Abstract
How genetic and environmental factors interact in Parkinson’s disease is poorly understood. We have now compared the patterns of vulnerability and rescue of C. elegans with genetic modifications of three different genetic factors implicated in PD. We observed that expressing α-synuclein, deleting parkin (K08E3.7) or knocking down DJ-1 (B0432.2) or parkin, produces similar patterns of pharmacological vulnerability and rescue. C. elegans lines with these genetic changes were more vulnerable than non-transgenic nematodes to mitochondrial complex I inhibitors, including rotenone, fenperoximate, pyridaben or stigmatellin. In contrast, the genetic manipulations did not increase sensitivity to paraquat, sodium azide, divalent metal ions (FeII or CuII) or etoposide compared to non-transgenic nematodes. Each of the PD-related lines was also partially rescued by the anti-oxidant probucol, the mitochondrial complex II activator, D-β-hydroxybutyrate (DβHB) or the anti-apoptotic bile acid tauroursodeoxycholic acid (TUDCA). Complete protection in all lines was achieved by combining DβHB with TUDCA but not with probucol. These results show that diverse PD-related genetic modifications disrupt mitochondrial function in C. elegans, and they raise the possibility that mitochondrial disruption is a pathway shared in common by many types of familial PD.
The etiology of Parkinson’s disease has both genetic and environmental components (1). Epidemiological studies show that PD is more common in rural areas, and increased rates of PD are associated with the use of agricultural toxins, such as pesticides and herbicides (2). Attention has focused on inhibitors of the mitochondrial electron transport chain because some of the agricultural toxins implicated in PD are complex I inhibitors (2). In addition, ingestion of complex I inhibitors causes syndromes related to PD. The complex I inhibitor 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) selectively kills dopaminergic neurons in many types of animals (1). Rotenone, another complex I inhibitor, also causes a PD-related syndrome in rats, and causes multiple changes in the mitochondria of cultured neurons relevant to PD (3-6). These factors implicate disruption of mitochondrial function, and particularly complex I inhibition, in the etiology of PD.
Many of the genes associated with familial cases of PD have been identified, but no clear consensus exists over whether the different disease-related proteins converge onto a common pathway. Mutation of α-synuclein (at A53T, A30P or K46E) or duplication of α-synuclein is associated with familial Parkinsonisms (7-10). Loss of the putative ubiquitin ligase, parkin, causes autosomal recessive juvenile Parkinsonism (11). Mutations in the genes coding for UCH-L1, DJ-1 and PINK1 are also all associated with autosomal recessive PD, and mutations in LRRK2 are associated with automal dominant PD (12-15). α-Synuclein is a small ubiquitous protein that binds lipids, and might regulate vesicular function, proteasomal activity and/or signal transduction (16-20). α-Synuclein has a tendency to form oligomers and fibrils, particularly after exposure to oxidative stress (21-24). α-Synuclein is most abundant in neurons in the brain, and is present in all mammals, but does not exist in C. elegans or Drosophila. Parkin is a putative E3 ubiquitin ligase that is neuroprotective in cell culture and of transgenic Drosophila (25-28). Over-expression of parkin protects against α-synuclein toxicity, which suggests that the two proteins affect intersecting biochemical pathways (27,28). DJ-1 is an oncogene that modulates oxidative stress possibly by affecting mitochondrial function (29-31). The varied functions of these proteins obscures any potential convergent pathways that might underly the pathophysiology of Parkinson’s disease.
In this manuscript, we demonstrate that genetic modulation of α-synuclein, parkin and DJ-1 all disrupt mitochondrial function in C. elegans. We also describe pharmacologic treatments that protect the mutant C. elegans strains against toxicity caused by the genetic mutations that support the mitochondrial convergence of the genetic pathways. These results point to disruption of mitochondrial function as a general cause of most, if not all, causes of PD.
MATERIALS AND METHODS
Generation of the α-synuclein transgenic lines
Human wild type α-synuclein cDNA was inserted into the vector pPD95.75 downstream of a synaptobrevin promoter and expressed in Bristol N2 non-tg lines by co-injecting with a pDPSU006-GFP vector to produce extrachromosomal arrays. The pDPSU006-GFP vector was used as a marker for gene transmission. The A53T α-synuclein expression is driven by an unc-119 neuronal promoter. GFP driven by the dopamine transporter promoter was used as a marker to identify those nematodes expressing the extrachromosomal array. Expression of β-synuclein expression was also is driven by an unc-119 neuronal promoter. Transgenes were integrated by exposing animals to 1,800 rads of γ irradiation, and animals were out-crossed five times.
Detection of the wild type and A53T α-synuclein transgene
Polymerase chain reaction (PCR) was performed to detect the transgene using he following primers to detect the transgene: sense primer 5′- ATTAATTCATAGCC-3′ and anti-sense primer 5′- CTGGGAGCAAAGATA-3′ (IDTDNA, Coralville, IA ).
Generation of the K08E3.7 knockout
A deletion library of nematodes derived from 460,000 trimethlypsoralen/UV mutagenized non-tg animals was used to generate the knockout. Previously described methods were used for library construction and screening (32). PCR with the primers listed below was used to verify a K08E3.7 deletion within the mutagenized animals. Animals verified as K08E3.7 knockouts were out-crossed five times to the wild type strain to produce a clean genetic background. The K08E3.7 knockout is a deletion of 1132 bp whose limits are GGACATTTCAAACTTTGAAT …AAAATAACTTCAGGAGTGGT. The following primers were used to detect the deletion: sense primer 5′- ATACAAGATAGAAAAACAGGTC-3′ and anti-sense primer 5′- GCAAAACGAAAAAAAAC A-3′ (IDTDNA, Coralville, IA ).
Other C. elegans lines
The ced-3 knockout strain used in this thesis was kindly given to us by Dr. Horvitz at Massachusetts Institute of Technology (Cambridge, MA). The BY200 lines was a generous gift of R. Blakely (Vanderbuilt U., TN). The tau P301L line was a generous gift of B. Kraemer (U. Washington). The Mito-GFP line was a generous gift of A. Van der Bliek (UCLA).
Lifespan Analysis
The life spans of the non-tg, strains expressing wild type and A53T α-synuclein, and the K08E3.7 KO strains were determined using previously described techniques (33). Three NGM plates (2.5 g bacto-peptone (Beckton Dickinson, Sparks, MD), 3 g NaCl, 20 g agar, 1 mM CaCl2, 1 mM MgSO4, 5 mg cholesterol, 25 mM KH2PO4, (Sigma, St. Louis, MO) ddH2O) seeded with OP50-1 E. coli) were seeded with 40 nematodes each and were monitored throughout the lifecycle for each strain.
Culturing of Nematodes and Isolation of Nematode Lysates
The Bristol N2 non-tg strain was used as the wild-type strain. Growth and culture of nematodes were performed using standard techniques (34,35). Previously described methods were used to isolate Mixed stage nematode lysates were prepared as described previously, except that the isolated pellet of nematodes was further washed with 5 ml cold 1X PBS + Protease Inhibitor Cocktail (Sigma, St. Louis, MO), spun down and sonicated for lysis (36).
Immunoblot Analysis
Protein concentration of nematode lysates was determined using BCA protein assay (Pierce, Rockford, IL). 30 μg of protein were used for immunoblot assays. 2× dithiothreitol protein loading buffer was added to each sample. The samples were then heated for 5 min at 90 °C, and run on 8-16% sodium dodecyl sulfate (SDS) gradient polyacrylamide gels (BioWhitaker, Walkersville, MD). Proteins on the polyacrylamide gels were then transferred to polyvinylidene difluoride (BioRad, Hercules, CA) overnight at 4 °C at 0.1 A/gel in transfer buffer. The immunoblot was blocked in 5% milk in 100 mM Tris-buffered saline/0.1% Tween 20 (TBST) for one hour at room temperature while shaking. The blots were then incubated overnight at 4 °C in primary antibody at appropriate concentration in 5% bovine serum albumin in TBST. The blots were washed three times, 10 min each, and incubated three hours in secondary antibody (1:5000) (Jackson Laboratories, West Grove, PA) in 5% milk in TBST at room temperature. Blots were washed three times and developed using a chemiluminescent reaction (PerkinElmer Life Sciences, Boston, MA).
Primary antibodies used were monoclonal anti-α-synuclein antibody (1:1000) (Zymed, San Francisco, CA) and monoclonal anti-ubiquitin antibody (1:1000) (Zymed, San Francisco, CA); secondary antibody was donkey anti-mouse (1:5000) (Jackson Laboratories, West Grove, PA). Resulting bands were analyzed using Image J (NIH).
Oxyblot Analysis
Protein lysates (20 μg) were derivatized with 2,4-dinitrophenyl hydrazine (DNPH) at room temperature (25°C) for 20 min, neutralized and then immunoblotted as described above. Peroxidase coupled anti-DNP antibody diluted 1:150 was used for immunoblotting as per manufacturers directions (Chemicon, Temecula, CA).
Chemical Treatments
For chemical treatments, 35 mm plates were poured with the indicated concentrations of chemicals described in the results section (Sigma, St. Louis, MO) diluted in NGM agar. The plates were seeded with bacteria medium containing rotenone, paraquat or metal. 40 nematodes of the appropriate strain were placed on each plate. Three plates were analyzed for each point; each experiment was repeated 3 - 6 times. Every other day surviving nematodes were transferred onto a new plate with the same concentrations of rotenone, paraquat, etoposide or metal. The number of surviving nematodes was counted on days 2 and 4. The number of live nematodes was determined by counting nematodes that were either moving or those that responded to light touch with a platinum wire. Toxicity was measured as a function of percent survival of the nematodes following treatment.
Treatment with siRNA
Generation of the B0432.2 Knockdown - E. coli containing a B0432.2 genomic fragment cloned into L4440 was used to generate the C. elegans knockdown (37). The bacteria was grown for 12 hours in LB + 50 μg/ml ampicillin (Sigm, St. Louis, MO). This culture was seeded onto NMG plates containing 25 μg/ml carbenicillin (Sigma, St. Louis, MO) and 1 mM Isopropyl-ß-D-thiogalactopyranoside (IPTG) (Research Products International Corporation, Mt. Prospect, IL). B0432.2 RNAi was induced overnight at room temperature. The following day five L3-L4 hermaphrodites were transferred onto the NGM plates. These nematodes were grown for 72 hours at 15°C for the RNAi to take effect. Single adults were then plated onto new individual NGM plates with additives and RNAi containing bacteria. Progeny of these adults were used in subsequent assays.
Quantification of knockdown
N2 worms were grown from hatching on HT115(DE3) bacteria containing L4440 B0432.2 (DJ-1) plasmid for knockout; worms were grown on freshly prepared bacterial plates containing 1mM IPTG for induction of B0432.2. For quantification of B0432.2 (DJ-1) RNA, adult worms (six days old) were transferred to micro-tubes with 30 mL PBS. Single worms were placed in tubes and dissolved in 300 mL TRIzol (Invitrogen) and 12 mg of linear polyacrylamide (GenElute LPA, Sigma, Inc.) was added to it as a carrier for RNA extraction. Aqueous phase with RNA was precipitated with isopropanol and washed once with 70% ethanol. The RNA pellet was dried at RT and dissolved in DEPC treated water. The extracted RNA was immediately reverse transcribed with a 3′ DJ-1 primer (TGC CAC TGA CAA CAA CAC GA) for one hour at 50° C. The reverse transcribed products were PCR analyzed with a BioRad real time PCR. A 5′ primer (CAA GTC ATC CAA GTG TTA AGG AGA AA) and a Taqman probe (TCG AGA AAG GAG GCT ACA AGT ACT CGG AGG) were added to the PCR mix to monitor the threshold cycles for DJ-1 amplifications. PCR products were analyzed with a 3% agarose gel electrophoresis. Band intensities were measured by Labworks software (UVP Inc.) DNA sequencing was also performed to confirm the identity of the amplified band.
Thioflavin S histochemistry
Worms were treated with 25 mM rotenone for 2 days, harvested with M9 media and fixed overnight in 4% paraformaldehyde at 4°C. After being washed three times with PBS buffer, the worms were incubated in a shaker at 37° C with TMT solution (1% Triton X-100, 5% b-Mercaptoethanol and 125mM Tris, pH 7.6) for three hours. The worms were then washed two times with a solution containing 100mM Tris (pH 7.6), 1mM CaCl2 and were incubated in the same solution with 2mg/ml collagenase for another three hours at 37° C with vigorous agitation. After washing three times with PBS buffer, animals were put on a poly D-Lysine coated slide in a 30mL volume and covered with a cover slip. The slides were kept at −80 overnight. Cover slips were removed quickly at −80° C and the slides were gradually hydrated with graded frozen methanol (100%-70%) followed by a 10 min wash with water. The slides were then incubated with 0.015% thioflavin S for 10 minutes, washed three times in 80% ethanol for 5 minutes. The slides were washed once with ddH2O. After ten minutes drying at RT, the slides were mounted with glass cover slips with thin layer of Fluoromount-G (Electron Microscopy Sciences, Hatfield, PA)
Dot Blot Analysis
The non-tg and A53T α-synuclein strains treated with rotenone were analyzed via dot blot for α-synuclein aggregation. Samples were vacuumed through a dot blot apparatus onto cellulose acetate membrane. Recombinant aggregated α-synuclein was used as a positive control. The cellulose acetate membrane was blocked and probed with the Syn303 antibody (1:500) (38) as described previously in the Immunoblot Analysis section.
Oxygen Consumption
2000 stage-synchronized adult worms were grown on large plates, collected in S-basal buffer and washed free of bacteria. Changes in oxygen concentration of the worms was performed in 1 ml S-basal buffer containing heat killed E. coli in a closed-chamber cuvette mixed with a mini–stirring bar, maintained at 25 °C and using a Clark-type electrode (Hansatech Instruments Ltd., Norfolk, United Kingdom). The worms were incubated in the chamber 5 minutes before measurement and then oxygen consumption measured for usually 5–10 min. The slope of the straight portion of the plot was used to derive the oxygen consumption rate. After the measurement, the worms were collected for protein quantification. Oxygen consumption rate for each population was normalized to its protein content.
Preparation of Mitochondria
The methods was followed as described by Kayser et al (39). Two grams of worms cleaned of E. Coli were suspended in MSM-E buffer (220 mM mannitol, 70 mM sucrose, 5 mM MOPS, 2 mM EDTA, pH 7.4). The worms were ruptured with a polytron, and then proteinase type XXVII (5 mg/g worms, Sigma) was added and incubated 10 min. Next the slurry was homogenized in a dounce. The slurry was mixed in 1 volume of MSM-E buffer containing 0.4% BSA, and the homogenate centrifuged (300g, 10 min, 4°C). The mitochondrial pellet was resuspended in MSM-E and washed twice by centrifugation (7000g, 10 min, 4°C). The final pellet was resuspended in 100 μl MSM-E, and total protein determined by BCA assay.
NADH-CoQ reductase assay
The assay was performed as described by Kramer et al (40). Mitochondria were mixed in freshly prepared assay buffer (potassium EDTA 0.25mM, BSA 0.2%, 1mM KCN, potassium phosphate 25mM pH 8.0, 100 μM NADH @ 30°C). After 3 min preincubation, 0.02 mM decylubiquinone was added for the measurement of complex I-III. All assays contained potassium cyanide to inhibit complex IV (1.0 mM final concentration). Upon addition of substrate, samples were placed in a spectrophotometer (Beckman) and oxidation of NADH was measured at 340nM for 15 min at 30°C. Rates of oxidation were calculated according to the equation:
where ΔABS/min is the change in absorbance, 1 cm is the cuvette pathlength and ε = 6.81, the extinction coefficient (in mM/L•cm).
Statistical Analysis
Statistical analysis on the raw numbers was done using an unpaired t-test or ANOVA analysis followed by a Newman Keuls post hoc analysis (GBSTAT), when necessary. All results experiments were performed in triplicate.
RESULTS
I. CHARACTERIZATION OF NEMATODE MODELS
Characterization of the wild type and A53T α-synuclein nematodes
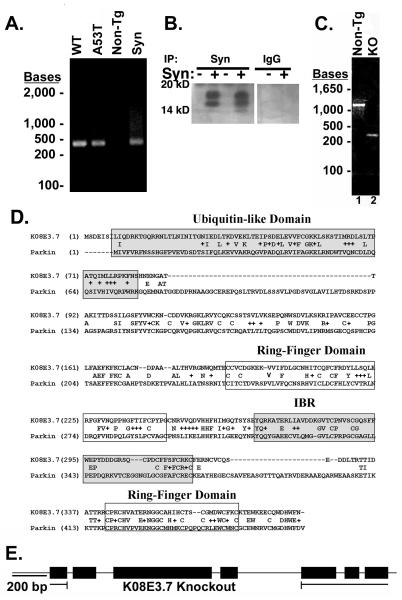
The presence of wild type or A53T α-synuclein in the C. elegans transgenic lines was demonstrated by PCR and immunoblot analysis (Figures 1A - C). PCR of the transgenic C. elegans genomes yielded 450 bp bands that corresponded to full-length wild type and A53T α-synuclein cDNA (Figure 1A, Lanes 1 and 2). The plasmid used to generate the wild type α-synuclein transgenic lines was used as a control to verify the size of α-synuclein (Figure 1A, Lane 4). No α-synuclein was detected through PCR analysis in the Bristol N2 non-transgenic (non-tg) strain, which is consistent with the absence of a homologue for α-synuclein in the C. elegans genome (Figure 1A, Lane 3). The presence of α-synuclein was validated by immunoprecipitating α-synuclein from the A53T α-synuclein transgenic or Bristol N2 non-tg lines. A 14 kD band, characteristic of α-synuclein was evident only in the A53T lines; no such band was present in the N2 line or in A53T α-synuclein lysates precipitated with pre-immune serum (fig. 1B).
Figure 1. Verification of α-synuclein expression and knockout of K08E3.7 in transgenic C. elegans strains.
(A) PCR analysis of expressed wild type and A53T α-synuclein transgenes. 450 bp band is present in the wild type (WT) and A53T α-synuclein expressing lines (lanes 1 and 2) and co-migrates with a band amplified from α-synuclein cDNA (lane 4). The 450 bp α-synuclein-specific band is absent from the non-tg lane (lane 3). (B) Immunoprecipitation of α-synuclein demonstrates the presence of the α-synuclein protein (lanes 2 and 4, left panel). No synuclein is present in lysates from non-tg worms (lanes 1 and 3, left panel) nor in lysates immunoprecipitated with non-specific IgG (right panel). The doublet pattern of reactivity is often seen in immunoblots of α-synuclein. (C) PCR of genomic DNA across the KO8E3.7 gene yields a 1.37 Kb band for non-tg nematodes (lane 1) and a 0.26 Kb band for the K08E3.7 KO strain (lane 2). (D) The amino acid sequence alignment of K08E3.7 and human parkin. Exact amino acid matches are indicated with the identical amino acid in between the K08E3.7 and human parkin sequences; conservative amino acid differences are identified by a ‘+’. The N-terminal ubiquitin-like domain (shaded box – upper right), RING finger domains (boxed), in-between RING domain (shaded box) are indicated. (E) Diagram of the full-length K08E3.7 gene and the K08E3.7 gene containing the 1132 bp deletion from the K08E3.7 knockout.
Characterization of the K08E3.7 knockout
Human parkin shows high homology to the KO8E3.7 gene in C. elegans (Figure 1D). Based on this homology, we searched for a strain of nematodes lacking KO8E3.7. Generation of the K08E3.7 knockout nematodes (K08E3.7 KO) was demonstrated by PCR based on the expected region deleted (Fig. 1C and E). A 1131 base pair region including exons 1-4 of the K08E3.7 gene was deleted to yield a non-functional form of K08E3.7 (Fig 1C). The non-tg nematodes yield a 1.37 kb band that corresponds to full length K08E3.7 (Figure 1C, Lane 1). Knockout of K08E3.7 yields a 0.26 kb band (Figure 1C, Lane 2).
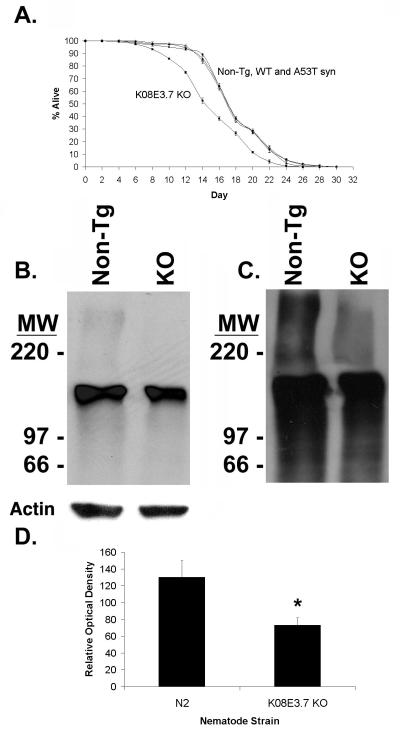
The life spans of the C. elegans strains were examined as a simple test to determine whether expression of α-synuclein or lack of K08E3.7 affects the metabolism of the nematodes, as is seen for loss of the parkin homologue in Drosophila [Greene, 2003 #2026]. The mean life span was similar among the non-tg, wild type and A53T α-synuclein expressing strains, however the K08E3.7 KO strain had a 15.4% shorter life span that the non-tg strain (Figure 2A). The mean survival for the non-tg strain was 18.5 ± 0.169 days, while that for the K08E3.7 KO strains was 15.65 ± 0.211 days, p<0.01 (Figure 2A). The transgenic and non-transgenic C. elegans strains were also examined visually at multiple life stages from larval development through adulthood. No defects were observed in any transgenic strains through development and into adulthood.
Figure 2. Characterization of the K08E3.7 KO transgenic strain.
(A) A life span curve shows that K08E3.7 KO (square) strain has life span that is 15.4% shorter than the non-tg (diamond), wild type (WT, triangle) and A53T (circle) α-synuclein expressing strains. Mean life span for the non-tg, WT and A53T α-synuclein expressing strains was 18.5 ± 0.169, while the mean life span of the K08E3.7 KO strain was 15.65 ± 0.211. p<0.01 compared to the non-tg. (B) Immunoblot probed with monoclonal ubiquitin antibody (upper panel) or actin (lower panel, loading control) showing higher levels of high molecular weight ubiquitin conjugates present in the non-tg (lane 1) strain than the K08E3.7 knockout (KO, lane 2) strain. (C) Longer exposure of immunoblot shows high molecular weight smears of ubiquitin conjugates in the stacking gel in the lysates from the non-tg strain. (D) Densitometric quantification of immunoblot from panel B blot; (*) p<0.01.
KO8E3.7/Parkin KO reduces ubiquitination in C. elegans
The K08E3.7 KO and non-tg nematodes were examined to explore the function of K08E3.7 in the nematode. The results show that the K08E3.7 KO strain exhibits reduced levels of basal ubiquitination (Fig. 2B-D). Basal ubiquitination in the K08E3.7 KO strain was 55% ± 6.5% (p<0.01) than of the non-tg strain (Fig. 2D); in comparison, basal ubiquitination was unchanged in the A53T α-synuclein line (data not shown). The reduction in high molecular weight ubiquitinated proteins in the K08E3.7 KO worm strain contrasts with results reported for parkin knockout mice (41). C. elegans might lack the intrinsic ability to compensate for lack of parkin, which could lead to a result similar to that observed for acute knockdown of parkin in cell culture. Reduced ubiquitination in the K08E3.7 KO strain lends support to the hypothesis that parkin is involved in the ubiquitin proteasomal system.
Other functions examined in the K08E3.7 knockout strain were unaffected. For instance, defecation and egg laying were not significantly changed (Supplemental fig. 1A and B). Chemotaxis to isoamylalcohol, trimethylthiazol and pyrazin also were not different than the N2 strain, although chemotaxis to diacetyl was significantly decreased in the K08E3.7 knockout line (Supplemental fig. 1C).
II. SURVEY OF TOXICOLOGY
Transgenic lines expressing PD-related genetic factors show selectively increased vulnerability to mitochondrial complex I inhibitors
Environmental factors are hypothesized to contribute to the pathophysiology of PD, but whether such factors interact with gene mutations associated with PD is poorly understood. To investigate the interaction of genes with environmental toxins, we examined the vulnerability of the A53T α-synuclein and K08E3.7 worms to a variety of toxins including inhibitors of complex I – IV, redox active metals and DNA repair inhibitors. For controls, we used the Bristol N2 non-transgenic worms, a transgenic worm line expressing GFP driven by a promoter for the dopamine transporter (BY200), a transgenic worm line expressing human β-synuclein driven by the neuron specific unc119 promoter and a transgenic worm line expressing human P301L tau driven by the muscle specific myo 3 promoter (42,43).
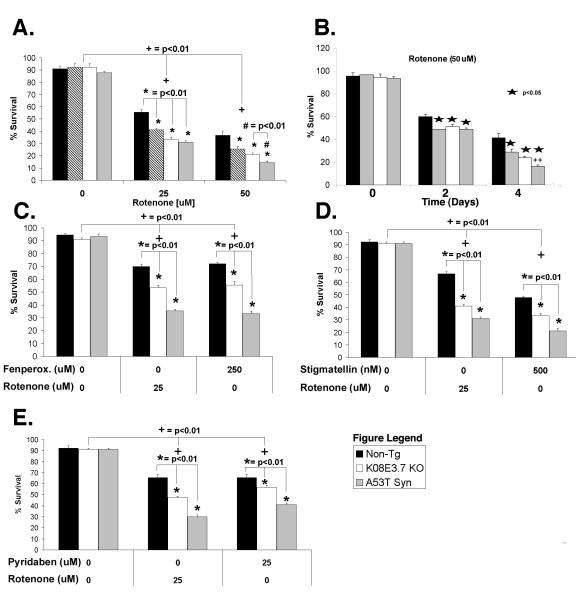
The first treatment was with rotenone, a known mitochondrial complex I inhibitor. Rotenone (25 or 50 μM) induced a significant dose dependent mortality in all the strains, which demonstrates that rotenone is toxic to the non-tg, K08E3.7 KO and α-synuclein expressing transgenic strains (Fig. 3A, dose response; Fig. 3B, time course). Because most of the worms were dead by 4 days, the life span analysis was stopped at 4 days. Interestingly, the K08E3.7 KO, wild type and A53T α-synuclein expressing strains showed reduced viability at each concentration of rotenone and at both 2 and 4 days of treatment compared to the non-tg strain or BY200 line at the same dose (Figure 3A & B, only the non-tg is shown as the control, see fig. 4E&F for BY200 data). After 25 μM rotenone treatment for 4 days, the K08E3.7, wild type and A53T α-synuclein expressing strains showed 74% ± 1.48%, 60% ± 1.2%, and 56% ± 1.12% survival, respectively (p<0.01). The A53T α-synuclein expressing strain showed greater vulnerability to rotenone compared to the wild type α-synuclein expressing strain with 50 μM rotenone treatment, exhibiting 68.4% (p<0.01) lower survival after 4 days of 50 μM rotenone (Fig. 3B). Because the K08E3.7 KO represents just one line or worms, we also examined the effects of knockdown with RNAi to K08E3.7 or Sel-9 (the latter representing a gene that should not affect the response to rotenone and is a control for RNAi). Worms that were treated with RNAi for K08E3.7 showed 43 ± 1.3% ( p<0.01) more toxicity than worms treated with RNAi for Sel-9, confirming the effects of K08E3.7 on rotenone toxicity.
Figure 3. K08E3.7 KO, wild type and A53T α-synuclein expressing lines are selectively vulnerable to rotenone-induced toxicity.
(A & B.) K08E3.7 KO, wild type and A53T α-synuclein expressing lines show increased vulnerability to rotenone at varying doses (A; 25 or 50 μM rotenone, 4 days treatment), and at different times (B; 50 μM rotenone, 2 and 4 days treatment). The PD-related lines show enhanced vulnerability after 4 days of rotenone treatment; (*) p<0.01. At 50 μM rotenone, the A53T α-synuclein expressing strain is also more vulnerable than the wild type α-synuclein strain; (#) p<0.01. (+) p <0.01 compared to untreated of the same strain. (C – E) The K08E3.7 KO, wild type and A53T α-synuclein expressing strains show enhanced toxicity compared to the non-tg line after treatment for 4 days with complex I inhibitors fenperoximate (C), stigmatellin (D) and pyridaben (E), (*) p<0.01, (+) p<0.01 compared to untreated nematodes of the same line.
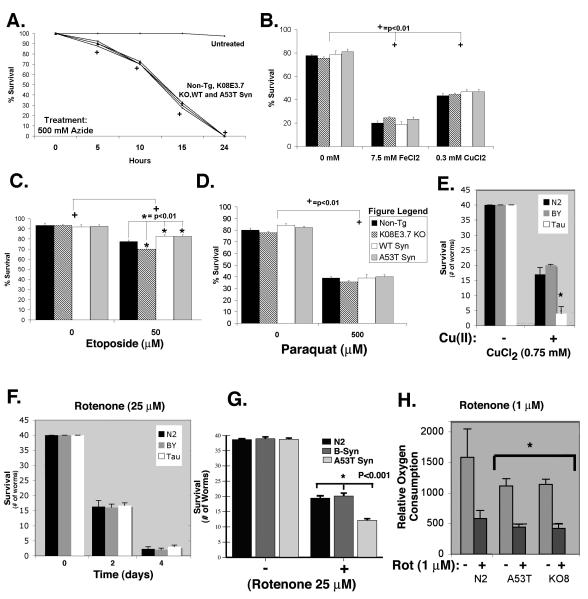
Figure 4. Vulnerability to other toxins unchanged in K08E3.7 KO, wild type and A53T α-synuclein expressing lines.
(A.) Kill curve over a 24-hour treatment with 500mM sodium azide. Neither the K08E3.7 KO, wild type nor A53T α-synuclein expressing strains show increased sensitivity to sodium azide compared to the non-tg strain; (+) p<0.01. (B. & C.) No difference in sensitivity to iron (II) (B), copper (II) (B), or paraquat (C) compared to the non-tg strain. (D) Both wild type and A53T α-synuclein are slightly protective against etoposide treatment, while K08E3.7 KO strain is slightly vulnerable compared to the non-tg strain. (E) The P301L tau C. elegans line shows increased vulnerability to copper compared to non-tg or BY200 worm lines after 6 days of exposure to 0.75 mM CuCl2. (F) The P301L tau C. elegans line shows no difference in vulnerability to rotenone (25 μM) compared to non-tg or BY200 worm lines (*) p<0.01 compared to non-tg. (G) The β-synuclein expressing C. elegans line shows no difference in vulnerability to rotenone (25 μM) compared to non-tg, while the A53T α-synuclein line shows increased toxicity (*) p<0.001 compared to non-tg & β-syn, N=8. (H) The K08E3.7 KO and A53T α-synuclein show reduced oxygen consumption under basal conditions compared to the non-tg line. (+) p <0.01 compared to untreated nematodes of the same strain.
To determine whether the increased vulnerability of the PD-related nematodes generalized to other complex I inhibitors, we tested other known mitochondrial complex I inhibitors. We examined the sensitivity to fenperoximate, pyridaben, stigmatellin, piericidin, papaverin, capsaicin and 4-phenylpyridin all of which are known to inhibit complex I (44). Three of these compounds were toxic to C. elegans: fenperoximate, pyridaben, and stigmatellin. We observed that the PD-related nematode lines showed increased vulnerability to each of these complex I inhibitors, similar to that observed with rotenone (Fig. 3C-E). These data suggest that the PD-related C. elegans lines are generally more vulnerable to complex I inhibitors.
Next we tested whether the sensitivity of the PD-related C. elegans was restricted to complex I inhibition or extended to inhibition mitochondrial complexes II – IV. Each of the C. elegans lines was exposed to antimycin A, 3-nitroproprionic acid (3-NP) or sodium azide, which inhibit mitochondrial complexes II, III and IV, respectively. The non-tg and transgenic strains were equally vulnerable to 500 mM azide (Figure 4A). We saw 50% survival for the non-tg and transgenic strains at 12.5 hours following azide treatment. Antimycin A and 3-NP had no effect on the nematodes, suggesting that the worms do not take up the compounds (data not shown).
PD-Related genetic factors do not increase vulnerability to redox active metals, paraquat or etoposide
To test whether PD-related genetic factors affect the vulnerability of nematodes to other toxins, the each strain was exposed to different concentrations of CuCl2 or FeCl2 (Fig. 4B), paraquat (Fig. 4C) or etoposide (Fig. 4D). The synuclein-expressing or K08E3.7 KO lines did not exhibit higher sensitivity to the metals or paraquat compared to the non-tg strain, and the α-synuclein expressing strains actually showed slightly reduced vulnerability to etoposide, a topoisomerase II inhibitor (Figure 4D, 4 day treatment). The data suggests that α-synuclein can be mildly protective against etoposide toxicity when expressed in the transgenic strains. Loss of K08E3.7 was associated with a small increase in vulnerability to etoposideinduced toxicity compared to the non-tg strain (Fig. 4C). These results indicate that the PD-related genetic changes cause increases in vulnerability to toxins that are largely selective for inhibition of mitochondrial complex I in C. elegans, although we cannot rule out the possibility that the vulnerability might also include inhibition of complexes II or III, because the worms were not susceptible to 3-NP or antimycin A.
As further controls, we examined the toxicity profile of worm strains expressing either human P301L tau driven by a muscle specific promoter (myo-3) or human β-synuclein driven by the unc119 neuronal specific promoter (43). Under basal conditions, the P301L worms moved slower and laid fewer eggs that the other lines, suggesting that they might be somewhat less healthy. The β-synuclein worms exhibited no gross abnormalities; they moved at normal speeds and laid normal numbers of eggs. The pattern of toxicity for the tau worm line differed distinctly from that of the PD-related lines. The P301L tau worms showed increased toxicity to copper (CuCl2, 0.75 mM) but no difference in vulnerability to rotenone (25 μM), compared to Bristol N2 worms or BY200 worms (fig. 4E, F). In contrast, rotenone (25 μM) did not induce any extra toxicity in the β-synuclein worms compared to the Bristol N2 worms (fig. 4G). These data demonstrate that expression of a protein prone to aggregation, such as P301L tau, does not necessarily cause a selective vulnerability to complex I inhibitors.
III. Mechanism of Toxicity: Production of free radicals, activation of apoptosis and formation of protein aggregates
Rotenone inhibits respiration
The vulnerability to complex I inhibitors raises the possibility that the function of the electron transport chain might be reduced in worms with PD-related genetic changes. To investigate this, we examined whether the doses of rotenone used inhibited oxygen consumption. C. elegans lines that non-tg, or expressing A53Tα-synuclein or lacking K08E3.7 were exposed to rotenone (1 μM) for 1 hr and oxygen consumption was measured; the line expressing P301L tau driven by a muscle promoter was used as an additional control in this experiment. Lower doses and shorter exposures of rotenone were used based on titrations of the non-tg line to determine doses of rotenone that would yield detectable oxygen consumption signals over the course of the assay. The results indicated that the worms expressing A53T α-synuclein or lacking K08E3.7 showed lower levels of oxygen consumption under basal conditions or after treatment with rotenone compared to the non-transgenic line (Fig. 4H).
To verify the mechanism of action of the other complex I inhibitors, we also investigated whether pyridaben and phenylpyridin inhibited respiration in the worm. Bristol N2 non-transgenic C. elegans were incubated in rotenone (50 μM, 1 hr), pyridaben (25 μM, 1 hr) or phenylpyridin (10 mM, 1 hr) after which respiration was measured. Both compounds significantly decreased respiration (Sup. fig. 2). In contrast, etoposide (50 μM, 1 hr) did not significantly decrease respiration (Sup. fig. 2). To further verify that rotenone was acting by inhibiting complex I, we isolated mitochondria from Bristol N2 nontransgenic C. elegans, and monitored mitochondrial NADH-CoQ reductase activity using decylubiquinone as the electron acceptor (40,45). Under control conditions, C. elegans exhibited an activity of 44 U/mg, while after treatment with rotenone (50 μM) no activity was detectable.
Altered morphology of dopaminergic neurons and aggregation of synuclein during rotenone treatment in A53T α-synuclein expressing worms
To investigate whether rotenone was affecting neurons in C. elegans lines, we subjected C. elegans line BY200 (expressing GFP driven by the dopamine transporter promoter), to 25 μM rotenone for 1-4 days. The morphology of the dopaminergic neurons was not changed by 2 days rotenone treatment (Figure 5A, B). However, staining with thioflavine S revealed the presence of multiple thioflavine positive inclusions that were present in 38.7 ± 1.3% of the worms (Fig. 5C). In addition, after thioflavine staining, occasional worms showed processes that were strongly thioflavine positive (Fig. 5D). We also examined mitochondrial morphology in response to 25 μM rotenone, by examining the response of a C. elegans line expressing a GFP with a mitochondrial promoter (provided by A. van der Bliek) (46). However, no changes in morphology were observed in response to rotenone, and we did not observe selective mitochondrial degeneration in any particular anatomic entity in the worm (Supplemental fig. 3)
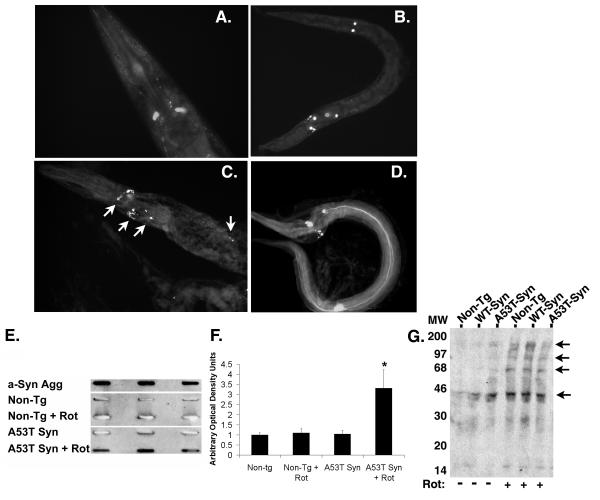
Figure 5. Increased fibrillogenisis in the A53T α-synuclein expressing line.
(A) GFP fluorescence present in dopaminergic neurons of BY200 worms after treatment with 25 μM rotenone for 2 days. (B) GFP fluorescence present in dopaminergic neurons of A53T α-synuclein worms after treatment with 25 μM rotenone for 2 days. (C & D) Staining with thioflavine S highlights additional fluorescence (arrows) present in A53T α-synuclein worms after treatment with 25 μM rotenone for 2 days, suggesting the presence of protein aggregates. (E) Slot blot analysis of non-tg and A53Tα-synculein expressing strain lysates using the Syn303 antibody that recognizes fibrillar α-synuclein. Row 1 – Recombinant aggregated α-synuclein (aged 1 month); Row 2 – non-tg strain; Row 3 – non-tg treated with rotenone (25 μM, 4 days); Row 4 – A53T α-synuclein expressing strain; Row 5 – A53T α-synuclein expressing strain treated with rotenone (25 μM, 4 days). Lysates were analyzed in triplicate. Aggregated α-synuclein was observed in the recombinant α-synuclein and in the A53T α-synuclein expressing strain treated with rotenone. (F) Quantification of slot blot analysis; (*) p<0.01 compared to non-tg. (G) Oxyblot analysis of total lysates from C. elegans treated with 25 μM rotenone for 2 days shows increased protein oxidation (arrows).
Presence of thioflavine positive aggregation of α-synuclein in response to rotenone
To test whether the α-synuclein was aggregating, the non-tg and A53T α-synuclein lines were exposed to rotenone (25 μM, 4 days) after which treated and untreated worms were homogenized in PBS. The insoluble material was captured with cellulose acetate membrane and probed with the Syn303 antibody that selectively recognizes aggregated α-synuclein (38). The Syn303 antibody reacted with lysates from the rotenone treated A53T α-synuclein line, but did not react with lysates from untreated worms or the rotenone treated non-tg line (fig. 5E, F). The synuclein aggregates from either total lysates or a sarkosyl insoluble fraction were not detectable following PAGE electrophoresis and immunoblotting (with or without prior immunoprecipitation), suggesting that the aggregates were not SDS-resistant; in addition, immunoprecipitated α-synuclein was not ubiquitinated (data not shown). Previous studies suggest that oxidation stimulates synuclein aggregation. To determine whether the aggregation was associated with increased protein oxidation, we performed oxyblots on the worm lysates (fig. 5G). The results demonstrated a clear increase in the amount of oxidation in the lysates (fig. 5G). These results indicate that aggregation accompanies rotenone treatment in the nematodes expressing A53T α-synuclein and is consistent with prior studies showing that α-synuclein aggregates in response to oxidative stresses (21,24,47-50).
Rotenone induces caspase activation
Next we explored whether the rotenone activated apoptosis. Non-tg, A53T-α-synuclein expressing worms or worms lacking K08E3.7 were treated with 25 μM rotenone for two days, and levels of caspase activity were compared to untreated worms. Caspase activity showed a trend toward elevation in the non-tg worms (33 ± 19 %, p=0.88, N=4), but was significantly elevated in the worms expressing A53T α-synuclein (101 ± 22%, p<0.001, N=4); caspase activation was not observed in the K08E3.7 KO worm. This suggests that significant levels of apoptosis were induced by rotenone in the worms expressing A53T α-synuclein, and identifies a difference in the types of cell death processes activated by rotenone among the worm strains.
IV. RESCUE FROM COMPLEX I INHIBITION
Rescue of mitochondrial function protects against rotenone-induced toxicity
Rotenone is known to induce free radical generation, inhibition of complex I, and apoptosis. To test whether preventing these processes protects against rotenone toxicity, the non-tg and PD-related lines (A53T α-synuclein strain and KO8E3.7 KO strain) were co-treated with rotenone and putative protective compounds such as anti-oxidants, mitochondrial complex II activators, or anti-apoptotic compounds. Testing of the α-synuclein lines was limited to the A53T α-synuclein line to limit the complexity of the analysis.
Two known anti-oxidants, N-acetyl cysteine (NAC) and probucol, were tested for protection against rotenone-induced free radical generation (51). NAC was unable to protect any of the strains against rotenone toxicity (data not shown). In contrast, probucol significantly reduced rotenone toxicity, yielding 12.13%, 13.34%, and 24.45% higher survival rates for the non-tg, K08E3.7 KO and A53T α-synuclein lines than treatment with rotenone alone (Figure 6A). However, probucol was unable to fully protect any of the nematode strains against rotenone-induced toxicity. The ability of probucol to partially protect against rotenone-induced toxicity suggests that the mechanism of toxicity involves generation of free radicals, although direct measurement of free radical production would be required to prove this hypothesis.
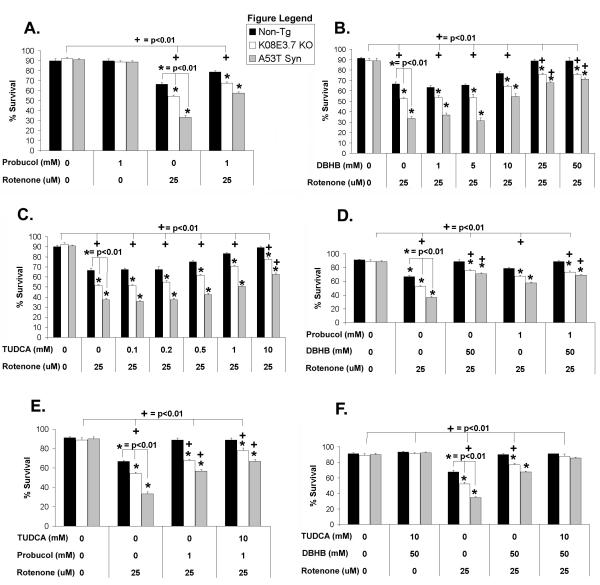
Figure 6. Protection against rotenone-induced toxicity.
(A) Co-treatment of the K08E3.7 KO and A53T α-synuclein expressing strains with rotenone and probucol yielded partial protection of the non-tg, K08E3.7 KO and A53T α-synuclein strains compared to untreated of the same strain; (+) p<0.01. (B & C) Co-treatment of the non-tg, K08E3.7 KO and A53T α-synuclein expressing strains with rotenone and DβHB (panel B) or TUDCA (panel C) fully protected the non-tg strain, but partially protected the K08E3.7 KO and A53T α-synuclein expressing strains compared to untreated nematodes of the same strain (+) p<0.01. Treatment with probucol, DβHB or TUDCA was unable to completely overcome the enhanced rotenone-induced toxicity of the transgenic strains as compared to the non-tg strain in any treatment group; (*) p<0.01. (D) Both probucol and DβHB provided partial protection against rotenone for K08E3.7 KO and A53T α-synuclein expressing strains, but did not show any additive benefit when combined together; (+) p<0.01 comparing the treatments, and (*) p<0.01 comparing the strains. (E) Probucol also did not show any additive benefit against rotenone toxicity when combined with TUDCA; (+) p<0.01 comparing the treatments, and (*) p<0.01 comparing the strains. (F) Combining DβHB and TUDCA fully protected against rotenone-induced toxicity in all the strains compared to untreated of the same strain; (+) p<0.01.
To test a strategy of bypassing rotenone-mediated inhibition of mitochondrial complex I, we utilized D-β-hydroxybutyrate (DβHB), which increases cellular succinate, a substrate for complex II (52,53). Treatment with DβHB protected the non-tg and PD-related lines against rotenone-induced toxicity in a dose-dependent manner, fully protecting the non-transgenic strain and partial protecting the K08E3.7 KO and the A53T α-synuclein lines (Figure 6B). Further studies in C. elegans suggest that the DβHB is acting by stimulating respiration, consistent with a mechanisms of action via the electron transport chain. Treating C. elegans with rotenone (50 μM, 1hr) blocked respiration, but concurrent treatment with 50 mM DβHB partially restored respiration (Supplemental Fig. 4). Full protection by DβHB in the non-tg strain is consistent with results from prior studies indicating that rotenone acts via inhibition of mitochondrial complex I.
The cytoprotective bile acid tauroursodeoxycholic acid (TUDCA), which is a more soluble form of ursodeoxycholic acid (a medicine used clinically for treatment of certain cholestatic liver diseases) was examined next for protection against rotenone-induced toxicity. TUDCA is a putative anti-apoptotic compound that inhibits Bax activity in eukaryotes, and might affect the apoptotic system in nematodes (54-56). Treatment with TUDCA yielded dose-dependent protection against rotenone-induced toxicity with full protection of the non-tg line occurring at 10 mM TUDCA. Treatment with 10 mM TUDCA also increased survival of the K08E3.7 KO and A53T α-synuclein expressing strains by 23.8% and 25%, respectively, but was unable to provide full protection (Figure 6C). We also tested other putative cytoprotective agents, including radicicol (0.1 – 10 mM), resverotrol (0.5 – 10 mM) and taxol (25 – 2000 μM), however none of these agents protected against rotenone toxicity.
DβHB shows additive protection in combination with TUDCA but not probucol
No dose of probucol, DβHB, or TUDCA, provided complete protection against rotenone-induced toxicity in the K08E3.7 KO or A53T α-synuclein expressing transgenic strains (Figure 6A-C). To test whether protection by the different agents was additive, the nematode lines were treated with different combinations of probucol, DβHB or TUDCA. Probucol did not supplement the protection against rotenone-induced toxicity provided by either DβHB or TUDCA in the K08E3.7 KO and A53T α-synuclein expressing lines (Figure 6D and E). However, cotreatment with DβHB and TUDCA fully prevented against rotenone-induced toxicity in the non-tg and transgenic lines (Figure 6F). This result suggests that a combined pharmacological approach based on activating mitochondrial complex II and inhibiting apoptosis in C. elegans can prevent the toxicity induced by rotenone in the presence of PD-related genetic changes, assuming that TUDCA acts in the worm by inhibiting apoptosis.
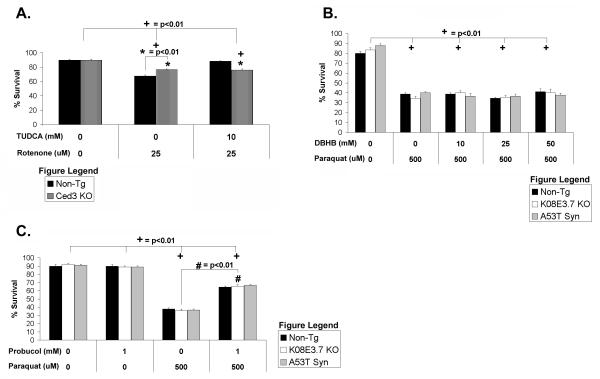
To test whether TUDCA inhibits apoptosis in C. elegans, we treated C. elegans lacking the executioner caspase ced-3 with 25 μM rotenone ± 10 mM TUDCA (Figure 7A). The worms lacking ced-3 showed less vulnerability to rotenone treatment, suggesting that the apoptotic machinery contributes to rotenone induced cell death (Fig. 7A). TUDCA did not provide any additional protection from rotenone-induced toxicity to the Ced-3 knockout nematodes although it did partially protect the non-tg worms (Figure 7A). The inability of TUDCA to add to the protection provided by Ced3 suggests that the mechanism of protection by TUDCA occurs at least in part through a pathway that involves the apoptotic apparatus.
Figure 7. Analysis of the mechanisms of action of TUDCA and paraquat.
(A) Treatment with TUDCA does not protect against rotenone-induced toxicity in the Ced-3 knockout worm compared to the untreated group; (+) p<0.01. The Ced-3 knockout worms was less vulnerable to rotenone toxicity than the non-tg strain, but addition of TUDCA did not provide any additional protection; (*) p<0.01. (B) Treatment with DβHB did not protect the non-tg, K08E3.7 KO or A53T α-synuclein expressing strain against paraquat induced toxicity. (+) p<0.01 compared to untreated of the same strain. (C) Treatment with probucol partially protects against paraquat induced toxicity in the non-tg, K08E3.7 KO and A53T α-synuclein expressing strains; (#) p<0.01 compared to paraquat treated of the same strain. (+) p<0.01 compared to untreated of the same strain.
Toxicity from paraquat is partially protected by probucol but not DβHB
Treatment of the non-tg and transgenic strains with paraquat indicated that all the strains were equally vulnerable to paraquat treatment (Figure 3B). Because the mechanism of paraquat toxicity is not fully understood, we sought to use the nematodes to explore the mechanism of toxicity. Co-treatment of non-tg nematodes with 25 μM paraquat ± 25 or 50 mM DβHB did not attenuate paraquat toxicity (Figure 7B). However, co-treatment with paraquat and probucol increased survival of all strains by 30% compared to paraquat treated alone (Figure 7C). The partial protection provided by probucol indicates that free radical generation contributes to paraquat toxicity. The inability of DβHB to protect against paraquat toxicity suggests that the toxicity in C. elegans is independent of complex I inhibition. A lack of full protection by probucol suggests either that paraquat has a secondary unknown mechanism of toxicity or that multiple free radical scavengers are required to minimize oxidation.
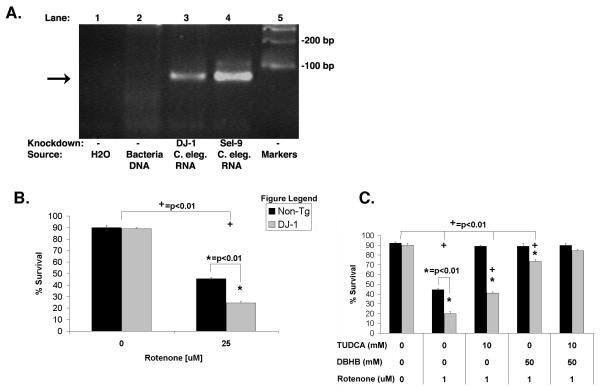
Knockdown of DJ-1 (B0432.2) increases vulnerability to rotenone, which is rescued by a combination of DβHB and TUDCA
The results described above show that C. elegans lines expressing α-synuclein (wild type or A53T) respond to complex I inhibition in much the same manner as C. elegans lines lacking parkin. To investigate whether this might be a trait that generalizes to other PD-related genes, we examined the responses of nematodes following knockdown of the B0432.2, the C. elegans homologue of DJ-1. The non-tg Bristol N2 C. elegans line was grown on HT115(DE3) E. Coli containing the L4440 vector coding for RNAi for B0432.2 (DJ-1). Two days later, the nematodes were exposed to 25 μM rotenone, and after four days the viability of the nematodes was scored. Knockdown of B0432.2 (DJ-1) produced a 53% reduction in transcript level (fig. 8A). The response of the nematodes resembled that of the other PD-related lines because the nematodes with B0432.2 (DJ-1) knockdown were observed to be significantly more sensitive to rotenone treatment than control nematodes (fig. 8B); knockdown by a bacterial line carrying a control vector (Sel-9) had no effect on the vulnerability to rotenone (data not shown). Rescue by DβHB and TUDCA was also examined. Nematodes with B0432.2 (DJ-1) knockdown were exposed to rotenone and either 25 mM DβHB, 10 mM TUDCA or both. Treatment with either DβHB or TUDCA alone induced a partial rescue of the B0432.2 (DJ-1) knockdown nematodes, but treatment with DβHB and TUDCA together elicited full protection (fig. 8C). These results provide evidence that PD-related lines of C. elegans carrying three different PD-related genetic factors exhibit similar responses to complex I inhibition.
Figure 8. Knockdown of DJ-1 increases vulnerability to rotenone and is rescued by DβHB and TUDCA.
(A) Knockdown of DJ-1 increased the vulnerability to rotenone-induced toxicity. Lane 1: H2O alone, Lane 2: RNA from the bacteria containing the B0432.2 (DJ-1) fragment used for the knockdown, Lane 3: RNA from C. elegans used for B0432.2 (DJ-1) knockdown, Lane 4: RNA from C. elegans used for Sel-9 knockdown, Lane 5: DNA standards. Lane 2 did not produce an amplification product because the procedure for isolating RNA effectively removed the DNA plasmid used to generate the RNAi. (B) Knockdown of DJ-1 increased the vulnerability to rotenone-induced toxicity. (C) Treatment with DβHB and TUDCA fully protected both the non-tg strain and the DJ-1 knockdown nematodes; (+) p<0.01 compared to untreated nematodes of the same line. (*) p<0.01 as compared to non-tg of the same treatment group.
DISCUSSION
Studies implicate a diverse array of environmental and genetic factors in the pathophysiology of PD. Increasing lines of evidence suggest that mitochondrial toxicity represents a common target of each of these agents. The research described above investigated the toxicological profile of three different genetic models in C. elegans related to PD. We examined C. elegans lines over-expressing wild type or A53T human α-synuclein, or lacking KO8E3.7, the homologue of parkin in C. elegans. Each line showed a selective increase in the vulnerability to inhibitors of the mitochondrial complex I, including rotenone, fenperoximate, pyridaben or stigmatellin. Knockdown of B0432.2, the homologue of DJ-1 in C. elegans also increased the vulnerability to complex I inhibition. The sensitivity of C. elegans to rotenone is less than that of mammalian organisms; but lower sensitivity to toxins is commonly observed in worms and might reflect the thick protective cuticle present in worms. In contrast, the C. elegans lines did not show increased sensitivity to other types of toxins, including inhibition of complex IV, oxidative stress or DNA damage. The convergence of the toxicology profiles on complex I prompted us to examine whether treatments that improve mitochondrial function might be applicable to nematode models related to PD. First we examined DβHB, which increases succinate levels and provides a secondary source of substrate for complex II (53). Treating the nematodes with DβHB fully protected the non-transgenic nematodes against rotenonemediated toxicity, and partially protected the PD-related lines against complex I inhibition. Combining DβHB with TUDCA, which inhibits apoptosis - another mitochondrial function, fully protected all the lines against complex I inhibition. These results suggest that PD-related genetic changes interfere with mitochondrial function, and disrupt complex I function. Whether they also affect the function of complexes II and/or III remains to be determined because the worms were not sensitive to 3-NP or antimycin A.
Each aspect of the genetic and toxic models studied above provide potentially important insights into the pathophysiology of human disease. The C. elegans line with the KO8E3.7/parkin deletion shows reduced levels of high molecular weight ubiquitin conjugates. This represents the first in vivo evidence that loss of a parkin homologue affects the ubiquitin proteasomal system. The studies reporting knockout of parkin in mice and knockout of parkin in Drosophila did not report any changes in ubiquitination, although levels of some mitochondrial proteins were noted to be changed by Palacino and colleagues (41,57,58). Once antibodies to the nematode homologues of parkin substrates are available it will be of great interest to study the turnover of these proteins.
The pathophysiological processes associated with rotenone toxicity in C. elegans bear many similarities to those occurring in mammals and provide valuable insights into the pathophysiology of complex I inhibition. Prior studies have shown that compounds such as rotenone and MPTP inhibit complex I of the mitochondrial electron transport chain in many species, including C. elegans (1). The ability of DβHB to protect against rotenone mediated toxicity provides further evidence that rotenone acts by inhibiting complex I activity. The secondary affects of complex I inhibition also appear to be similar in mammals and C. elegans. Rotenone has been shown to cause oxidative damage in rats in addition to inhibiting electron transport, and our results indicate that rotenone causes oxidative damage in C. elegans (4). Oxyblots of lysates from rotenone treated nematodes showed increased reactivity compared to untreated nematodes. Rotenone treatment causes the accumulation of α-synuclein aggregates in rats, possibly through a mechanism mediated by oxidative stress, and similar processes occur in C. elegans (3). Thioflavine positive deposits and α-synuclein aggregation were apparent following rotenone treatment of the A53T C. elegans line. In addition, fragmentation of the GFP signal in the A53T α-synuclein worms following rotenone treatment suggests damage to dopaminergic neurons. The pathology that we observed complements a prior report of α-synuclein-induced dopaminergic dysfunction in a transgenic synuclein worm line generated by Lasko and colleagues (42). The synuclein aggregates developed following rotenone treatment did not show SDS resistance, unlike that which occurs in humans, but this susceptibility to SDS might reflect the acute nature of the rotenone treatment paradigm used in this study. SDS resistant α-synuclein aggregates only appear in Drosophila after 3 weeks of aging, in rat after 6 weeks of rotenone treatment and in transgenic mice after 6 – 11 months of aging (42,47,50,59-61). These results suggest that the pathophysiology of rotenone toxicity in C. elegans shares important similarities with that occurring in mammals.
The specificity of rotenone and MPTP for complex I raises the possibility that treatments that act distal to complex I to enhance the function of the electron transport chain might be beneficial. DβHB is a ketone that increases the levels of succinate, which is a substrate for complex II (52). By increasing substrate levels for complex II, DβHB increases the activity of complex II and protects against a variety of toxic insults (52,53,62). Recent studies demonstrated that DβHB partially protects against MPTP in cell culture and in vivo (52,53). However, humans with PD might have genetic changes that predispose to the disease, which could render them less responsive to DβHB. The nematode models of PD have allowed us to explore how PD-related genetic changes alter the response to DβHB. The studies confirm that DβHB effectively protects non-transgenic nematode against complex I inhibition. However, DβHB was unable to fully protect against complex I inhibition in nematodes containing PD-related genetic changes. Although most patients with PD do not have mutations in parkin, synuclein or DJ-1, patients with PD might have other genetic changes that affect the mitochondria in a similar manner and render them less responsive to DβHB.
A limited screen of other known neuroprotective compounds identified two compounds, probucol and TUDCA, that partially protected against mitochondrial toxicity. Probucol was designed to lower cholesterol and has been used to treat hyperlipidemia in humans, but is also a potent anti-oxidant (51). Protection by probucol is consistent with the suggestion by Sherer and colleagues that rotenone induces free radical production and oxidative injury (4). However, probucol did not show protection that was additive with that of DβHB, which suggests that DβHB reduces oxidative stress in addition to increasing substrate availability for complex II. In contrast, TUDCA did show protection that was additive with that of DβHB. TUDCA has been shown to inhibit apoptosis in mammals, and its cousin, UDCA is clinically approved for use in humans (55,56,63). TUDCA has not been studied in C. elegans previously, but our studies indicated that TUDCA does not protect C. elegans lacking Ced 3, which suggest that TUDCA acts in part by inhibiting apoptosis. A corollary of these results is the likelihood that complex I inhibition induces apoptosis in the nematode as it does in mammal cells; measurement of caspase activity supports this hypothesis. The complex I inhibitor MPTP induces apoptosis in vivo, and mice lacking the pro-apoptotic protein Bax are less vulnerable to MPTP (64,65). Post-mortem studies of patients who died from PD also show histochemical evidence of apoptosis (66,67). Together, these results suggest that combination therapy that stimulates complex II function and inhibits apoptosis enhances protection against complex I inhibition.
Supplementary Material
Supplemental Figure 1: Functional analysis of K08E3.7 worm: defecation, egg laying and chemotaxis. A.) Defecation rate provided in feces per minute. B.) Egg laying is provided in duration of time needed for production of 100 eggs. C.) Chemotactic response. Methods: For the chemotactic response, worms were placed on plates dotted with diacetyl (DIA, diluted 1:1000), isoamylalcohol (IAA; diluted 1:200 in ethanol), trimethylthiazol (TMT; diluted 1:1000 in ethanol) and pyrazin (10 mg/ml. The attractant was placed at the end of the plate just past a 1.5 cm diameter ring of sodium azide (1M). The worms were placed on the other side of the plate). The assay was performed for 90 min at room temperature with 50 worms, and worms that were attracted were killed in the sodium azide. At the end of the assay chloroform was put on top of the dish, the dish was inverted to cover the dish which killed all remaining worms. The worms number of worms in the sodium azide area and out of the area were counted. The chemotaxis index is defined as A−B/A+B, where A is the +attractant, and B is just carrier (ethanol). count only the worms within 1.5 cm on a 10 cm plate. The response of the K08E3.7 KO worm was unchanged except for diacetyl.
Supplemental Figure 2: Complex I inhibitors, but not etoposide, inhibit respiration. C. elegans were incubated for 1 hr with differing complex I inhibitors (roteonone, 50 mM; pyridaben, 25 μM; phenylpyridin, 10 mM) or etoposide (50 μM) and respiration was measured. Each of the complex I inhibitors significantly decreased respiration, while etoposide did not exhibit a significant effect on respiration. P<0.001 compared to control.
Supplemental Figure 3. C. elegans expressing GFP targeted to the mitochondria show robust fluorescence in the mitochondrial after treatment with rotenone for either 1 day (A, 40X magnification) or 4 days (B, 10X magnification).
Supplemental Figure 4: DβHB partially protects C. elegans from inhibition of respiration by rotenone. C. elegans were incubated with rotenone (50 μM) ± DβHB (10 mM) for 1 hr and respiration was monitored. C. elegans treated with rotenone + DBHB showed significantly mored respiratory activity than worms treated with rotenone alone. P<0.05.
The abbreviations used are
- DβHB
D-β-hydroxybutyrate
- DNPH
2,4-dinitrophenyl hydrazine
- GFP
green fluorescent protein
- KO
knockout
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NAC
N-acetyl cysteine
- non-tg
non-transgenic
- TUDCA
tauroursodeoxycholic acid
Footnotes
Supported in part by grants from NIH grants NS41786, AG/NS17485 and USAMRC 17-01-1-0781. We would also like to thank Rosemary Bryant and Andrew Ferree for their assistance in preparing the manuscript. We also gratefully thank the following individuals for providing C. elegans strains: Brian Kraemer (U. Washington), Alexander Van der Bliek (UCLA) and Roger Horvitz (MIT). We also wish to acknowledge Claudia Rudolph at Elegene (Martinsried, Germany) for making/providing the KO8E3.7 deletion and A53T a-synuclein worm lines. We would also like to thank the Caenorhabditis Genetics Center (U. Minnesota) for providing the Bristol N2 C. elegans strain.
REFERENCES
- 1.Dauer W, Przedborski S. Neuron. 2003;39(6):889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 2.Langston J. Ann. Neurol. 1998;44(Suppl. 1):S45–52. doi: 10.1002/ana.410440707. [DOI] [PubMed] [Google Scholar]
- 3.Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Nat Neurosci. 2000;3(12):1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 4.Sherer TB, Betarbet R, Stout AK, Lund S, Baptista M, Panov AV, Cookson MR, Greenamyre JT. J Neurosci. 2002;22(16):7006–7015. doi: 10.1523/JNEUROSCI.22-16-07006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sherer TB, Betarbet R, Testa CM, Seo BB, Richardson JR, Kim JH, Miller GW, Yagi T, Matsuno-Yagi A, Greenamyre JT. J Neurosci. 2003;23(34):10756–10764. doi: 10.1523/JNEUROSCI.23-34-10756.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoglinger GU, Carrard G, Michel PP, Medja F, Lombes A, Ruberg M, Friguet B, Hirsch EC. J Neurochem. 2003;86(5):1297–1307. doi: 10.1046/j.1471-4159.2003.01952.x. [DOI] [PubMed] [Google Scholar]
- 7.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Science. 1997;276(5321):2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 8.Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen J, Schols L, Riess O. Nature Gen. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 9.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. Science. 2003;302(5646):841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 10.Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Tortosa EG, Del Ser T, Munoz DG, De Yebenes JG. Ann Neurol. 2004;55(2):164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 11.Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 12.Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, Gonzalez-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, Wood NW. Science. 2004 doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 13.Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P. Science. 2003;299(5604):256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- 14.Maraganore DM, Farrer MJ, Hardy JA, Lincoln SJ, McDonnell SK, Rocca WA. Neurology. 1999;53(8):1858–1860. doi: 10.1212/wnl.53.8.1858. [DOI] [PubMed] [Google Scholar]
- 15.Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Muller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, Gasser T. Neuron. 2004;44(4):601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Sharon R, Goldberg MS, Bar-Josef I, Betensky RA, Shen J, Selkoe DJ. Proc Natl Acad Sci U S A. 2001;98(16):9110–9115. doi: 10.1073/pnas.171300598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snyder H, Mensah K, Theisler C, Lee J, Matouschek A, Wolozin B. J Biol Chem. 2003;278(14):11753–11759. doi: 10.1074/jbc.M208641200. [DOI] [PubMed] [Google Scholar]
- 18.Ostrerova N, Petrucelli L, Farrer M, Mehta N, Alexander P, Choi P, Palacino J, Hardy J, Wolozin B. J. Neurosci. 1999;19:5782–5791. doi: 10.1523/JNEUROSCI.19-14-05782.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perez RG, Waymire JC, Lin E, Liu JJ, Guo F, Zigmond MJ. J Neurosci. 2002;22(8):3090–3099. doi: 10.1523/JNEUROSCI.22-08-03090.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Souza JM, Giasson BI, Lee VM, Ischiropoulos H. FEBS Lett. 2000;474(1):116–119. doi: 10.1016/s0014-5793(00)01563-5. [DOI] [PubMed] [Google Scholar]
- 21.Conway K, Harper J, Lansbury P. Nature Med. 1998;4:1318–1320. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- 22.Conway K, Rochet J, Bieganski R, Lansbury P. Science. 2001;294:1346–1349. doi: 10.1126/science.1063522. [DOI] [PubMed] [Google Scholar]
- 23.Conway KA, Lee SJ, Rochet JC, Ding TT, Williamson RE, Lansbury PT., Jr. Proc Natl Acad Sci U S A. 2000;97(2):571–576. doi: 10.1073/pnas.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ostrerova-Golts N, Petrucelli L, Hardy J, Lee J, Farrer M, Wolozin B. J. Neuroscience. 2000;20:6048–6054. doi: 10.1523/JNEUROSCI.20-16-06048.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimura H, Hattori N, Kubo S, Mizuno Y, Asakawa S, Minoshima S, Shimizu N, Iwai K, Chiba T, Tanaka K, Suzuki T. Nat Genet. 2000;25(3):302–305. doi: 10.1038/77060. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Gao J, Chung KK, Huang H, Dawson VL, Dawson TM. Proc Natl Acad Sci U S A. 2000;97(24):13354–13359. doi: 10.1073/pnas.240347797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petrucelli L, O’Farrell C, Lockhart PJ, Baptista M, Kehoe K, Vink L, Choi P, Wolozin B, Farrer M, Hardy J, Cookson MR. Neuron. 2002;36(6):1007–1019. doi: 10.1016/s0896-6273(02)01125-x. [DOI] [PubMed] [Google Scholar]
- 28.Yang Y, Nishimura I, Imai Y, Takahashi R, Lu B. Neuron. 2003;37(6):911–924. doi: 10.1016/s0896-6273(03)00143-0. [DOI] [PubMed] [Google Scholar]
- 29.Nagakubo D, Taira T, Kitaura H, Ikeda M, Tamai K, Iguchi-Ariga SM, Ariga H. Biochem Biophys Res Commun. 1997;231(2):509–513. doi: 10.1006/bbrc.1997.6132. [DOI] [PubMed] [Google Scholar]
- 30.Mitsumoto A, Nakagawa Y. Free Radic Res. 2001;35(6):885–893. doi: 10.1080/10715760100301381. [DOI] [PubMed] [Google Scholar]
- 31.Yokota T, Sugawara K, Ito K, Takahashi R, Ariga H, Mizusawa H. Biochem Biophys Res Commun. 2003;312(4):1342–1348. doi: 10.1016/j.bbrc.2003.11.056. [DOI] [PubMed] [Google Scholar]
- 32.Dong MQ, Chase D, Patikoglou GA, Koelle MR. Genes Dev. 2000;14(16):2003–2014. [PMC free article] [PubMed] [Google Scholar]
- 33.Tissenbaum HA, Guarente L. Nature. 2001;410(6825):227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 34.Wood WB. Cold Spring Harbor Laboratory; Cold Spring Harbor, N.Y.: 1988. [Google Scholar]
- 35.Brenner S. Genetics. 1974;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slice LW, Freedman JH, Rubin CS. J Biol Chem. 1990;265(1):256–263. [PubMed] [Google Scholar]
- 37.Fraser AG, Kamath RS, Zipperlen P, Martinez-Campos M, Sohrmann M, Ahringer J. Nature. 2000;408(6810):325–330. doi: 10.1038/35042517. [DOI] [PubMed] [Google Scholar]
- 38.Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM. Science. 2000;290(5493):985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- 39.Kayser EB, Morgan PG, Sedensky MM. Anesthesiology. 2004;101(2):365–372. doi: 10.1097/00000542-200408000-00017. [DOI] [PubMed] [Google Scholar]
- 40.Kramer KA, Oglesbee D, Hartman SJ, Huey J, Anderson B, Magera MJ, Matern D, Rinaldo P, Robinson BH, Cameron JM, Hahn SH. Clin Chem. 2005 doi: 10.1373/clinchem.2005.050146. [DOI] [PubMed] [Google Scholar]
- 41.Goldberg MS, Fleming SM, Palacino JJ, Cepeda C, Lam HA, Bhatnagar A, Meloni EG, Wu N, Ackerson LC, Klapstein GJ, Gajendiran M, Roth BL, Chesselet MF, Maidment NT, Levine MS, Shen J. J Biol Chem. 2003;278(44):43628–43635. doi: 10.1074/jbc.M308947200. [DOI] [PubMed] [Google Scholar]
- 42.Lakso M, Vartiainen S, Moilanen AM, Sirvio J, Thomas JH, Nass R, Blakely RD, Wong G. J Neurochem. 2003;86(1):165–172. doi: 10.1046/j.1471-4159.2003.01809.x. [DOI] [PubMed] [Google Scholar]
- 43.Kraemer BC, Zhang B, Leverenz JB, Thomas JH, Trojanowski JQ, Schellenberg GD. Proc Natl Acad Sci U S A. 2003;100(17):9980–9985. doi: 10.1073/pnas.1533448100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schuler F, Casida JE. Biochim Biophys Acta. 2001;1506(1):79–87. doi: 10.1016/s0005-2728(01)00183-9. [DOI] [PubMed] [Google Scholar]
- 45.Ladha JS, Tripathy MK, Mitra D. Cell Death Differ. 2005;12(11):1417–1428. doi: 10.1038/sj.cdd.4401668. [DOI] [PubMed] [Google Scholar]
- 46.Labrousse AM, Zappaterra MD, Rube DA, van der Bliek AM. Mol Cell. 1999;4(5):815–826. doi: 10.1016/s1097-2765(00)80391-3. [DOI] [PubMed] [Google Scholar]
- 47.Feany MB, Bender WW. Nature. 2000;404(6776):394–398. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- 48.Kahle PJ, Neumann M, Ozmen L, Muller V, Jacobsen H, Spooren W, Fuss B, Mallon B, Macklin WB, Fujiwara H, Hasegawa M, Iwatsubo T, Kretzschmar HA, Haass C. EMBO Rep. 2002;3(6):583–588. doi: 10.1093/embo-reports/kvf109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee M, Stirling W, Xu Y, Xu X, Qui D, Mandir A, Dawson T, Copeland N, Jenkins N, Price D. PNAS. 2002;99:8968–8973. doi: 10.1073/pnas.132197599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, Lee VM. Neuron. 2002;34(4):521–533. doi: 10.1016/s0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- 51.Barnhart RL, Busch SJ, Jackson RL. J Lipid Res. 1989;30(11):1703–1710. [PubMed] [Google Scholar]
- 52.Kashiwaya Y, Takeshima T, Mori N, Nakashima K, Clarke K, Veech RL. Proc Natl Acad Sci U S A. 2000;97(10):5440–5444. doi: 10.1073/pnas.97.10.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tieu K, Perier C, Caspersen C, Teismann P, Wu DC, Yan SD, Naini A, Vila M, Jackson-Lewis V, Ramasamy R, Przedborski S. J Clin Invest. 2003;112(6):892–901. doi: 10.1172/JCI18797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodrigues CM, Fan G, Ma X, Kren BT, Steer CJ. J Clin Invest. 1998;101(12):2790–2799. doi: 10.1172/JCI1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodrigues CM, Sola S, Nan Z, Castro RE, Ribeiro PS, Low WC, Steer CJ. Proc Natl Acad Sci U S A. 2003;100(10):6087–6092. doi: 10.1073/pnas.1031632100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodrigues CM, Sola S, Sharpe JC, Moura JJ, Steer CJ. Biochemistry. 2003;42(10):3070–3080. doi: 10.1021/bi026979d. [DOI] [PubMed] [Google Scholar]
- 57.Palacino JJ, Sagi D, Goldberg MS, Krauss S, Motz C, Klose J, Shen J. J Biol Chem. 2004 doi: 10.1074/jbc.M401135200. [DOI] [PubMed] [Google Scholar]
- 58.Greene JC, Whitworth AJ, Kuo I, Andrews LA, Feany MB, Pallanck LJ. Proc Natl Acad Sci U S A. 2003;100(7):4078–4083. doi: 10.1073/pnas.0737556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Auluck PK, Chan HY, Trojanowski JQ, Lee VM, Bonini NM. Science. 2002;295(5556):865–868. doi: 10.1126/science.1067389. [DOI] [PubMed] [Google Scholar]
- 60.Kahle PJ, Neumann M, Ozmen L, Muller V, Odoy S, Okamoto N, Jacobsen H, Iwatsubo T, Trojanowski JQ, Takahashi H, Wakabayashi K, Bogdanovic N, Riederer P, Kretzschmar HA, Haass C. Am J Pathol. 2001;159(6):2215–2225. doi: 10.1016/s0002-9440(10)63072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee D, Lee SY, Lee EN, Chang CS, Paik SR. J Neurochem. 2002;82(5):1007–1017. doi: 10.1046/j.1471-4159.2002.01024.x. [DOI] [PubMed] [Google Scholar]
- 62.Kashiwaya Y, Sato K, Tsuchiya N, Thomas S, Fell DA, Veech RL, Passonneau JV. J Biol Chem. 1994;269(41):25502–25514. [PubMed] [Google Scholar]
- 63.Keene CD, Rodrigues CM, Eich T, Chhabra MS, Steer CJ, Low WC. Proc Natl Acad Sci U S A. 2002;99(16):10671–10676. doi: 10.1073/pnas.162362299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chun HS, Gibson GE, DeGiorgio LA, Zhang H, Kidd VJ, Son JH. J Neurochem. 2001;76(4):1010–1021. doi: 10.1046/j.1471-4159.2001.00096.x. [DOI] [PubMed] [Google Scholar]
- 65.Vila M, Jackson-Lewis V, Vukosavic S, Djaldetti R, Liberatore G, Offen D, Korsmeyer SJ, Przedborski S. Proc Natl Acad Sci U S A. 2001;98(5):2837–2842. doi: 10.1073/pnas.051633998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dragunow M, Faull RL, Lawlor P, Beilharz EJ, Singleton K, Walker EB, Mee E. Neuroreport. 1995;6(7):1053–1057. doi: 10.1097/00001756-199505090-00026. [DOI] [PubMed] [Google Scholar]
- 67.Mochizuki H, Goto K, Mori H, Mizuno Y. Journal of the Neurological Sciences. 1996;137(2):120–123. doi: 10.1016/0022-510x(95)00336-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Functional analysis of K08E3.7 worm: defecation, egg laying and chemotaxis. A.) Defecation rate provided in feces per minute. B.) Egg laying is provided in duration of time needed for production of 100 eggs. C.) Chemotactic response. Methods: For the chemotactic response, worms were placed on plates dotted with diacetyl (DIA, diluted 1:1000), isoamylalcohol (IAA; diluted 1:200 in ethanol), trimethylthiazol (TMT; diluted 1:1000 in ethanol) and pyrazin (10 mg/ml. The attractant was placed at the end of the plate just past a 1.5 cm diameter ring of sodium azide (1M). The worms were placed on the other side of the plate). The assay was performed for 90 min at room temperature with 50 worms, and worms that were attracted were killed in the sodium azide. At the end of the assay chloroform was put on top of the dish, the dish was inverted to cover the dish which killed all remaining worms. The worms number of worms in the sodium azide area and out of the area were counted. The chemotaxis index is defined as A−B/A+B, where A is the +attractant, and B is just carrier (ethanol). count only the worms within 1.5 cm on a 10 cm plate. The response of the K08E3.7 KO worm was unchanged except for diacetyl.
Supplemental Figure 2: Complex I inhibitors, but not etoposide, inhibit respiration. C. elegans were incubated for 1 hr with differing complex I inhibitors (roteonone, 50 mM; pyridaben, 25 μM; phenylpyridin, 10 mM) or etoposide (50 μM) and respiration was measured. Each of the complex I inhibitors significantly decreased respiration, while etoposide did not exhibit a significant effect on respiration. P<0.001 compared to control.
Supplemental Figure 3. C. elegans expressing GFP targeted to the mitochondria show robust fluorescence in the mitochondrial after treatment with rotenone for either 1 day (A, 40X magnification) or 4 days (B, 10X magnification).
Supplemental Figure 4: DβHB partially protects C. elegans from inhibition of respiration by rotenone. C. elegans were incubated with rotenone (50 μM) ± DβHB (10 mM) for 1 hr and respiration was monitored. C. elegans treated with rotenone + DBHB showed significantly mored respiratory activity than worms treated with rotenone alone. P<0.05.