Abstract
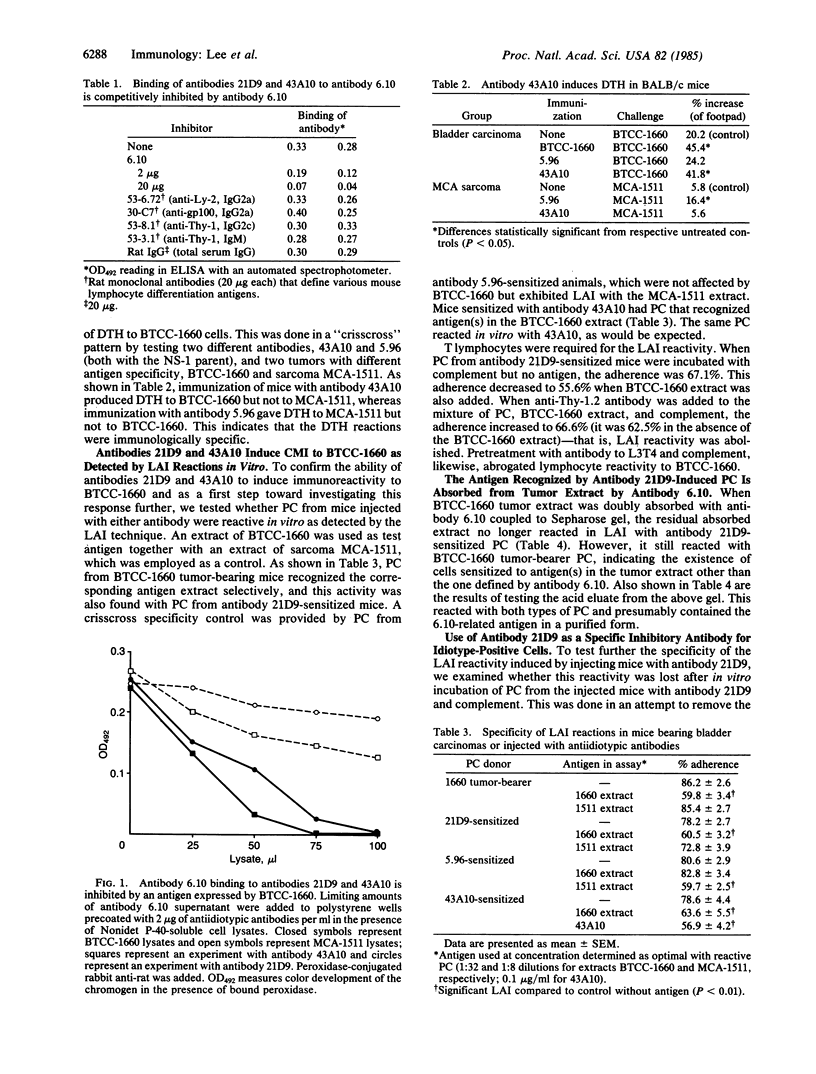
Rat monoclonal antibody 6.10 recognizes a 175-kDa protein expressed in all BALB/c mouse transitional cell bladder carcinomas tested, in epithelial cells of the mouse embryo, and in a few epithelial cells of adult mice. The antibody was used as an immunogen to generate two mouse monoclonal antibodies, 21D9 and 43A10, which bind to idiotopes on antibody 6.10 associated with the binding site for the 175-kDa antigen. The antiidiotypic antibodies induced bladder tumor-specific, cell-mediated immunity when injected into syngeneic mice, as shown by delayed-type hypersensitivity reactions in vivo and leukocyte adherence inhibition reactions in vitro. Tumor specificity was demonstrated by employing as controls a chemically induced BALB/c fibrosarcoma, MCA-1511 (MCA, 3-methylcholanthrene), and its corresponding antiidiotypic antibody, 5.96. Lymphocytes from mice sensitized with antibody 21D9 or 5.96 specifically recognized antigens in extracts of BALB/c bladder carcinoma BTCC-1660 (BTCC, bladder transitional cell carcinoma) and sarcoma MCA-1511, respectively, as shown by leukocyte adherence inhibition reactivity. This reactivity was selectively abrogated by prior treatment of the sensitized cells with the appropriate antiidiotypic antibodies and complement. An antigen recognized in vitro by antibody 21D9-sensitized lymphocytes could be separated from BTCC-1660 extract by immunoabsorption with antibody 6.10 and elution with acidic buffer. Our findings indicate that the oncofetal antigen defined by antibody 6.10 is recognized by the immune system of syngeneic mice and suggest that antiidiotypic antibodies related to certain oncofetal antigens can be used to immunize against syngeneic tumors.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binz H., Meier B., Wigzell H. Induction or elimination of tumor-specific immunity against a chemically-induced rat tumor using auto-anti-idiotypic immunity. Int J Cancer. 1982 Apr 15;29(4):417–423. doi: 10.1002/ijc.2910290410. [DOI] [PubMed] [Google Scholar]
- Binz H., Wigzell H. Shared idiotypic determinants on B and T lymphocytes reactive against the same antigenic determinants. I. Demonstration of similar or identical idiotypes on IgG molecules and T-cell receptors with specificity for the same alloantigens. J Exp Med. 1975 Jul 1;142(1):197–211. doi: 10.1084/jem.142.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPMAN W. H. A method for producing bladder tumors in mice, with a technique allowing easy interpretation of the difference between hyperplasia and true tumor. J Urol. 1962 Oct;88:518–526. doi: 10.1016/S0022-5347(17)64836-5. [DOI] [PubMed] [Google Scholar]
- Flood P. M., Kripke M. L., Rowley D. A., Schreiber H. Suppression of tumor rejection by autologous anti-idiotypic immunity. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2209–2213. doi: 10.1073/pnas.77.4.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstrom J. W., Nelson K. A., Nepom G. T., Hellström I., Hellström K. E. Immunization to a syngeneic sarcoma by a monoclonal auto-anti-idiotypic antibody. Nature. 1983 Jun 16;303(5918):627–629. doi: 10.1038/303627a0. [DOI] [PubMed] [Google Scholar]
- Halliday W. J., Maluish A. E., Little J. H., Davis N. C. Leukocyte adherence inhibition and specific immunoreactivity in malignant melanoma. Int J Cancer. 1975 Oct 15;16(4):645–658. doi: 10.1002/ijc.2910160415. [DOI] [PubMed] [Google Scholar]
- Halliday W. J., Maluish A., Miller S. Blocking and unblocking of cell-mediated anti-tumor immunity in mice, as detected by the leucocyte adherence inhibition test. Cell Immunol. 1974 Mar 15;10(3):467–475. doi: 10.1016/0008-8749(74)90138-5. [DOI] [PubMed] [Google Scholar]
- Hellström I., Hellström K. E., Rollins N., Lee V. K., Hudkins K. L., Nepom G. T. Monoclonal antibodies to cell surface antigens shared by chemically induced mouse bladder carcinomas. Cancer Res. 1985 May;45(5):2210–2218. [PubMed] [Google Scholar]
- Hellström I., Hellström K. E., Shepard T. H. Cell-mediated immunity against antigens common to human colonic carcinomas and fetal gut epithelium. Int J Cancer. 1970 Nov 15;6(3):346–351. doi: 10.1002/ijc.2910060304. [DOI] [PubMed] [Google Scholar]
- Hellström I., Rollins N., Settle S., Chapman P., Chapman W. H., Hellström K. E. Monoclonal antibodies to two mouse bladder carcinoma antigens. Int J Cancer. 1982 Feb 15;29(2):175–180. doi: 10.1002/ijc.2910290211. [DOI] [PubMed] [Google Scholar]
- Hellström K. E., Hellström I. Cellular immunity against tumor antigens. Adv Cancer Res. 1969;12:167–223. doi: 10.1016/s0065-230x(08)60331-0. [DOI] [PubMed] [Google Scholar]
- Herberman R. B. Cell-mediated immunity to tumor cells. Adv Cancer Res. 1974;19(0):207–263. doi: 10.1016/s0065-230x(08)60055-x. [DOI] [PubMed] [Google Scholar]
- Holbeck S. L., Nepom G. T. Enhanced Detection of immunoglobulin binding by a modified ELISA. J Immunol Methods. 1983 May 27;60(1-2):47–52. doi: 10.1016/0022-1759(83)90333-2. [DOI] [PubMed] [Google Scholar]
- Koppi T. A., Halliday W. J. Further characterization of the cells involved in leukocyte adherence inhibition with murine tumor extracts. Cell Immunol. 1982 Jan 15;66(2):394–406. doi: 10.1016/0008-8749(82)90189-7. [DOI] [PubMed] [Google Scholar]
- Koppi T. A., Halliday W. J. Regulation of cell-mediated immunologic reactivity to Moloney murine sarcoma virus-induced tumors. I. Cell and serum activity detected by leukocyte adherence inhibition. J Natl Cancer Inst. 1981 Jun;66(6):1089–1096. doi: 10.1093/jnci/66.6.1089. [DOI] [PubMed] [Google Scholar]
- Nepom G. T., Nelson K. A., Holbeck S. L., Hellström I., Hellström K. E. Induction of immunity to a human tumor marker by in vivo administration of anti-idiotypic antibodies in mice. Proc Natl Acad Sci U S A. 1984 May;81(9):2864–2867. doi: 10.1073/pnas.81.9.2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele G., Jr, Sjögren H. O., Rosengren J. E., Lindström C., Larsson A., Leandoer L. Sequential studies of serum blocking activity in rats bearing chemically induced primary bowel tumors. J Natl Cancer Inst. 1975 Apr;54(4):959–967. [PubMed] [Google Scholar]
- Taranger L. A., Chapman W. H., Hellström I., Hellström K. E. Immunological studies on urinary bladder tumors of rats and mice. Science. 1972 Jun 23;176(4041):1337–1340. doi: 10.1126/science.176.4041.1337. [DOI] [PubMed] [Google Scholar]


