Abstract
Alzheimer's disease is the most common form of dementia. Abnormal hyperphosphorylation of Microtubule associated protein tau (MAPT) is one of the hallmarks of Alzheimer's disease and related tau pathies. CDK5 and GSK3B are the two main protein kinases that have an important role in the abnormal hyperphosphorylation of MAPT which leads to Alzheimer's disease. Structural information for both MAPT-CDK5 and MAPT-GSK3B complexes being absent, we resorted to molecular modeling for gaining insight into the mechanism of implication of hyperphosphorylation of MAPT by both enzymes. First the tertiary structure of MAPT was modeled and its active regions were defined. This was followed by molecular docking and interaction studies of MAPT with CDK5 and GSK3B kinases to infer the role of these kinases in abnormal hyperphosphorylation of MAPT protein. In addition, we have investigated the characteristic features such as phosphorylation sites and ATP binding sites of MAPT and two kinases. Further we computed the stabilization centers and stabilization residues of the MAPT protein and two kinases before and after docking process. The overall results portray that CDK5 is strongly involved in the hyperphosphorylation of MAPT when compared to GSK3B.
Keywords: Alzheimer's, MAPT, CDK5, GSK3B, hyperphosphorylation
Background
Alzheimer's disease (AD) is the leading neurological and psychiatric brain disorder characterized by learning and memory destruction affecting more than 24 million people worldwide [1]. Abnormally hyperphosphorylated microtubule associated protein tau (MAPT) is the hallmark of Alzheimer's disease and related taupathies [2]. Pathologically AD is characterized by the formation of two aggregates namely neurofibrillary tangles and senile plaques. Neurofibrillary tangles consisting of filamentous aggregates of hyperphosphorylated tau in microtubules [3, 4]. Certain post translational modifications of tau like phosphorylation allows forming of characteristic paired helical filaments. Tau is the major component of Alzheimer paired helical filaments. MAPT (Microtubule associated protein tau) is a kind of tau protein which occurs in healthy brain and functions in axonal transport, assembly and stabilization of microtubules, in certain pathological conditions it gets hyperphosphorylated in AD brains. The hyperphosphorylation of MAPT leads to form aggregates in neurofibrillary tangles, which are the main causes of AD [5]. Two proline rich kinases such as Cyclin dependent kinase5 (CDK5) and Glycogen synthase kinase3B (GSK3B) act as putative mediators in aberrant hyperphosphorylation of MAPT protein at proline directed sites [6]. Over activation of CDK5 and GSK3B kinases enhances the formation of neurofibrillary tangles in AD brain by MAPT hyperphosphorylation [7]. CDK5 is a serine/threonine protein kinase which involves in the processes of neuronal migration, synaptic function, myogenesis, neurite outgrowth and controlling the differentiation of nerve cells [8, 9]. The activity of CDK5 depends on interaction with an activator. P25 and P35 are the activators of CDK5 that functionally involve in the hyperphosphorylation of tau protein [10, 11]. Calpain is a calcium dependent protease which is responsible for the progression of p35/p25 activators; moreover increase in calpain activity enhancing the p25 levels and contributes to CDK5 activity in the AD brain. Tau phosphorylated by CDK5, loses its ability to bind with microtubules, revealing that CDK5- mediated tau phosphorylation disrupts the normal functions of tau. Therefore CDK5 is coined as a potential candidate for a significant role in the hyperphophorylation of MAPT in AD [12]. GSK3B (Glycogen Synthase Kinase B) is a multifunctional serine/ threonine protein kinase which is involved in the regulation of glycogen synthesis and has the ability to phosphorylate and inactivates the glycogen synthase [13, 14, 15]. In addition to glucose metabolism it intermediates in many extra cellular pathways, involved in energy metabolism, neuronal cell development and body pattern formation [16]. Deregulation of GSK3B kinase contributes to the pathogenesis of Alzheimer's disease by decreasing the levels of nuclear β- catenin and results in tau phosphorylation [17].
Overexpression of GSK3B decreases the levels of nuclear β- catenin, and results in tau hyperphosphorylation and neurodegeneration in AD brain [18]. Shi Jie Liu et al. suggested that inhibition of GSK3B activity abolishes hyperphosphorylation of MAPT and spatial memory impairment. From these results it is strongly implied that the role of GSK3B is important for the formation of neurofibrillary tangles, abnormalities of AD as well as the spatial memory impairment. Both GSK3B and CDK5 are involved in the regulation of hyperphosphorylation of MAPT by catalyzing the phosphorylation of serine, threonine and tyrosine residues of MAPT using ATP as phosphate donor .The association between GSK3B, CDK5 and tau has been confirmed by isolating them in a complex with neurofibrillary tangles from AD affected brain. The two proline directed protein kinases, GSK3B and CDK5 are strongly associated with hyperphosphorylation of MAPT and also implicates in AD pathiese [19, 20]. In this present study, the various structural and functional characteristics of MAPT, GSK3B and CDK5 proteins have been analyzed. The tertiary structure of MAPT protein has been modeled and its interaction with GSK3B and CDK5 kinases has been analyzed using insilico docking methods. The important phosphorylation sites of MAPT and other two kinases were predicted and the significance of phosphorylated sites which leads to hyperphosphorylation of MAPT was analyzed. Active sites which associated in the binding of MAPT with GSK3B and CDK5 were also examined. The role of two protein kinases GSK3B and CDK5 in hyperphosphorylation of MAPT in Alzheimer's disease has been investigated.
Methodology
Sequence retrieval and modeling:
The sequences of the three proteins, namely MAPT, CDK5 and GSK3B were retrieved from NCBI (http://www. ncbi.nlm.nih.gov) protein database. The x- ray crystallographic structure of CDK5 and GSK3B were already available and were directly taken from PDB [21]. However, the x- ray crystallographic structure of MAPT protein is not available in PDB and other protein databases. So, we first modeled three dimensional structure of MAPT protein to analyze its interaction studies with other two proteins. The complete sequence of MAPT (358 aa) was submitted to NCBI BLAST [22] tool for detecting the homologues sequences against PDB database. However, there was no homologues were identified with the identity percentage of 40% for homology modeling. So protein threading method was used to construct the tertiary structure of MAPT using RAPTOR version 3.0 [23]. RAPTOR contains about 6000 non-redundant structural files within its template library for the modeling task. From the available structures, structures with CATH hierarchical classification alone were selected for protein threading. For this a list of CATH classified PDB codes were first cross-referenced with the RAPTOR structure library. In addition, templates with missing structural regions in the PDB files were excluded from the template set. The resulting template library contained 2938 structures. The protein sequence of MAPT with 358 amino acids was given as input for threading against the selected 2938 structures. The alignments between the target sequence and the template database were generated using the non pair wise core threading algorithm. The stereochemical quality of the modeled structure was validated with PROCHECK [24] and the best model was selected after stereo correctness. Finally, energy minimization was performed for the three protein molecules namely model of MAPT and crystal structure of CDK5 and GSK3B with the help of Swiss PDB Viewer [25].
Binding site prediction:
To determine the binding affinities of MAPT with two protein kinases CDK5 and GSK3B, the active sites of modeled MAPT protein was predicted using Q-Site Finder [26]. The Q-Site Finder algorithm is based on calculating the Vander walls interaction energy with the protein for aliphatic carbons for the probes on a grid and, retaining the probes with favorable interactions. Structural domains are the independently stable element in a protein structure. In Q-site finder the clusters of probes with favorable interactions are ranked according to their total interaction energies, ligand binding site tends to be among the highest ranking pockets.
Protein protein docking:
GRAMMX v.1.2 [27] was used for docking the two protein kinases GSK3B and CDK5 with the modeled MAPT protein. In protein docking studies, we consider MAPT protein as receptor and two kinases GSK3B and CDK5 as ligands. GRAMMX employs grid projection of a smoothed lennard jones potential, combined with a post docking procedure of rigid-body minimization and structure clustering for producing best docked complexes. The GRAMM-X output generates ten models and on the basis of hydrogen bond affinity between the atoms of two proteins; the best docked complex was selected for further analysis. The complex structures were viewed with Pymol viewer [28].
Prediction of phosphorylation profile:
Phosphorylation is an important nature of protein post translational modification (PTM). Protein phosphorylation plays a significant role in a wide range of cellular processes. Identification of phosphorylation sites of a protein is important for understanding and analyzing its functions. In this study, we used three different servers for predicting the phophorylation sites of MAPT protein namely NetPhos 2.0 [29], DISPHOS 1.3 [30], and PostMod [31]. NetPhos 2.0 [29] uses artificial neural networks to predict 17 kinase family specific phosphorylation sites in input protein sequence. DISPHOS [30] (DISorder enhanced PHOSphorylation site predictor) uses position specific amino acid composition method to predict structural disorder information for differentiate phosphorylation and non-phosphorylation sites of a given protein sequence. PostMod [31] uses the BLOSUM62 matrix-based similarity measure and profile-profile alignment scores with a noise- reducing algorithm to predict post translational modifications of the query protein with a protein kinase.
Binding affinity analysis:
ATP binding residues of GSK3B and CDK5 protein kinases were predicted by using ATPint online server [32]. ATPint is a web based tool for the prediction of ATP binding residue in a protein sequence. In addition stabilization potential of the complex structure is computed by two programs namely Stabilization center (Scide) [33] and Stabilization residue (Sride) [34]. Scide program predicts the number of residues which acts as stabilization center in the complex as well as in the individual molecule. Stabilization center is an artificial network to predict the key residues acting as stabilization centers of the complex protein. Sride program calculates the number of stabilization residues in the complex. Both the algorithms can predict the stability of the structures by numbering the residues.
Results
Protein Models:
The crystal structures of CDK5 (PDB id: 1UNH_A) and GSK3B (PDB id: 1UV5) being available were directly retrieved from PDB database [21]. However for MAPT Protein neither crystal structure nor template is available for homology modeling. So, protein threading method was used to construct the 3D structure of MAPT using RAPTOR version 3.0 [22]. RAPTOR successfully generated 20 structural models (sequence-template alignments) for the target protein MAPT and a model with the highest Z-score of 0.39 was selected as the tertiary structure for MAPT. The modeled tertiary structure of MAPT protein is shown in (Figure 1). The stereo chemical quality of the developed model was validated by PROCHECK program [24] using Ramachandran plots. The Ramachandran plot analysis revealed that in the backbone dihedral angle distributions of amino acid residues 77.1% in the most favored, 17.5% in additional allowed, and 3.3 % in generously allowed regions. This ensures that the quality of the generated model was good. The binding sites of the developed model were predicted using Q-site finder [26]. There were in total 10 ligand binding site pockets in the MAPT protein. The binding pockets are shown in (Figure 2).
Figure 1.
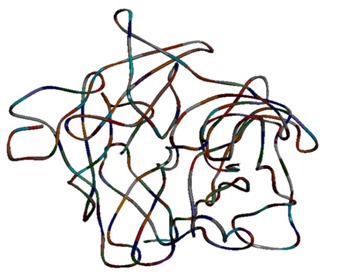
Modelled tertiary structure of human MAPT
Figure 2.
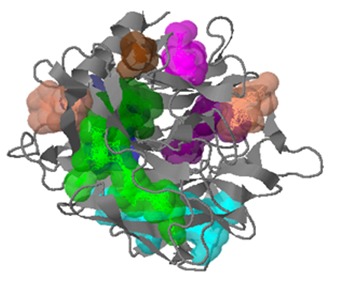
Predicted binding pockets of MAPT showing all its 10 binding sits in different colors
Docking studies of MAPT with CDK5 and GSK3B:
We have analyzed the mode of interaction of MAPT with two kinases CDK5 and GSK3B individually using GRAMM-X program [27] to find their association in hyperphosphorylation. Binding conformations of MAPT-CDK5 complexes are displayed in Figure 3a and the individual hydrogen bond formation between the interacting residues of CDK5 and MAPT are shown in (Figure 3b, 3c, 3d, 3e, 3f, 3g & 3h). Hydrogen bonds create significant contributions to the interactions between receptor and ligand. The docking interactions of amino acid residues of MAPT and CDK5 with interatomic distances are presented in Table 1 (see supplementary material). The docking results of MAPT-CDK5 revealed that, polar amino acid residues present in the active site region of MAPT were strongly involved in the protein-protein interactions. The residues GLN6, GLY9, ARG15, ARG56, ALA66, LYS209 and THR205 were the indispensable residues for accounting hydrogen bonding with amino acid residues of CDK5. Further, all the docked regions of MAPT were in coil in structure and showed that coiled regions of MAPT were binding effectively with CDK5 when compared with α-helices and b-sheet regions. Binding conformations of MAPT-GSK3B complex is displayed in Figure 4a and the individual hydrogen bond formation between the interacting residues of MAPT and GSK3B are shown in Figure 4b & 4c. The docking interactions of amino acid residues of MAPT and GSK3B with interatomic distances are presented in Table 2 (see supplementary material). The docking results revealed that only two residues of MAPT namely THR95 and GLY76 were involved in hydrogen bonding interaction with the residues of GSK3B.
Figure 3.
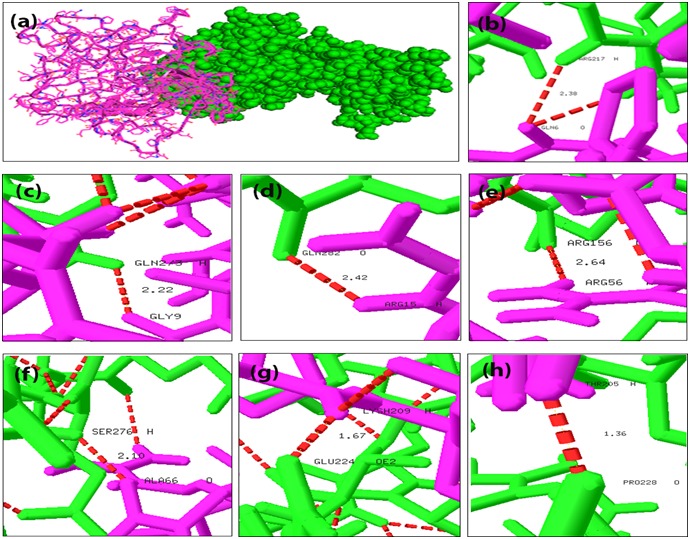
Docking interaction of MAPT with CDK5 and its interatomic hydrogen bonds with distances. Red color dash lines represents the hydrogen bond interactions between the amino acid residues: a) Docked complex structure of MAPT (cartoon representation in pink) and CDK5 (sphere representation in green); b) Interatomic hydrogen bonds between GLN6 and ARG217 with distance; c) Interatomic hydrogen bonds between GLY9 and GLN273; d) Interatomic hydrogen bonds between ARG15 and GLN282; e) Interatomic hydrogen bonds between ARG56 and ARG 156; f) Interatomic hydrogen bonds between ALA66 and SER276; g) Interatomic hydrogen bonds between LYS209 and GLU224; h) Interatomic hydrogen bonds between THR205 and PRO 228.
Figure 4.
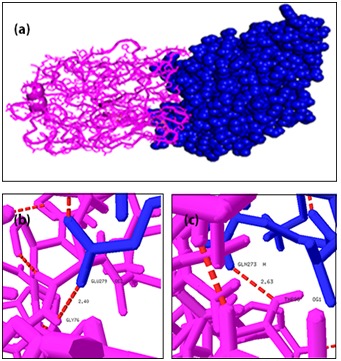
Docking interaction of MAPT with GSK3B and its interatomic hydrogen bonds with distances. Red color dash lines represent the hydrogen bonds interactions between the amino acid residues: a) Docked complex structure of MAPT (cartoon representation in pink) and GSK3B (sphere representation in blue); b) Interatomic hydrogen bonds between THR95 and GLN 273; c) Interatomic hydrogen bonds between GLY 76 and GLU 279.
Phosphorylation site predictions:
Recognition of phosphorylation sites are related to many functional features. The phosphorylation sites of Microtubule associated protein tau (MAPT) predicted using three different severs namely NetPhos2.0 [29], DISPHOS1.3 [30], and PostMod [31]. Netphos and DISPHOS predict the phosphorylation sites of MAPT protein and are shown in Figure 5 & Figure 6 respectively. In (Figure 5) sequence position is plotted against phosphorylation potential of the MAPT protein. In Figure 6 the phosphorylation sites are plotted as amino acid position and DISPHOS score. The servers predict overall 39 (27 Serine, 9 threonine and 3 tyrosine) phosphorylation sites for MAPT. PostMod predicts the post translational modification sites of MAPT protein with the influence of CDK5 and GSK3B kinases. The specific phosphorylation sites for CDK5 and GSK3B kinases with MAPT were analyzed using PostMod. When CDK5 was selected as candidate for phosphorylating MAPT, out of the total 39 phosphorylation sites of MAPT, 14 of them were recognized as phosphorylation sites for CDK5. However, in case of GSK3B, only five sites were recognized as phosphorylation sites. These results imply that hyperphosphorylation of MAPT is regulated by both CDK5 and GSK3B, however the activity of CDK5 is more than that of GSK3B. The three residues of MAPT such as T53, T117 and S315 are commonly phosphorylated by both kinases. The result of post translational modification of MAPT with CDK5 and GSK3B are presented in Tables 3 & 4 (see supplementary material) respectively.
Figure 5.
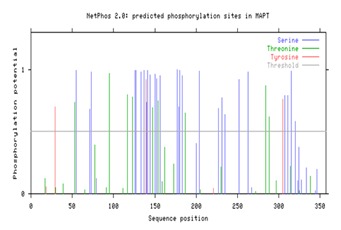
Phosphorylation sites present in MAPT protein. Serine, Threonine and Tyrosine sites are displayed in colours blue, green and red respectively.
Figure 6.
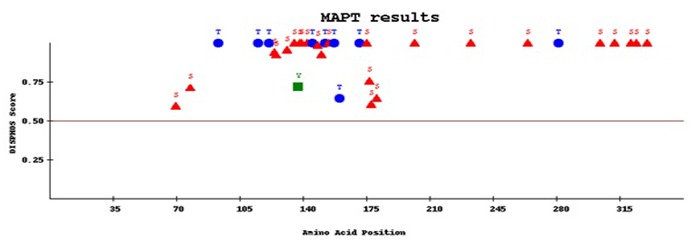
The number of disorder enhanced phosphorylation sites of MAPT. Red colored triangles denotes the serine residues, blue colored elliptical shapes denotes threonine sites and green colored square areas denoted the tyrosine sites.
ATP Binding site analysis:
Predicting Adenosine Tri Phosphate (ATP) binding sites is essential for analyzing the phosphorylation process and function of the particular protein. ATP interacting residues in the CDK5 and GSK3B protein kinases were predicted by using ATPint server. The numbers of ATP interacting residues in CDK5 were 70 and in GSK3B were 102. ATP binding pockets were located around the active sites of the both protein kinases. The predicted ATP interacting residues of CDK5 and GSK3B are presented in Table 5 (see supplementary material).
Prediction of stabilization center and stabilizing residues in binding residues:
The stabilization center for the two complexes MAPT-CDK5 and MAPT-GSK3B was computed using the Scide [33] program. The results of stabilization center are presented in Table 6 (see supplementary material). In case of CDK5 many number of residues which act as stabilization center were also involved in binding with the MAPT molecule. Among the CDK5 and GSK3B kinases, CDK5 has the highest number of 21 residues which act as stabilization center as well as ATP binding sites. In case of GSK3B there were 19 residues acting as stabilization residues as well as ATP interacting sites. We have predicted the stabilizing residues in the CDK5 and GSK3B molecules and also in the complex using Sride [34] program. The results of the stabilizing residues are presented in Table 7 (see supplementary material).
Discussion
Microtubule associated protein tau (MAPT) was coined to be abnormally hyperphosphorylated in Alzheimer's disease. Inge GI et al. reported that CDK5 and GSK3B are the two important kinases regulating the hyperphosphorylation of MAPT [5]. However it is currently contested as to which kinase is strongly associated with tangle formation, which plays a key role in regulation of hyperphosphorylation of MAPT. So it is necessary to evaluate the role of two protein kinases in hyperphosphorylation of MAPT protein that leads to tangle formation. In this study, we have investigated various insilico strategies to find the role of CDK5 and GSK3B kinases in hyperphosphorylation of microtubule associated protein tau (MAPT) in Alzheimer's disease.
The initial docking study reveals the important binding sites of MAPT protein and their association with CDK5 and GSK3B kinases. The docked regions of MAPT were located within the binding cleft. Higher number of inter molecular hydrogen bonds were observed in the MAPT-CDK5 complex but in the case of MAPT-GSK3B complex the hydrogen bonding interactions were less. Next, the numbers of sites to be phosphorylated on MAPT with the influence of CDK5 and GSK3B kinases were found to be 14 and 5 respectively. Further, some of the ATP binding interacting sites of CDK5 and GSK3B kinases also acted as stabilization centers after the complex formation. There were more number of binding residues of CDK5 that acted as stabilization residues and stabilization centers when compared to GSK3B. Certain residues that act as stabilizing residues in the GSK3B were not seen as the stabilizing residue when they formed the complex with the MAPT protein. In the case of CDK5, it shows the best results for three residues acting as stabilizing residues before and after binding. Moreover some of the residues that act as stabilizing centers in the GSK3B were not seen in the ATP binding sites when they formed the complex with the MAPT receptor. Again the CDK5 showed the best result for 21 residues acting as stabilizing residues before and after binding and also acts as ATP binding sites. These stabilization centers may increase stability of the MAPT-CDK5 complex during the docking process. The overall results from various insilico analysis discussed are summarized in Table 8 (see supplementary material) and suggest that CDK5 is strongly involved in the hyperphosphorylation of MAPT than GSK3B.
Conclusion
MAPT is the key protein which is involved in the pathogenesis of Alzheimer's disease. This present insilico study helps to understand the structural and functional basis of MAPT protein with CDK5 and GSK3B kinases. The docking study of MAPT protein showed 7 binding confirmations for CDK5 and only two for GSK3B. The coiled nature of the MAPT protein is effectively phosphorylated by ATP binding sites of CDK5 and moreover these residues play a major role in hyperphosphorylation of MAPT than GSK3B. Some of the ATP binding sites of CDK5 kinases acted as the stabilization centers before and after formation of the complex. The numbers of sites to be phosphorylated on MAPT with the influence of CDK5 and GSK3B kinases were found to be 14 and 5 respectively. Eventhough both kinases are regulating the hyperphosphorylation of MAPT, with a small extent CDK5 showed more binding affinity towards the active sites of the target protein MAPT, when compared to GSK3B. With the overall results from different insilico analysis it can be suggested that CDK5 is strongly involved in the hyperphosphorylation of MAPT than GSK3B. So the ATP binding sites of CDK5 can be focused as drug targets for Alzheimer's disease.
Supplementary material
Footnotes
Citation:Jayapalan & Natarajan, Bioinformation 9(20): 1023-1030 (2013)
References
- 1.Ferri CP, et al. Lancet. 2005;366:2112. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braak H, et al. Neurosci Lett. 1986;65:351. doi: 10.1016/0304-3940(86)90288-0. [DOI] [PubMed] [Google Scholar]
- 3.Lovestone S, Reynolds CH. Neuroscience. 1997;78:309. doi: 10.1016/s0306-4522(96)00577-5. [DOI] [PubMed] [Google Scholar]
- 4.Goedert M, et al. Neuron. 1989;3:519. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- 5.Grundke-Igbal I, et al. J Bio Chem. 1986;261:6084. [PubMed] [Google Scholar]
- 6.Ballatore C, et al. Nat Rev Neurosci. 1989;8:663. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- 7.Liu SJ, et al. J Neurochem. 2003;87:1333. doi: 10.1046/j.1471-4159.2003.02070.x. [DOI] [PubMed] [Google Scholar]
- 8.Jessberger S, et al. PLoS Biol. 2008;6:e272. doi: 10.1371/journal.pbio.0060272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith DS, Tsai LH. Trends Cell Biol. 2002;12:28. doi: 10.1016/s0962-8924(01)02181-x. [DOI] [PubMed] [Google Scholar]
- 10.Alvarez A, et al. FEBS Lett. 1999;459:421. doi: 10.1016/s0014-5793(99)01279-x. [DOI] [PubMed] [Google Scholar]
- 11.Patrick GN, et al. Nature. 1999;402:615. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- 12.Kusakawa G, et al. J Biol Chem. 2000;275:17166. doi: 10.1074/jbc.M907757199. [DOI] [PubMed] [Google Scholar]
- 13.Lau LF, et al. J Mol Neurosci. 2002;19:267. doi: 10.1385/JMN:19:3:267. [DOI] [PubMed] [Google Scholar]
- 14.Chin PC, et al. Brain Res Mol Brain Res. 2005;137:193. doi: 10.1016/j.molbrainres.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Balaraman Y, et al. Cell Mol Life Sci. 2006;63:1226. doi: 10.1007/s00018-005-5597-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jope RS, et al. Neurochem Res. 2007;32:577. doi: 10.1007/s11064-006-9128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Embi N, et al. Eur J Biochem. 1980;107:519. [PubMed] [Google Scholar]
- 18.Giese KP. IUBMB Life. 2009;61:516. doi: 10.1002/iub.187. [DOI] [PubMed] [Google Scholar]
- 19.Lucas JJ, et al. EMBO J. 2001;20:27. doi: 10.1093/emboj/20.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pei JJ, et al. Am J Pathol. 2003;163:845. doi: 10.1016/S0002-9440(10)63445-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernstein FC, et al. J Mol Biol. 1977;112:535. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- 22.Altschul SF, et al. Nucleic Acids Res. 1997;25:3389. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu J, et al. J Bioinform Comput Biol. 2003;1:95. doi: 10.1142/s0219720003000186. [DOI] [PubMed] [Google Scholar]
- 24.Laskowski RA. Journal of Applied Crystallography. 1993;26:283. [Google Scholar]
- 25.Guex N, Peitsch MC. Electrophoresis. 1997;18:2714. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- 26.Alasdair TRL, Richard MJ. Bioinformatics. 2005;21:1908. [Google Scholar]
- 27.Tovchigrechko A, Vakser IA. Nucleic Acids Res. 2006;34:W310. doi: 10.1093/nar/gkl206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeLano WL, et al. The PyMOL Molecular Graphics System on World Wide Web. 2002 http://www.pymol.org. [Google Scholar]
- 29.Blom N, et al. J Mol Biol. 1999;294:1351. doi: 10.1006/jmbi.1999.3310. [DOI] [PubMed] [Google Scholar]
- 30.Lakoucheva LM, et al. Nucleic Acids Res. 2004;32:1037. doi: 10.1093/nar/gkh253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jung I, et al. BMC Bioinformatics. 2010;11:S10. doi: 10.1186/1471-2105-11-S1-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chauhan JS, et al. BMC Bioinformatics. 2009;10:434. doi: 10.1186/1471-2105-10-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dosztanyi Z, et al. J Mol Biol. 1997;272:597. doi: 10.1006/jmbi.1997.1242. [DOI] [PubMed] [Google Scholar]
- 34.Maqyar C, et al. Nucleic Acids Res. 2005;33:W303. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


