Abstract
Background
Few studies have examined the changes in lipoproteins over time and how inflammation is associated with lipoprotein concentrations among patients with end-stage renal disease on dialysis. One possible explanation for the association of low LDL cholesterol concentration and adverse outcomes is that inflammation reduces selected apolipoprotein concentrations.
Methods
Serum samples were collected from a subsample of patients enrolled into the Comprehensive Dialysis Study every 3 months for up to 1 year. We examined the relation between temporal patterns in levels of inflammatory markers and changes in apolipoproteins (apo) A1 and B and the apo B/A1 ratio using linear mixed effects modeling and adjusting for potential confounders.
Results
We enrolled 266 participants from 56 dialysis facilities. The mean age was 62 years, 45% were women and 26% were black. Apo A1 was lower among patients with higher Quetelet's (body mass) index (BMI), diabetes mellitus and atherosclerosis. Apo B was lower among older patients, patients with higher serum creatinine and patients with lower BMI. Over the course of a year, apo A1 changed inversely with serum concentrations of the acute phase proteins C-reactive protein (CRP) and α1 acid glycoprotein (α1AG), while apo B did not. Changes in α1AG were more strongly associated with changes in apolipoprotein concentrations than were changes in CRP; increases in α1AG were associated with decreases in apo A1 and increases in the apo B/A1 ratio.
Conclusions
Changes in inflammatory markers were associated with changes in apo A1, but not apo B over 1 year, suggesting that reductions in high-density lipoprotein cholesterol are associated with inflammation, either of which could mediate cardiovascular risk, but not supporting a hypothesis linking increased risk of low levels of apo B containing lipoproteins to the risk associated with inflammation.
Keywords: apolipoprotein A1, apolipoprotein B, α1 acid glycoprotein, CRP, HDL
INTRODUCTION
Chronic kidney disease (CKD) is associated with high cardiovascular risk [1] and exhibits a characteristic pattern of cardiovascular risk markers, including heightened inflammation and alterations in lipid and lipoprotein levels and structure, typically described as dyslipidemia rather than hyperlipidemia [2] with higher triglyceride levels and lower high-density lipoprotein (HDL) cholesterol levels associated continuously with estimated glomerular filtration rate (eGFR) [3, 4]. Triglycerides are elevated, whereas HDL and low-density lipoprotein (LDL) cholesterol concentrations are usually low [5]. Although lipids themselves manifest expected associations with measures of vascular structure and function, specifically aortic pulse wave velocity [6] and carotid intimal media thickness [7], the relationships among lipoprotein concentrations and mortality in patients on dialysis differ from the relationship observed in the general population, inasmuch as patients on dialysis or with advanced chronic kidney disease with the lowest cholesterol and triglyceride concentrations are those at highest risk [8, 9]. There is evidence that inflammation may modify the associations of lipoproteins with cardiovascular end points in end-stage renal disease (ESRD). Patients with little or no indication of inflammatory activity exhibit the expected relations among lipoproteins and cardiovascular events (i.e. higher levels = higher risk). In contrast, patients with heightened inflammatory activity show paradoxical relations (lower levels = higher risk) [10–14]. Other studies show no persistent inverse association [15–17] in this patient population. An understanding of whether inflammation affects the levels of lipoproteins or modifies lipoprotein-associated risk in another way is essential in order to make sense of these conflicting results, but there have been few cross-sectional [18] and no longitudinal studies relating inflammation to lipoprotein concentrations to our knowledge.
The Comprehensive Dialysis Study (CDS) was a prospective cohort study in which serum samples were collected every 3 months for 1 year from a group of patients initiating dialysis in facilities within the USA. We sought to examine the temporal patterns in levels of apolipoproteins (apo) A1 and B and the apo B/A1 ratio and to characterize the associations among these apolipoproteins and concurrent measures of acute phase proteins, C-reactive protein (CRP) and α1 acid glycoprotein (α1AG).
MATERIALS AND METHODS
Design and participants
The CDS is a prospective cohort study of adults with ESRD who initiated hemodialysis or peritoneal dialysis between June 2005 and June 2007 in dialysis facilities throughout the USA designed to examine the nutritional status, physical activity and health-related quality of life among incident dialysis patients [19, 20]. The CDS has been previously described in detail, including sampling of dialysis facilities, recruitment and measures [19, 20]. In brief, participants were successfully recruited from 297 dialysis facilities out of 335 selected facilities. Fifty-six of 73 facilities subsampled to participate in the nutrition substudy agreed to participate and provide serum samples. Participants (n = 266) from the 56 facilities provided up to five serum samples each, one at enrollment and quarterly thereafter for up to a year. Facilities were selected a priori by systematic probability sampling proportional to estimated size sampling to participate in the nutrition substudy. The biomarkers chosen for study, albumin, prealbumin, apo B, apo A1, CRP and α1AG were predetermined to establish the longitudinal trajectory of these patients once dialysis was initiated and the relationship between inflammation and both lipoproteins and nutritional biomarkers, as previously reported [20]. Sex, age and the comorbidities used for analysis were selected and predetermined during the design of this study.
The study was approved by the Institutional Review Boards of the University of California, San Francisco Emory Universityand and the University of California Davis and all patients provided informed consent for participation.
Data collection
Data on demographics, body composition (height and weight), dialysis modality and access, comorbidities and serum creatinine at dialysis initiation were collected from the CMS Medical Evidence Form (CMS 2728) and a telephone interview administered by DataBanque Research Services (Pittsburgh, PA). Blood samples were collected at enrollment and quarterly for up to 1 year.
Exposure and outcome
The primary exposures of interest were longitudinal measures of acute phase proteins, CRP and α1AG. The primary outcomes of interest were longitudinal measures of apolipoprotein A1, B and the ratio of apolipoproteins (apo B/apo A1). Apo A1, CRP and α1AG concentrations were measured in duplicate on each serum sample using a Beckman Array 360 nephelometer (Beckman, La Brea, CA), and apo B was measured in duplicate on each serum sample with a Poly-Chem chemical analyzer (Polymedco Cortlandt Manor, NY). We used the mean concentration of the duplicate serum concentrations of each analyte in analyses. Intra-assay coefficients of variation (CoV) were as follows: apo A1 1%, apo B 1.1%, CRP 3.6% and α1 AG 0.14%. Inter-assay CoV were as follows: apo A1 3.2%, apo B 5.4%, CRP 9.2% and α1AG 3.1–5.5%.
Statistical analyses
We considered how changes in acute phase proteins were associated with changes in each apolipoprotein during 1 year of dialysis. Statistical modeling was based on linear mixed effects models with random intercept terms for dialysis center to account for within-center correlations (clustering by dialysis center). We adjusted for confounding by including baseline patient characteristics as covariates. We preselected demographic (age, sex, race) and clinical covariates (diabetes mellitus, atherosclerotic vascular disease, modality and vascular access) and nutritional status (BMI and serum creatinine concentration), which might confound the relations among inflammatory markers and lipoprotein concentrations in the initial design of this study. We additionally incorporated sampling weights based on the CDS survey design in order to generate population representative estimates from the original stratified sampling (using robust standard error estimates).
Lowess curves of each apolipoprotein versus each of the two time-varying independent variables, CRP and α1AG, were examined to determine whether the assumption of linearity was appropriate. CRP was log-transformed for analyses. The linear mixed effects models included time specified as equally spaced units representing each quarterly blood draw, time varying longitudinal measures of CRP and α1AG (measured at enrollment and quarterly for up to 1 year) and potential confounders measured only at baseline (age, sex, race, body mass index (BMI), dialysis modality and access, serum creatinine and comorbidities).
Baseline measured covariates were included as fixed effects with an interaction with time. Longitudinal measures were included as (fixed-effect) time-varying covariates. Our model building strategy was to include all covariates in an initial model without any interactions and add in the baseline covariate interactions with time individually. All the baseline by time interactions with P < 0.05 from the individual models were then included together in a single model, from which we dropped terms one at a time (based on dropping the term with the highest P-value) and refitting the model until all remaining interaction terms had P-values < 0.05. We further examined the inclusion of quadratic terms for continuous baseline predictors and their interactions with time in the model to account for any potential non-linearity, and retained the quadratic terms when appropriate. Diagnostic tests of the final fitted models (QQ plots, Cook's D statistics) were performed to check that modeling assumptions were met and that results were not unduly influenced by outlying centers.
All measurements were included from all patients in the final analyses. Two sensitivity analyses were performed to address the issue of bias arising from patient dropout. First, models were run including measurements from only those participants who provided a serum sample at 12 months (end of study). As a further check, final models were run including all the data and two additional predictors, a predictor equal to the number of the last blood draw for each individual and a predictor equal to the interaction of last draw with time. Results from both analyses were similar to that resulted from the primary analyses. All statistical analyses were performed using SAS 9.2 (Cary, NC).
RESULTS
We included 266 CDS nutrition substudy participants from a total of 56 dialysis facilities (Table 1). The mean age was 62 years, 45% were women and 26% were black.
Table 1.
Baseline characteristics of Comprehensive Dialysis Study nutrition participants
| Baseline characteristic, n = 266 | |
| Age, years | 62 (14) |
| Male | 147 (55) |
| Race | |
| White | 189 (71) |
| Black | 68 (26) |
| Other | 9 (3) |
| BMI, kg/m2 | 29.8 (7.8)a |
| Serum creatinine, mg/dLb | 6.20 [4.70, 8.20] |
| Diabetes | 152 (57) |
| Atherosclerosis | 96 (36) |
| Heart failure | 85 (32) |
| Dialysis modality and access | |
| Hemodialysis, AVF or AVG | 63 (24) |
| Hemodialysis, catheter | 181 (68)c |
| Peritoneal dialysis | 22 (8) |
| Baseline concentration of time-varying analyte | |
| Baseline apo A1, mg/dL | 141 (34.7) |
| Baseline apo B, mg/dL | 84 (27.2) |
| Baseline ratio apo B/apo A1 | 0.58 [0.18, 1.74] |
| Baseline α1AG, mg/dL) | 115(35.9) |
| Baseline C reactive protein, mg/L) | 7.45 [3.95, 12.75] |
Data presented as mean (SD), number (%) or median [interquartile range]; AVF or AVG: arteriovenous fistula or arteriovenous graft.
aOne male with missing BMI was assigned average BMI value for males.
bFrom USRDS 2728 form.
cOne hemodialysis patient with missing access type was assigned catheter.
Association of baseline patient characteristics with apolipoproteins during follow-up
Associations of apo A1 with patient characteristics seen in the final multiple predictor model were similar to those observed in the general population, with statistically significantly higher levels among women and lower levels among patients with higher BMI, diabetes mellitus and atherosclerosis (Table 2). Furthermore, age was a statistically significant correlate of apo A1 concentrations.
Table 2.
Baseline and time-varying correlates of apolipoproteins
| Variable | Apo A1 |
Apo B |
Apo B/apo A1 |
|||
|---|---|---|---|---|---|---|
| Coefficient (95% CI) | P-value | Coefficient (95% CI) | P-value | Coefficient (95% CI) | P-value | |
| Fixed covariates | ||||||
| Age, years | 0.52 (0.30, 0.74) | <0.0001 | −0.26 (−0.49, −0.03) | 0.02 | −0.007 (−0.01, <0.01) | 0.0006 |
| Age2 | – | – | −0.02 (−0.03, <0.01) | 0.009 | −0.0002 (>−0.01, <−0.01) | 0.01 |
| BMI, kg/m2 | −0.60 (−1.13, −0.17) | 0.01 | 0.48 (0.13, 0.84) | 0.007 | 0.01 (0.01, 0.01) | <0.0001 |
| Sex, female | 18.07 (11.10, 25.04) | <0.0001 | 1.79 (−6.09, 9.67) | 0.66 | −0.10 (−0.20, <0.01) | 0.05 |
| Race, white | −6.32 (−12.80, 0.15) | 0.06 | 3.50 (−3.99, 10.98) | 0.36 | 0.09 (≥0.01, 0.18) | 0.06 |
| Modality/access | ||||||
| HD, AVF or AVG | Reference | Reference | Reference | |||
| HD, catheter | −6.15 (−14.74, 2.44) | 0.16 | −2.93 (−9.39, 3.52) | 0.37 | 0.003 (−0.10, 0.10) | 0.98 |
| PD | −1.83 (−15.96, 12.30) | 0.80 | 20.38 (1.94, 38.82) | 0.03 | 0.20 (−0.04, 0.44) | 0.11 |
| Diabetes | −10.83 (−20.22, −1.44) | 0.02 | −6.70 (−14.76, 1.37) | 0.10 | −0.04 (−0.16, 0.08) | 0.47 |
| Atherosclerosis | −8.49 (−16.32, −0.65) | 0.03 | −5.20 (−14.62, 4.22) | 0.28 | −0.01 (−0.14, 0.11) | 0.88 |
| CHF | −0.02 (−7.20, 7.17) | >0.99 | 1.69 (−5.11, 8.50) | 0.63 | 0.05 (−0.04, 0.13) | 0.31 |
| Serum creatinine, mg/dL | −0.45 (−1.23, 0.32) | 0.25 | −0.76 (−1.15, −0.36) | 0.0002 | −0.005 (−0.01, <0.01) | 0.05 |
| Time and time-varying covariates | Change in apo A1 | Change in apo B | Change in apo B/apo A1 | |||
| Time, per quarter | −10.76 (−14.42, −7.10) | <0.0001 | 0.80 (0.01, 1.59) | 0.05 | 0.11 (0.07, 0.14) | <0.001 |
| Time2 | 2.81 (2.05, 3.57) | <0.0001 | – | – | −0.03 (−0.03, −0.02) | <0.001 |
| α1AG, mg/dL | −0.16 (−0.25, −0.08) | 0.0003 | 0.03 (−0.05, 0.10) | 0.44 | 0.001 (<0.01, <0.01) | 0.03 |
| Time*creatinine | 0.35 (−0.07, 0.77) | 0.10 | – | – | −0.007 (−0.01, −0.002) | 0.01 |
| Time2*creatinine | −0.14 (−0.25, −0.02) | 0.02 | – | – | 0.003 (−0.01, ≥0.01) | 0.001 |
BMI, body mass index; HD, hemodialysis; AVF, arteriovenous fistula; AVG, arteriovenous graft; PD, peritoneal dialysis; CHF, congestive heart failure; α1AG, alpha 1 acid glycoprotein.
Patients with lower BMI, older individuals and patients with higher serum creatinine concentration had lower levels of apo B. Patients on peritoneal dialysis had significantly higher apo B concentrations when compared with patients on hemodialysis with an arteriovenous fistula or graft. The ratio of apo B/apo A1 was significantly lower in older patients, marginally lower in women and higher among patients with higher BMI.
Changes in lipoproteins over time
During 1 year of observation, the mean apo A1 concentrations decreased from the baseline during the first two quarters and returned to baseline values by the last quarter. Apo B showed no consistent change over time. Since apo B, unlike apo A1, did not vary substantially over time, the temporal relationship of the ratio was mostly related to changes in apo A1 and exhibited a similar (but inverted) curvilinear pattern. Serum creatinine was statistically significantly associated with the pattern of change of apo A1 and the ratio. Specifically, there were statistically significant interactions between creatinine and time, leading to a pattern where the U-shape was ‘wider’ among patients with higher serum creatinine concentrations. However, the inclusion or exclusion of these creatinine interactions with time did not substantively change the other primary associations of interest in the models (data not shown). We retained these terms in our models because of our prespecified modeling strategy.
Association of lipoprotein changes with inflammation
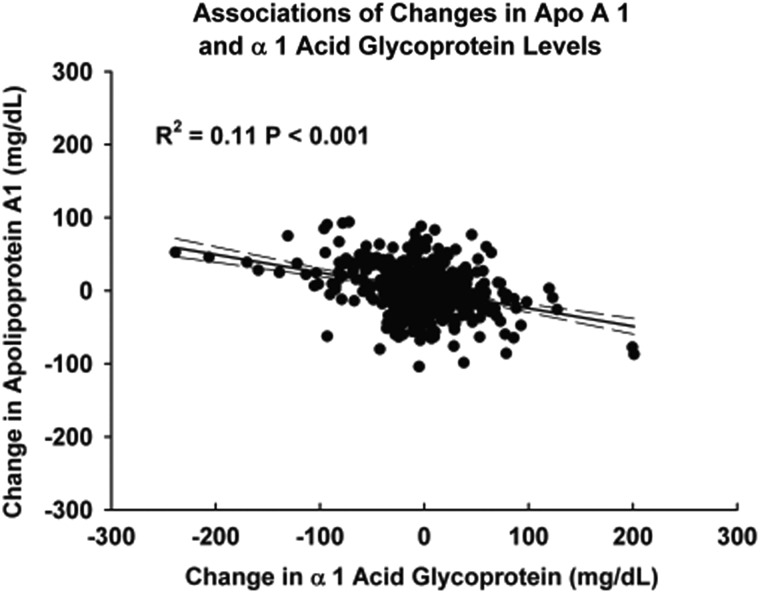
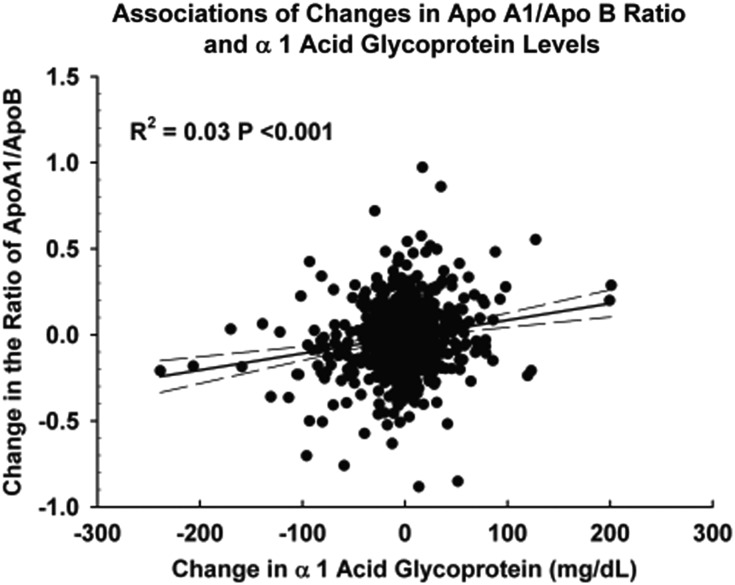
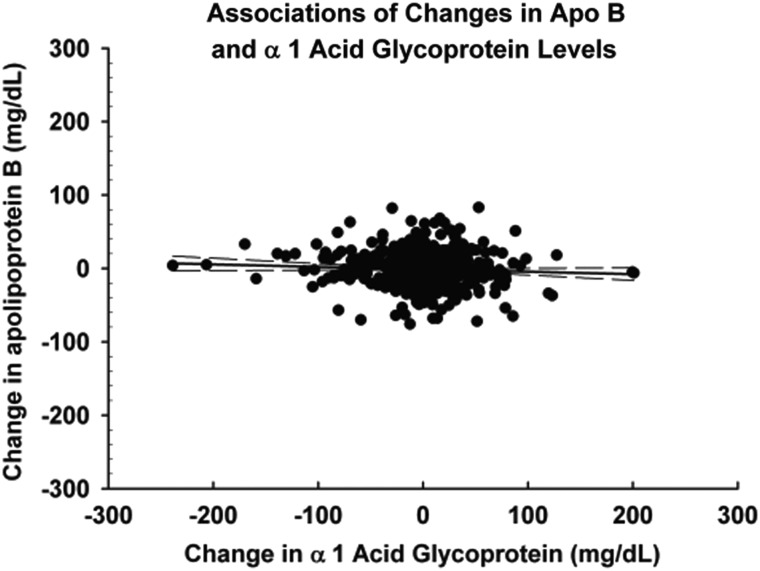
Figures 1–3 demonstrate the unadjusted relationships between changes in the levels of α1AG and changes in apolipoprotein concentrations. Increases in α1AG were associated with decreases in apo A1 (Figure 1) (r2 = 0.11, P < 0.0001) and increases in the ratio of apo B/apo A1 (Figure 2) (r2 = 0.03 P < 0.0001). Changes in CRP exhibited similar associations (data not shown). In contrast to apo A1, changes in neither α1AG (Figure 3) (r2 = 0.003 P = 0.13) nor in CRP (data not shown) were associated with changes in apo B concentration.
FIGURE 1:
Association of changes in the apo A1 concentration and changes in the α1AG concentration between serial measurements. Changes in the levels of the two proteins were statistically significantly inversely correlated. r2 = 0.11, P < 0.0001.
FIGURE 2:
Association of changes in the apoB/Apo A1 ratio and changes in the α1 AG concentration between serial measurements. Changes in the ratio of the two apolipoproteins and changes in the concentration of α1AG were statistically significantly directly correlated. r2 = 0.03, P < 0.0001.
FIGURE 3:
Association of changes in the apo B concentration and changes in the α1AG concentration between serial measurements. There was no significant relationship between changes in α1AG and changes in the apo B concentration. r2 = 0.003, P = 0.13.
Adjusted analyses are presented in Table 2 and Supplementary data, Tables S1 and S2. Changes in α 1AG were associated inversely with changes in apo A1 and directly with that in the ratio of apo B/apo A1, and were not independently associated with changes in apo B (Table 2). Changes in CRP were significantly inversely associated with changes in apo A1, but not with apo B or the ratio of apo B/apo A1 (Supplementary data, Table S1). When changes in both inflammatory markers were considered simultaneously, the estimated associations with both inflammatory markers were attenuated (Supplementary data, Table S2). The associations of changes in α1AG with changes in apo A1 and changes in the ratio of apo B/apo A1 remained statistically significant. Although changes in CRP were also inversely related to changes in apo A1, the association was no longer significant once α1AG was accounted for in the model (Supplementary data, Table S2). Consequently, based on our model building process, our final models included only α 1AG as a measure of inflammation (Table 2).
DISCUSSION
In this cohort of patients new to dialysis, we found that associations of lipoproteins with patient characteristics mostly paralleled those observed in the general population. Longitudinal data showed that apo A1 concentrations exhibited a curvilinear trajectory, first decreasing, then increasing and returning to approximately baseline levels after 1 year. In contrast, apo B levels did not show a consistent pattern of change over a 1-year period, and consequently the apo B/apo A1 ratio varied mostly according to changes in the apo A1 concentration. Changes in apo A1, but not apo B showed a significant pattern of association with markers of inflammation.
Apo A1 is the principal apolipoprotein contained in HDL [21], and apo B occurs as a single molecule in LDL, very-low-density lipoprotein (VLDL) and chylomicron and VLDL remnant particles making up intermediate density lipoproteins. Apo A1, apo B and the ratio of apo B/A1 are associated with cardiovascular risk in non-ESRD and ESRD populations [22]. HDL levels decrease with increasing adiposity in individuals without CKD [23], and low HDL cholesterol and low apo A1 are risk factors for loss of kidney function [24, 25] as is a higher apoB/A1 ratio [26]. Our study indicated that Apo A1, but not apo B, was associated with the comorbidity of atherosclerosis, in contrast to what was reported in the ARIC study [27]. The relation between high BMI and low HDL is progressively attenuated as eGFR declines [4], and so it is interesting that we observed an inverse association between BMI and apo A1 even in this cohort of patients on dialysis. HDL cholesterol has not been previously observed to be associated with age in cross-sectional studies [28–31], and HDL levels have been observed to decrease with time in prospective observational studies [32–34] among individuals without kidney disease. In contrast, we found that apo A1 was higher among older patients in our cohort.
The relation between inflammation and apolipoprotein levels may be complex and bi-directional. Inflammation and the acute phase response cause increases in triglyceride levels as a consequence of increased synthesis of VLDL [35], while both LDL and HDL cholesterol concentrations are decreased in the setting of inflammation in the general population [36, 37]; these changes may play a role in altering vascular structure [36, 38]. While apo AI has been described as an agent that directly suppresses cytokine production [39, 40] and thus potentially plays an anti inflammatory role, infectious events have been documented to both alter endothelial function and suppress apo A1 levels and increase apo B/A1 ratios in patients without kidney disease [41]. TNF α has been shown to directly downregulate Apo A1 gene expression [42, 43] and HDL levels have been reported to be decreased following infectious events in previously healthy children [44]. In addition, inflammation could act indirectly to lower HDL and LDL by causing anorexia. On the other hand, atherosclerotic plaques resulting from increased levels of triglyceride-rich and apo B-rich lipoproteins are thought to be one source of inflammation [45–47], linking lipoprotein levels to inflammation through a causal path. In addition, adiposity may increase levels of apo B-containing lipoproteins, decrease HDL and also be a source of inflammation [48, 49]. Our data support an inverse association between episodic inflammation and HDL levels among patients on dialysis, consistent with the possibility that decreases in HDL could mediate some of the increased cardiovascular risk observed in this population. Although the correlation between changes in apo A1 and α1AG was statistically significant, indicating that there is enough evidence in the data to demonstrate a non-zero correlation between them at the α = 0.05 level, the estimated r2 was quite modest at 0.11 (corresponding to a correlation of 0.33). Thus, only an estimated 11% of the variability in changes in apo A1 was linked to changes in inflammation identified by changes in α1AG. Apo A1 levels as well as HDL levels are controlled by the activities of enzymes [cholesterol ester transfer protein (CETP), lecithin cholesterol acyltransferase protein (LCAT), the activity of the ATP binding cassette transporter (ABCA1)], as well as the rate of catabolism of HDL and of its constitutive apolipoproteins. These effects may not be directly associated with changes in inflammation. In the case of LCAT, some enzymes are suppressed in the presence of renal failure [50]. Activity of the ABCA1 cassette has anti-inflammatory effects in some tissues, and heterogeneity in the expression of this protein may therefore modify the effect of inflammation [40, 51]. Although CETP activity and mass are lower during acute inflammation [52], it would be incorrect to assert that all of the longitudinal variability in HDL or in its constitutive apolipoproteins is directly caused by the inflammatory response or that the quantitative effect of inflammation would be numerically identical in all individuals.
Acute inflammation in experimental models results in increased VLDL secretion accompanied by an increase in apo B [53, 54]. However, we did not find a change in apo B during episodes of inflammation [0.03 (95% CI −0.05 to 0.10) per 1 mg/dL change in α1AG]. Apo B does not appear to be functioning as an acute phase protein, and our data do not support inflammation as an explanation for the paradoxical association of high LDL with lower mortality. It has been hypothesized that the paradoxical association of high LDL with lower mortality could be related to suppression of LDL in the setting of inflammation and anorexia. However, our data do not support this hypothesis since apo B did not change significantly with changes in markers of inflammation (Figure 3). The direct relation between BMI and apo B is likely the result of increases in apo B containing lipoproteins associated with adiposity [55]; the inverse relation with creatinine may reflect a modification of this effect by representing that part of higher BMI is due to larger muscle mass [56]. These findings raise the possibility that body composition or nutritional status may be a more important mediator than inflammation of the association of lower LDL with higher mortality among patients on dialysis or that effects of inflammation on apolipoproteins are mediated by changes in body composition.
The apo B/A1 ratio has been found to be a stronger predictor of myocardial infarction and cardiovascular death than the ratio of total cholesterol to HDL cholesterol in persons with normal kidney function [22, 57, 58], and the apo B/A1 ratio may be more practical in the research setting because determinations are unaffected by whether samples are obtained in the fasting state [59]. The changes in the ratio of apo B/A1 that we observed were largely related to changes in apo A1, which themselves varied inversely with inflammation as observed here and reported by others [35–38].
This study has several strengths, including the relatively large sample size, national sampling frame and repeated measures of apolipoproteins and inflammatory markers. However, important limitations of our study should be acknowledged. First, we did not update information on weight or comorbidities after baseline assessment, which could lead to underestimation of associations with changes in apolipoproteins and which precluded us from assessing the extent to which changes in BMI are associated with changes in apolipoprotein concentrations. In addition, we did not make direct measures of body composition, instead using BMI and serum creatinine concentration as surrogates for adiposity and muscle mass, respectively, again potentially underestimating associations among body composition and apolipoproteins. Finally, while we adjusted for several important covariates in our multivariable analyses, we did not collect an infinite number of covariates in the CDS owing to its design; residual confounding could explain some of the associations described here. While the association between markers of inflammation and lipoprotein concentrations was modest, these findings do support a meaningful role of inflammation in determining the characteristic findings of dyslipidemia in the ESRD population.
In conclusion, we found that body composition was associated with apolipoprotein concentrations, with lower apo A1, higher apo B and a lower ratio of apo B/A1 among patients with higher BMI. Inflammatory markers were clearly associated with changes in apo A1 but not apo B levels over 1 year, suggesting that reduction in HDL cholesterol may be one mechanism by which inflammation confers higher cardiovascular risk among patients with ESRD and that, in contrast, apo B concentrations may be more closely related to nutritional status than to inflammation.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
ACKNOWLEGEMENTS
This study was supported by the National Institutes of Health (Contracts N01-DK-1-2450 to G.M.C. and N01-DK-7-0005 to K.L.J.). The interpretation and reporting of the data presented here are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government. The authors would like to acknowledge the technical contribution of Tjien Dwyer.
CONFLICT OF INTEREST STATEMENT
None of this material has been published elsewhere other than in abstract form.
None of the authors have any conflict of interest with the content of this manuscript. Specifically, no authors are shareholders of companies engaged in measuring acute phase proteins, apolipoproteins or other cholesterol-related serum parameters or engaged in the development, production or distribution of pharmaceutical products related to lowering lipoprotein-containing cholesterol particles. Dr Kaysen serves as a consultant for Merck and for Renal Research Institute and has grant support from Dialysis Clinics Incorporated (DCI).
Supplementary Material
REFERENCES
- 1.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. Erratum in: N Engl J Med 2008;18(4):4 doi:10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Bakris G, Vassalotti J, Ritz E, et al. CKD Consensus Working Group. National Kidney Foundation consensus conference on cardiovascular and kidney diseases and diabetes risk: an integrated therapeutic approach to reduce events. Kidney Int. 2010;78:726–736. doi: 10.1038/ki.2010.292. doi:10.1038/ki.2010.292. [DOI] [PubMed] [Google Scholar]
- 3.de Boer IH, Astor BC, Kramer H, et al. Lipoprotein abnormalities associated with mild impairment of kidney function in the multi-ethnic study of atherosclerosis. Clin J Am Soc Nephrol. 2008;3:125–132. doi: 10.2215/CJN.03390807. doi:10.2215/CJN.03390807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lo JC, Go AS, Chandra M, et al. GFR, body mass index, and low high-density lipoprotein concentration in adults with and without CKD. Am J Kidney Dis. 2007;50:552–558. doi: 10.1053/j.ajkd.2007.07.011. doi:10.1053/j.ajkd.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Vaziri ND. Dyslipidemia of chronic renal failure: the nature, mechanisms, and potential consequences. Am J Physiol Renal Physiol. 2006;290:F262–F272. doi: 10.1152/ajprenal.00099.2005. doi:10.1152/ajprenal.00099.2005. [DOI] [PubMed] [Google Scholar]
- 6.Shoji T, Nishizawa Y, Kawagishi T, et al. Intermediate-density lipoprotein as an independent risk factor for aortic atherosclerosis in hemodialysis patients. J Am Soc Nephrol. 1998;9:1277–1284. doi: 10.1681/ASN.V971277. [DOI] [PubMed] [Google Scholar]
- 7.Kuang D, You H, Ding F, et al. Intima-media thickness of the carotid artery and its correlation factors in maintenance hemodialysis patients: a cross-sectional study. Blood Purif. 2009;28:181–186. doi: 10.1159/000227787. doi:10.1159/000227787. [DOI] [PubMed] [Google Scholar]
- 8.Lowrie EG, Lew NL. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis. 1990;15:458–482. doi: 10.1016/s0272-6386(12)70364-5. [DOI] [PubMed] [Google Scholar]
- 9.Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Inverse association between lipid levels and mortality in men with chronic kidney disease who are not yet on dialysis: effects of case mix and the malnutrition-inflammation-cachexia syndrome. J Am Soc Nephrol. 2007;18:304–311. doi: 10.1681/ASN.2006060674. doi:10.1681/ASN.2006060674. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Coresh J, Eustace JA, et al. Association between cholesterol level and mortality in dialysis patients: role of inflammation and malnutrition. JAMA. 2004;291:451–459. doi: 10.1001/jama.291.4.451. doi:10.1001/jama.291.4.451. [DOI] [PubMed] [Google Scholar]
- 11.Kwan BC, Kronenberg F, Beddhu S, et al. Lipoprotein metabolism and lipid management in chronic kidney disease. J Am Soc Nephrol. 2007;18:1246–1261. doi: 10.1681/ASN.2006091006. doi:10.1681/ASN.2006091006. [DOI] [PubMed] [Google Scholar]
- 12.Kilpatrick RD, McAllister CJ, Kovesdy CP, et al. Association between serum lipids and survival in hemodialysis patients and impact of race. J Am Soc Nephrol. 2007;18:293–303. doi: 10.1681/ASN.2006070795. doi:10.1681/ASN.2006070795. [DOI] [PubMed] [Google Scholar]
- 13.Bowden RG, La Bounty P, Shelmadine B, et al. Reverse epidemiology of lipid-death associations in a cohort of end-stage renal disease patients. Nephron Clin Pract. 2011;119:c214–c219. doi: 10.1159/000329509. doi:10.1159/000329509. [DOI] [PubMed] [Google Scholar]
- 14.Chmielewski M, Carrero JJ, Qureshi AR, et al. Temporal discrepancies in the association between the apoB/apoA-I ratio and mortality in incident dialysis patients. J Intern Med. 2009;265:708–716. doi: 10.1111/j.1365-2796.2009.02074.x. doi:10.1111/j.1365-2796.2009.02074.x. [DOI] [PubMed] [Google Scholar]
- 15.Shoji T, Masakane I, Watanabe Y, et al. Committee of Renal Data Registry, Japanese Society for Dialysis Therapy. Elevated non-high-density lipoprotein cholesterol (non-HDL-C) predicts atherosclerotic cardiovascular events in hemodialysis patients. Clin J Am Soc Nephrol. 2011;6:1112–1120. doi: 10.2215/CJN.09961110. doi:10.2215/CJN.09961110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chmielewski M, Verduijn M, Drechsler C, et al. Low cholesterol in dialysis patients—causal factor for mortality or an effect of confounding? Nephrol Dial Transplant. 2011;26:3325–3331. doi: 10.1093/ndt/gfr008. doi:10.1093/ndt/gfr008. [DOI] [PubMed] [Google Scholar]
- 17.Krane V, Winkler K, Drechsler C, et al. German Diabetes and Dialysis Study Investigators. Association of LDL cholesterol and inflammation with cardiovascular events and mortality in hemodialysis patients with type 2 diabetes mellitus. Am J Kidney Dis. 2009;54:902–911. doi: 10.1053/j.ajkd.2009.06.029. doi:10.1053/j.ajkd.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 18.Psychari SN, Sinos L, Iatrou C, et al. Relations of inflammatory markers to lipid levels and autonomic tone in patients with moderate and severe chronic kidney disease and in patients under maintenance hemodialysis. Clin Nephrol. 2005;64:419–427. doi: 10.5414/cnp64419. [DOI] [PubMed] [Google Scholar]
- 19.Kutner NG, Johansen KL, Kaysen GA, et al. The comprehensive dialysis study (CDS): a USRDS special study. Clin J Am Soc Nephrol. 2009;4:645–650. doi: 10.2215/CJN.05721108. doi:10.2215/CJN.05721108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dalrymple LS, Johansen KL, Chertow GM, et al. Longitudinal measures of serum albumin and prealbumin concentrations in incident dialysis patients: the Comprehensive Dialysis Study. J Ren Nutr. 2013;23:91–7. doi: 10.1053/j.jrn.2012.03.001. doi:10.1053/j.jrn.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips MC. New insights into the determination of HDL structure by apolipoproteins. J Lipid Res. 2013;54:2034–48. doi: 10.1194/jlr.R034025. doi:10.1194/jlr.R034025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sniderman AD, Holme I, Aastveit A, et al. Relation of age, the apolipoprotein B/apolipoprotein A-I ratio, and the risk of fatal myocardial infarction and implications for the primary prevention of cardiovascular disease. Am J Cardiol. 2007;100:217–221. doi: 10.1016/j.amjcard.2007.02.086. doi:10.1016/j.amjcard.2007.02.086. [DOI] [PubMed] [Google Scholar]
- 23.Grundy SM, Brewer HB, Cleeman JI, et al. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. doi:10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 24.Goek ON, Köttgen A, Hoogeveen RC, et al. Association of apolipoprotein A1 and B with kidney function and chronic kidney disease in two multiethnic population samples. Nephrol Dial Transplant. 2012;27:2839–2847. doi: 10.1093/ndt/gfr795. doi:10.1093/ndt/gfr795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J, Muntner P, Hamm LL, et al. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med. 2004;140:167–174. doi: 10.7326/0003-4819-140-3-200402030-00007. doi:10.7326/0003-4819-140-3-200402030-00007. [DOI] [PubMed] [Google Scholar]
- 26.Lundberg S, Gunnarsson I, Jacobson SH. Impact of the apolipoprotein B/apolipoprotein A-I ratio on renal outcome in immunoglobulin A nephropathy. Scand J Urol Nephrol. 2012;46:148–155. doi: 10.3109/00365599.2011.644635. doi:10.3109/00365599.2011.644635. [DOI] [PubMed] [Google Scholar]
- 27.Muntner P, He J, Astor BC, et al. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: results from the atherosclerosis risk in communities study. J Am Soc Nephrol. 2005;16:529–538. doi: 10.1681/ASN.2004080656. doi:10.1681/ASN.2004080656. [DOI] [PubMed] [Google Scholar]
- 28.Heiss G, Tamir I, Davis CE, et al. Lipoprotein-cholesterol distributions in selected North America populations: the Lipid Research Clinics Program Prevalence Study. Circulation. 1980;61:302–315. doi: 10.1161/01.cir.61.2.302. doi:10.1161/01.CIR.61.2.302. [DOI] [PubMed] [Google Scholar]
- 29.Heitmann BL. The effects of gender and age on associations between blood lipid levels and obesity in Danish men and women aged 35–65 years. J Clin Epidemiol. 1992;45:693–702. doi: 10.1016/0895-4356(92)90046-p. doi:10.1016/0895-4356(92)90046-P. [DOI] [PubMed] [Google Scholar]
- 30.Abbott RD, Garrison RJ, Wilson PW, et al. Joint distribution of lipoprotein cholesterol classes: the Framingham Study. Arteriosclerosis. 1983;3:260–272. doi: 10.1161/01.atv.3.3.260. doi:10.1161/01.ATV.3.3.260. [DOI] [PubMed] [Google Scholar]
- 31.Ettinger WH, Wahl PW, Kuller LH, et al. for the CHS Collaborative Research Group. Lipoprotein lipids in older people: results from the Cardiovascular Health Study. Circulation. 1992;86:858–869. doi: 10.1161/01.cir.86.3.858. doi:10.1161/01.CIR.86.3.858. [DOI] [PubMed] [Google Scholar]
- 32.Anderson KM, Wilson PWF, Garrison RJ, et al. Longitudinal and secular trends in lipoprotein cholesterol measurements in a general population sample: the Framingham offspring study. Atherosclerosis. 1987;68:59–66. doi: 10.1016/0021-9150(87)90094-3. doi:10.1016/0021-9150(87)90094-3. [DOI] [PubMed] [Google Scholar]
- 33.Hubert HB, Eaker ED, Garrison RJ, et al. Life-style correlates of risk factor change in young adults: an eight-year study of coronary heart disease risk factors in the Framingham offspring. Am J Epidemiol. 1987;125:812–831. doi: 10.1093/oxfordjournals.aje.a114598. [DOI] [PubMed] [Google Scholar]
- 34.Wilson PWF, Anderson KM, Harris T, et al. Determinants of change in total cholesterol and HDLC with age: the Framingham study. J Gerontol. 1994;49:M252–M257. doi: 10.1093/geronj/49.6.m252. doi:10.1093/geronj/49.6.M252. [DOI] [PubMed] [Google Scholar]
- 35.Khovidhunkit W, Kim MS, Memon RA, et al. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res. 2004;45:1169–1196. doi: 10.1194/jlr.R300019-JLR200. doi:10.1194/jlr.R300019-JLR200. [DOI] [PubMed] [Google Scholar]
- 36.Liuba P, Persson J, Luoma J, et al. Acute infections in children are accompanied by oxidative modification of LDL and decrease of HDL cholesterol, and are followed by thickening of carotid intima-media. Eur Heart J. 2003;24:515–521. doi: 10.1016/s0195-668x(02)00750-9. doi:10.1016/S0195-668X(02)00750-9. [DOI] [PubMed] [Google Scholar]
- 37.van Leeuwen HJ, Heezius EC, Dallinga GM, et al. Lipoprotein metabolism in patients with severe sepsis. Crit Care Med. 2003;31:1359–1366. doi: 10.1097/01.CCM.0000059724.08290.51. doi:10.1097/01.CCM.0000059724.08290.51. [DOI] [PubMed] [Google Scholar]
- 38.Sammalkorpi K, Valtonen V, Kerttula Y, et al. Changes in serum lipoprotein pattern induced by acute infections. Metabolism. 1988;37:859–865. doi: 10.1016/0026-0495(88)90120-5. doi:10.1016/0026-0495(88)90120-5. [DOI] [PubMed] [Google Scholar]
- 39.Yin K, Deng X, Mo ZC, et al. Tristetraprolin-dependent post-transcriptional regulation of inflammatory cytokine mRNA expression by apolipoprotein A-I: role of ATP-binding membrane cassette transporter A1 and signal transducer and activator of transcription 3. J Biol Chem. 2011;286:13834–13845. doi: 10.1074/jbc.M110.202275. doi:10.1074/jbc.M110.202275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yin K, Chen WJ, Zhou ZG, et al. Apolipoprotein A-I inhibits CD40 proinflammatory signaling via ATP-binding cassette transporter A1-mediated modulation of lipid raft in macrophages. J Atheroscler Thromb. 2012;19:823–836. doi: 10.5551/jat.12823. doi:10.5551/jat.12823. [DOI] [PubMed] [Google Scholar]
- 41.Kilickap M, Goksuluk H, Candemir B, et al. Evaluation of acute infection-induced endothelial dysfunction and its potential mediators. Acta Cardiol. 2011;66:581–587. doi: 10.1080/ac.66.5.2131082. [DOI] [PubMed] [Google Scholar]
- 42.Orlov SV, Mogilenko DA, Shavva VS, et al. Effect of TNFalpha on activities of different promoters of human apolipoprotein A-I gene. Biochem Biophys Res Commun. 2010;398:224–230. doi: 10.1016/j.bbrc.2010.06.064. doi:10.1016/j.bbrc.2010.06.064. [DOI] [PubMed] [Google Scholar]
- 43.Beers A, Haas MJ, Wong NCW, et al. Inhibition of apolipoprotein AI gene expression by tumor necrosis factor α: roles for MEK/ERK and JNK signaling. Biochemistry. 2006;45:2408–2413. doi: 10.1021/bi0518040. doi:10.1021/bi0518040. [DOI] [PubMed] [Google Scholar]
- 44.Liubaa P, Perssonb J, Luomac J, et al. Acute infections in children are accompanied by oxidative modification of LDL and decrease of HDL cholesterol, and are followed by thickening of carotid intima–media. European Heart Journal. 2003;24:515–521. doi: 10.1016/s0195-668x(02)00750-9. doi:10.1016/S0195-668X(02)00750-9. [DOI] [PubMed] [Google Scholar]
- 45.Epstein F. Atherosclerosis an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. doi:10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 46.Haverkate F, Thompson SG, Pyke SD, et al. Production of C-reactive protein and risk of coronary events in stable and unstable angina: European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. Lancet. 1997;349:462–466. doi: 10.1016/s0140-6736(96)07591-5. doi:10.1016/S0140-6736(96)07591-5. [DOI] [PubMed] [Google Scholar]
- 47.Toss H, Lindahl B, Siegbahn A, et al. Prognostic influence of increased fibrinogen and C-reactive protein levels in unstable coronary artery disease. Circulation. 1997;96:4204–4210. doi: 10.1161/01.cir.96.12.4204. doi:10.1161/01.CIR.96.12.4204. [DOI] [PubMed] [Google Scholar]
- 48.Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miida T, Miyazaki O, Hanyu O, et al. LCAT-dependent conversion of prebeta1-HDL into alpha-migrating HDL is severely delayed in hemodialysis patients. J Am Soc Nephrol. 2003;14:732–738. doi: 10.1097/01.asn.0000046962.43220.8a. doi:10.1097/01.ASN.0000046962.43220.8A. [DOI] [PubMed] [Google Scholar]
- 51.Westerterp M, Murphy AJ, Wang M, et al. Deficiency of ABCA1 and ABCG1 in macrophages increases inflammation and accelerates atherosclerosis in mice. Circ Res. 2013;112:1456–65. doi: 10.1161/CIRCRESAHA.113.301086. doi:10.1161/CIRCRESAHA.113.301086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jahangiri A, de Beer MC, Noffsinger V, et al. HDL remodeling during the acute phase response arterioscler. Thromb Vasc Biol. 2009;29:261–267. doi: 10.1161/ATVBAHA.108.178681. doi:10.1161/ATVBAHA.108.178681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lippi G, Braga V, Adami S, et al. Modification of serum apolipoprotein A-I, apolipoprotein B and lipoprotein(a) levels after bisphosphonates-induced acute phase response. Clin Chim Acta. 1998;271:79–87. doi: 10.1016/s0009-8981(97)00212-x. doi:10.1016/S0009-8981(97)00212-X. [DOI] [PubMed] [Google Scholar]
- 54.Cabana VG, Siegel JN, Sabesin SM. Effects of the acute phase response on the concentration and density distribution of plasma lipids and apolipoproteins. J Lipid Res. 1989;30:39–49. [PubMed] [Google Scholar]
- 55.Kesäniemi YA, Beltz WF, Grundy SM. Comparisons of metabolism of apolipoprotein B in normal subjects, obese patients, and patients with coronary heart disease. J Clin Invest. 1985;76:586–595. doi: 10.1172/JCI112010. doi:10.1172/JCI112010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Noori N, Kopple JD, Kovesdy CP, et al. Mid-arm muscle circumference and quality of life and survival in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2010;5:2258–2268. doi: 10.2215/CJN.02080310. doi:10.2215/CJN.02080310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walldius G, Jungner I, Aastveit AH, et al. The apoB/apoA-I ratio is better than the cholesterol ratios to estimate the balance between plasma proatherogenic and antiatherogenic lipoproteins and to predict coronary risk. Clin Chem Lab Med. 2004;42:1355–1363. doi: 10.1515/CCLM.2004.254. doi:10.1515/CCLM.2004.254. [DOI] [PubMed] [Google Scholar]
- 58.Sniderman AD, Jungner I, Holme I, et al. Errors that result from using the TC/HDL C ratio rather than the apoB/apoA-I ratio to identify the lipoprotein-related risk of vascular disease. J Intern Med. 2006;259:455–461. doi: 10.1111/j.1365-2796.2006.01649.x. Erratum in: J Intern Med 2006 Aug;260(2):186 doi:10.1111/j.1365-2796.2006.01649.x. [DOI] [PubMed] [Google Scholar]
- 59.Wen ZZ, Geng DF, Luo JG, et al. Combined use of high-sensitivity C-reactive protein and apolipoprotein B/apolipoprotein A-1 ratio prior to elective coronary angiography and oral glucose tolerance tests. Clin Biochem. 2011;44:1284–1291. doi: 10.1016/j.clinbiochem.2011.08.1142. doi:10.1016/j.clinbiochem.2011.08.1142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.