percutaneous renal angioplasty failed to detect significant differences in systemic levels of inflammatory biomarkers [24]. Taken together, these observations imply that the net release of inflammatory markers assessed in RV samples might represent inflammatory damage beyond the stenotic lesion more closely than their systemic levels.
In the current study, we assessed the release of inflammatory markers in a large animal model of chronic experimental RAS and correlated their levels with their tissue expression. We used MSC as an anti-inflammatory strategy, due to their immunomodulatory properties [11, 12]. We found that MSC attenuated the release of several inflammatory markers from the post-stenotic kidney as well as their tissue expression. Conversely, the net release of the anti-inflammatory cytokine IL-10 was lower in RAS, and although it was not improved by MSC, it directly correlated with its renal expression. Although the observed correlations were generally modest, these findings imply that changes in net cytokine release may be a useful index of tissue inflammation in the post-stenotic kidney. Furthermore, the lateralization of the release of inflammatory cytokines toward the stenotic kidney and its restoration after MSC therapy underscore the improvement of renal parenchymal inflammation in the progression to chronic renal failure in RAS, and its potential modulation with anti-inflammatory interventions. Pertinently, urine and IVC cytokine levels (which sample both the kidneys) were similar among the groups and did not correlate with their renal release, arguing against their use as reliable markers of kidney inflammation in asymmetric kidney disease.
Notably, this study showed the robust expression of IF-γ in renal tubules [25] that was elevated in the stenotic kidney, as was TNF-α and MCP-1 expression in interstitial cells. Similar to IF-γ, IL-10 was mostly expressed in renal tubules, as observed in the human kidney [26], suggesting that renal parenchymal cells can modulate their release from the stenotic kidney. These results extend previous studies that have shown that in situ production of pro-inflammatory and anti-inflammatory cytokines by renal tubular epithelial cells mediates tissue repair [25–27]. In addition, a small proportion of IL-10 was expressed at the interstitium and co-stained with CD68, possibly reflecting M2 macrophages. Our group has also shown in experimental murine RAS elevated renal expression of TNF-α and MCP-1 [5, 28]. Thus, the interstitial production of these cytokines may reflect secretion by infiltrating inflammatory cells. Furthermore, TNF-α increases the expression of adhesion molecules and chemokines, including MCP-1, a key regulator of macrophage recruitment [29]. Interestingly, IL-10 expression was decreased in both RAS groups, whereas its net release was elevated in RAS + MSC. Therefore, IL-10 net release might reflect its level of activity instead of the number of IL-10-expressing cells in the renal tissue.
Macrophages exhibit great plasticity in their surface marker expression profile [30], with M1 macrophages expressing inflammatory cytokines like IL-1β and iNOS, whereas M2 express arginase-1, mannose receptor or IL-10 [10]. In models of acute kidney injury, M1 are involved in initiation of the inflammatory process and aggravating renal damage, whereas M2 participate in subsequent tissue remodeling and repair [10]. The current study shows that in chronic renal ischemia the kidney shows a mixed macrophage phenotype with greater predominance of M1 macrophages, which might be regulated by treatment with MSC. Furthermore, our in vitro study confirmed the ability of MSC to switch macrophages from the M1 to M2 phenotype. The higher numbers of M2c macrophages in RAS + MSC animals suggest a selective contribution of this M2 macrophage subtype to renal repair in this group [31]. Interestingly, we found that the renal release of MCP-1 tended to directly correlate with the ratio of M1/M2 macrophages populating the stenotic kidney, whereas the release of the anti-inflammatory IL-10 correlated inversely with the M1/M2 ratio. Hence, the induction of the inflammatory macrophage phenotype within the kidney might be reflected in the corresponding renal venous profiles for M1 (TNF-α, IF-γ) and M2 (IL-10) cytokines.
Previous studies have suggested an important role for T cells in the pathogenesis of hypertensive disorders [32]. Peripheral blood of RAS patients also contains increased numbers of cells expressing CD3 and CD4 markers, which correlate with their expression in post-mortem samples, interpreted to suggest that some circulating T cells derive from the renal vasculature [33]. In the current study, the number of T cells in the kidney was similarly elevated in RAS and RAS + MSC compared with normal, but did not correlate with the release of the measured inflammatory markers. However, we cannot rule out the possibility that the number of T cells might be related to different markers, whereas the markers we examined are more specific to macrophages.
Increased serum creatinine levels in RAS animals was associated with higher histological damage (tubulo-interstitial fibrosis and tubular injury), which was restored in MSC-treated pigs. Stenotic-kidney release of TNF-α and MCP-1 correlated directly, and IL-10 inversely, with both tubulo-interstitial fibrosis and tubular damage, underscoring the implication of these inflammatory mediators in the progression of the disease. Contrarily, lack of association between IF-γ release and either histological damage or its expression in the stenotic kidney argues against a major role of this cytokine in the progression of tissue injury.
The limitations of our study include the use of young animals with no co-morbidities (atherosclerosis, diabetes, essential hypertension), which may aggravate tissue damage in the stenotic kidney. In addition, the early stage of RAS and the short duration of the disease may modulate the release of individual markers. Despite these caveats, this study has a number of strengths. Our model recapitulates many characteristics observed in human RAS [7, 9, 34]. Indeed, our results correlate with our previous clinical study that showed a significant release of inflammatory cytokines in patients with RAS [7]. Collecting RV samples remains an invasive procedure, yet associated with minimal morbidity and few complications.
CONCLUSIONS
Our study demonstrates that renal parenchymal inflammation can be profiled by the measurement of the net release of cytokines from the stenotic kidney, including TNF-α, MCP-1 and IL-10. Moreover, our observations highlight the central role of macrophages in the pathogenesis of renal ischemic injury and the potential for MSCs to modulate expression of inflammatory pathways. These results may direct the development of novel and specific therapies aimed to improve the outcome of ischemic renovascular disease. The clinical implications of these findings remain to be established.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
FUNDING
This study was partly supported by the NIH (HL085307, DK73608, HL77131, UL1-RR000135 and UL1-RR024150) and by the American Heart Association.
CONFLICT OF INTEREST STATEMENT
A.B.D. has intellectual property assigned to and is a shareholder of Mill Creek Life Sciences. The results presented in this paper have not been published previously in whole or part, except in abstract format.(See related article by Tsuboi et al. A ray of light in the dark: alternative approaches to the assessment and treatment of ischemic nephropathy. Nephrol Dial Transplant 2014; 29: 228–231.)
Supplementary Material
REFERENCES
- 1.Hansen KJ, Edwards MS, Craven TE, et al. Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg. 2002;36:443–451. doi: 10.1067/mva.2002.127351. [DOI] [PubMed] [Google Scholar]
- 2.Kalra PA, Guo H, Kausz AT, et al. Atherosclerotic renovascular disease in united states patients aged 67 years or older: risk factors, revascularization, and prognosis. Kidney Int. 2005;68:293–301. doi: 10.1111/j.1523-1755.2005.00406.x. [DOI] [PubMed] [Google Scholar]
- 3.Lerman LO, Textor SC, Grande JP. Mechanisms of tissue injury in renal artery stenosis: ischemia and beyond. Prog Cardiovasc Dis. 2009;52:196–203. doi: 10.1016/j.pcad.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johns EJ. Inflammation: the underlying foe in renovascular hypertension? J Hypertens. 2009;27:1964–1965. doi: 10.1097/HJH.0b013e328331a881. [DOI] [PubMed] [Google Scholar]
- 5.Zhu XY, Chade AR, Krier JD, et al. The chemokine monocyte chemoattractant protein-1 contributes to renal dysfunction in swine renovascular hypertension. J Hypertens. 2009;27:2063–2073. doi: 10.1097/HJH.0b013e3283300192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Truong LD, Farhood A, Tasby J, et al. Experimental chronic renal ischemia: morphologic and immunologic studies. Kidney Int. 1992;41:1676–1689. doi: 10.1038/ki.1992.241. [DOI] [PubMed] [Google Scholar]
- 7.Eirin A, Gloviczki ML, Tang H, et al. Inflammatory and injury signals released from the post-stenotic human kidney. Eur Heart J. 2013;34:540–548a. doi: 10.1093/eurheartj/ehs197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eirin A, Ebrahimi B, Zhang X, et al. Changes in glomerular filtration rate after renal revascularization correlate with microvascular hemodynamics and inflammation in swine renal artery stenosis. Circ Cardiovasc Interv. 2012;5:720–728. doi: 10.1161/CIRCINTERVENTIONS.112.972596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gloviczki ML, Keddis MT, Garovic VD, et al. Tgf expression and macrophage accumulation in atherosclerotic renal artery stenosis. Clin J Am Soc Nephrol. 2013;8:546–553. doi: 10.2215/CJN.06460612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee S, Huen S, Nishio H, et al. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol. 2011;22:317–326. doi: 10.1681/ASN.2009060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu XY, Urbieta-Caceres V, Krier JD, et al. Mesenchymal stem cells and endothelial progenitor cells decrease renal injury in experimental swine renal artery stenosis through different mechanisms. Stem Cells. 2013;31:117–125. doi: 10.1002/stem.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eirin A, Zhu XY, Krier JD, et al. Adipose tissue-derived mesenchymal stem cells improve revascularization outcomes to restore renal function in swine atherosclerotic renal artery stenosis. Stem Cells. 2012;30:1030–1041. doi: 10.1002/stem.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eirin A, Zhu XY, Urbieta-Caceres VH, et al. Persistent kidney dysfunction in swine renal artery stenosis correlates with outer cortical microvascular remodeling. Am J Physiol Renal Physiol. 2011;300:F1394–F1401. doi: 10.1152/ajprenal.00697.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krier JD, Ritman EL, Bajzer Z, et al. Noninvasive measurement of concurrent single-kidney perfusion, glomerular filtration, and tubular function. Am J Physiol Renal Physiol. 2001;281:F630–F638. doi: 10.1152/ajprenal.2001.281.4.F630. [DOI] [PubMed] [Google Scholar]
- 15.Daghini E, Primak AN, Chade AR, et al. Assessment of renal hemodynamics and function in pigs with 64-section multidetector ct: comparison with electron-beam ct. Radiology. 2007;243:405–412. doi: 10.1148/radiol.2432060655. [DOI] [PubMed] [Google Scholar]
- 16.Eirin A, Li Z, Zhang X, et al. A mitochondrial permeability transition pore inhibitor improves renal outcomes after revascularization in experimental atherosclerotic renal artery stenosis. Hypertension. 2012;60:1242–1249. doi: 10.1161/HYPERTENSIONAHA.112.199919. [DOI] [PubMed] [Google Scholar]
- 17.Chade AR, Zhu X, Lavi R, et al. Endothelial progenitor cells restore renal function in chronic experimental renovascular disease. Circulation. 2009;119:547–557. doi: 10.1161/CIRCULATIONAHA.108.788653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez FO, Gordon S, Locati M, et al. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 19.Stouffer GA, Pathak A, Rojas M. Unilateral renal artery stenosis causes a chronic vascular inflammatory response in apoe-/- mice. Trans Am Clin Climatol Assoc. 2010;121:252–264. 264–256. [PMC free article] [PubMed] [Google Scholar]
- 20.Nobuhiko A, Suganuma E, Babaev VR, et al. Angiotensin ii amplifies macrophage-driven atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:2143–2148. doi: 10.1161/01.ATV.0000145607.03879.e0. [DOI] [PubMed] [Google Scholar]
- 21.Walker PD. The renal biopsy. Arch Pathol Lab Med. 2009;133:181–188. doi: 10.5858/133.2.181. [DOI] [PubMed] [Google Scholar]
- 22.Kapoor N, Fahsah I, Karim R, et al. Physiological assessment of renal artery stenosis: comparisons of resting with hyperemic renal pressure measurements. Catheter Cardiovasc Interv. 2010;76:726–732. doi: 10.1002/ccd.22731. [DOI] [PubMed] [Google Scholar]
- 23.Ratliff BB, Rabadi MM, Vasko R, et al. Messengers without borders: mediators of systemic inflammatory response in aki. J Am Soc Nephrol. 2013;24:529–536. doi: 10.1681/ASN.2012060633. [DOI] [PubMed] [Google Scholar]
- 24.Alhadad A, Guron G, Fortuna-Nowakowska E, et al. Renal angioplasty causes a rapid transient increase in inflammatory biomarkers, but reduced levels of interleukin-6 and endothelin-1 1 month after intervention. J Hypertens. 2007;25:1907–1914. doi: 10.1097/HJH.0b013e328244e2ca. [DOI] [PubMed] [Google Scholar]
- 25.Timoshanko JR, Holdsworth SR, Kitching AR, et al. Ifn-gamma production by intrinsic renal cells and bone marrow-derived cells is required for full expression of crescentic glomerulonephritis in mice. J Immunol. 2002;168:4135–4141. doi: 10.4049/jimmunol.168.8.4135. [DOI] [PubMed] [Google Scholar]
- 26.Niemir ZI, Ondracek M, Dworacki G, et al. In situ upregulation of il-10 reflects the activity of human glomerulonephritides. Am J Kidney Dis. 1998;32:80–92. doi: 10.1053/ajkd.1998.v32.pm9669428. [DOI] [PubMed] [Google Scholar]
- 27.Menke J, Iwata Y, Rabacal WA, et al. Csf-1 signals directly to renal tubular epithelial cells to mediate repair in mice. J Clin Invest. 2009;119:2330–2342. doi: 10.1172/JCI39087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng J, Zhou W, Warner GM, et al. Temporal analysis of signaling pathways activated in a murine model of two-kidney, one-clip hypertension. Am J Physiol Renal Physiol. 2009;297:F1055–F1068. doi: 10.1152/ajprenal.90439.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murao K, Ohyama T, Imachi H, et al. Tnf-alpha stimulation of mcp-1 expression is mediated by the akt/pkb signal transduction pathway in vascular endothelial cells. Biochem Biophys Res Commun. 2000;276:791–796. doi: 10.1006/bbrc.2000.3497. [DOI] [PubMed] [Google Scholar]
- 30.Ricardo SD, van Goor H, Eddy AA. Macrophage diversity in renal injury and repair. J Clin Invest. 2008;118:3522–3530. doi: 10.1172/JCI36150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mantovani A, Sica A, Sozzani S, et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 32.Harrison DG, Guzik TJ, Lob HE, et al. Inflammation, immunity, and hypertension. Hypertension. 2011;57:132–140. doi: 10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kotliar C, Juncos L, Inserra F, et al. Local and systemic cellular immunity in early renal artery atherosclerosis. Clin J Am Soc Nephrol. 2012;7:224–230. doi: 10.2215/CJN.06270611. [DOI] [PubMed] [Google Scholar]
- 34.Keddis MT, Garovic VD, Bailey KR, et al. Ischaemic nephropathy secondary to atherosclerotic renal artery stenosis: Cclinical and histopathological correlates. Nephrol Dial Transplant. 2010;25:3615–3622. doi: 10.1093/ndt/gfq269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.