Abstract
Aggression in humans and animals has been linked to androgens and serotonin function. To further our understanding of the effect of androgens on serotonin and aggression in male macaques, we sought to manipulate circulating androgens and the activity of aromatase; and to then determine behavior and the endogenous availability of serotonin. Male Japanese macaques (Macaca fuscata) were castrated for 5-7 months and then treated for 3 months with [1] placebo, [2] testosterone (T), [3] T+Dutasteride (5a reductase inhibitor; AvodartTM), [4] T+Letrozole (non-steroidal aromatase inhibitor; FemeraTM), [5] Flutamide+ATD (androgen antagonist plus steroidal aromatase inhibitor) or [6] dihydrotestosterone (DHT)+ATD (n=5/group). Behavioral observations were made during treatments. At the end of the treatment period, each animal was sedated with propofol and administered a bolus of fenfluramine (5 mg/kg). Fenfluramine causes the release of serotonin proportional to endogenous availability and in turn, serotonin stimulates the secretion of prolactin. Therefore, serum prolactin concentrations reflect endogenous serotonin. Fenfluramine significantly increased serotonin/prolactin in all groups (p <0.0001). Fenfluramine-induced serotonin/prolactin in the T-treated group was significantly higher than the other groups (p<0.0001). Castration partially reduced the serotonin/prolactin response; and Letrozole partially blocked the effect of T. Complete inhibition of aromatase with ATD, a non-competitve inhibitor, significantly and similarly reduced the fenfluramine-induced serotonin/prolactin response in the presence or absence of DHT. Neither aggressive behavior nor yawning (indicators of androgen activity) correlated with serotonin/prolactin, but posited aromatase activity correlated significantly with prolactin (p<0.0008; r2 =0.95). In summary, androgens induced aggressive behavior but they did not regulate serotonin. Altogether, the data suggest that aromatase activity supports serotonin production and that androgens increase aggression by another mechanism.
Keywords: Aggression, androgen, serotonin, fenfluramine, aromatase, letrozole, dutasteride
Introduction
A reduction in serotonin is thought to play a pivotal role in androgen-induced aggressive behavior in humans, non-human primates, foxes and rodents (G.L. Brown & LInnoila, 1990; Clark & Henderson, 2003; Coccaro, Kavoussi, Cooper, & Hauger, 1997; Dabbs, Frady, Carr, & Besch, 1987; Hall & Chapman, 2005; Higley, King, et al., 1996; Kreuz & Rose, 1972; McGinnis, Lumia, Breuer, & Possidente, 2002; Melloni & Ferris, 1996; Popova, 2006; Virkkunen, Goldman, Nielsen, & Linnoila, 1995; Wilson & Vessey, 1968). Studies with humans and macaques suggested that androgens decrease serotonin function, thereby increasing aggressive behavior (G.L. Brown & LInnoila, 1990; Coccaro et al., 1997; Virkkunen et al., 1995). However, in macaques it has also been suggested that aggression linked to low serotonin may be related to loss of inhibition, whereas aggression linked to testosterone may be derived from increased competition (Higley, Mehlman, et al., 1996). This report points to independent mechanisms of aggression for serotonin and testosterone.
Nonetheless, sparse data and inferences also suggest that serotonin is reduced in castrated or androgen-deprived men. An increased incidence of cognitive deficits and symptoms of depression/anxiety are observed in males in conditions of hypogonadism (Giltay et al., 2010; Ponholzer et al., 2009) and serotonin supports these functions. Castration increased the 5HT1A autoreceptor in the dorsal raphe, indicating lower extracellular serotonin (Zhang, Ma, Barker, & Rubinow, 1999). Moreover, castration reduced fenfluramine-induced prolactin secretion compared to normal men (Foresta, Indino, & Scandellari, 1987). Hence, low serotonin may be present in castrated males who exhibit low testosterone (T) and low aggression, as well as in intact males with high T and high aggression. In addition, several meta-analysis studies of aggression in humans and animals found little efficacy of SSRIs in the treatment of pathological aggression (Carrillo, Ricci, Coppersmith, & Melloni, 2009; Fava, 1997; Goedhard et al., 2006; Pappadopulos et al., 2006). Therefore, although many studies link androgens, aggression and reduced serotonin, other studies find reduced serotonin in the absence of androgens and aggression.
While androgens clearly increase aggression, apparently the regulation of serotonin by androgens is less clear. We hypothesized that androgens increase aggression, but that serotonin may not be directly regulated by androgens. Testosterone (T) can freely diffuse into the brain and T can be reduced to dihydrotestosterone (DHT) by 5α-reductase, or aromatized to estradiol, which acts through estrogen receptors (ER). Estradiol in the brain may also arise de novo from cholesterol. T and DHT both act through androgen receptors (AR), but DHT cannot be aromatized. We previously found ERβ in serotonin neurons of female macaques, which acted to increase serotonin synthesis (Gundlah, Lu, Mirkes, & Bethea, 2001), but AR did not colocalize with serotonin in rodents (Sheng et al., 2004). Thus, it was important to distinguish the effects of T, and its metabolites DHT and E, on serotonin and aggression.
We explored this issue in castrated male Japanese macaques and sought to manipulate brain androgens and aromatase activity with various treatments. During treatments, behavior was monitored; and at the end of treatment, serotonin was determined. Previous studies in macaques suggested that exogenous administration of estradiol differed from estradiol produced by aromatase in specific brain regions (Zumpe, Bonsall, & Michael, 1993). We termed the estradiol produced in the brain by aromatase activity as ‘neuroE’ to distinguish it from serum estradiol. Global serotonin availability was assessed with a fenfluramine challenge. Fenfluramine induces serotonin release, which stimulates prolactin secretion. Therefore, measurement of serum prolactin indicates the endogenous serotonin availability (Cleare, Murray, & O'Keane, 1998; O'Keane & Dinan, 1991; Rothman & Baumann, 2002). Behavior was observed for correlational analyses.
Methods and Materials
This experiment was approved by the IACUC of the Oregon National Primate Research Center and conducted in accordance with the 2011 Eight Edition of the National Institute of Health Guide for the Care and Use of Laboratory Animals. Male Japanese macaques (Macaca fuscata) were utilized for study.
Troop
The Japanese macaques were born and raised in a 2-acre outdoor corral at ONPRC with approximately 300 individuals. The troop has been the subject of extensive behavioral studies since it arrived at ONPRC in 1965 (Eaton & Resko, 1974; Eaton, Worlein, & Glick, 1990). The troop composition is relatively stable and the age structure is comparable to that of a natural troop (Maruhashi, 1982). Like other macaque species, the hierarchical organization of the troop is along matriarchal lineages. The matriarchal lines and dominance hierarchies within the troop are well documented, and have remained stable for the past 40 years. Males normally leave the natal troop and so their dominance is less a function of their mother’s status and more a function of their age, size and social skills.
Study Animals
Twenty adult male Japanese macaques were assigned to this project. The animals were aged from 4.9-9.8 years (average 7.1 years) and weighed between 7.7 and 16.2 kg (average of 12.81 kg). Microsatillite analysis of the entire Japanese macaque troop indicated that the animals were highly related. Table 1 contains the animal ages and weights as they progressed through the protocol. From birth, each animal was clinically checked, marked for observation purposes and administered a tuberculosis test each year as part of the basic husbandry procedures for the whole troop. This age range is considered adult.
Table 1.
Average and range of age and weight of the male Japanese macaques used in this study.
| Age (yrs) Mean (range) |
Weights (kg) Mean (range) |
|
|---|---|---|
| Year 1 (n=20) | 6.9±0.2 (4.9-9.8) |
|
| Intact | 12.8±0.6 (7.7-16.2) |
|
| Castrate 3 mo | 12.5±0.5 (8.5-16.8) |
|
| Castrate 5.5 mo | 13.1±1.3 (9.4-17.2) |
|
| Treated 3 mo | 12.8±0.9 (9.3-16.2) |
|
| Year 2 (n=10) | 7.7±0.4 (6-10) |
|
| Castrate Rested 7 mo |
12.9±0.5 (10.2-14.9) |
|
| Treated 3 mo | 13.9±1.1 (11.3-18.3) |
|
All the animals were born in an outdoor 2-acre corral. Several years prior to this study, 6 of the males were removed and put together into an indoor/outdoor pen and they stayed together as one group for the entire study. The remaining 14 animals were brought in from the corral at the start of the study.
All animals were castrated in the first week of May 2010. Then, all 20 monkeys were acclimated to new indoor group housing consisting of 3 adjacent pens (6-8 animals/pen) in a horseshoe configuration. The 6 animals previously housed together were in pen A. The 14 males from the corral were split into two groups (pen B and Pen C), one containing 6 individuals and the other containing 8. Each pen had 3 compartments devised with screens and small doors, which were left open, allowing individuals access to all 3 sections within their pen. These doors could be closed, preventing entry or escape from an individual compartment. The monkeys were trained to gather in one section and the doors were closed. The animals were further trained to respond to a hand signal and to individually move into the middle section, as the door was raised, to receive a food treat containing drugs. Then, they shifted into the final section until all animals were treated. The doors were re-opened and the animals resumed dispersion throughout the 3 sections. This process took approximately 20-30 min per group. Animal grouping and mechanisms of drug administration are illustrated in the Supplemental material.
In pens A and B, there were 2 dominant, 2 mid-ranking and 2 subordinate males. In pen C, there were 2 dominant, 4 mid-ranking and 2 subordinate males. The treatment groups also had representatives of each rank. The treatments were balanced among the ranks and within the pens so that different ranks and pens were represented in each treatment group. The win-loss results for determination of dominance and the distribution of ranks and treatments in the pens are presented in the Supplemental material.
Animal Treatments
The experimental period was conducted during the mating period when aggression is highest amongst males in the troop. Although the animals were housed indoors, their annual rhythms continue to be manifested (Rostal, Glick, Eaton, & Resko, 1986). The experimental treatments began in the second week of October 2010. The start time was approximately 2 weeks prior to mating season, which is considered November through February. The groups were treated with placebo (empty Silastic capsules), testosterone (T), T + Dutasteride and T + Letrozole (n=5/group). The placebo group was expected to have no androgen activity and no conversion of T to E in the brain. However, independent production of neuroE from cholesterol could remain. Therefore, the placebo group was predicted to have no AR activation and little ER activation in aromatase positive regions (low AR & reduced ER).
Testosterone was administered with 4 Silastic implants per animal (6-cm long; i.d. 0.132 in.; o.d. 0.183 in.; Dow Corning, Midland, MI) that were packed with crystalline T (Sigma, St. Louis, MO) and implanted subcutaneously on the back. With T only treatment, it was expected that the brain was exposed to T and DHT via neural 5α-reductase and neuroE via neural aromatase, which in turn fully activated AR and ER in a local manner (high AR & high ER).
Dutasteride is a 5α-reductase inhibitor. The pharmaceutical form (AvodartTM) is a gelatin capsule with liquid drug inside. Humans may be prescribed 0.5 – 1.0 mg/day; and we chose the higher dose for the monkeys. Each capsule contained 0.5 mg/350μl liquid. With an average human weight of 70 kg, humans receive 14 μg/kg/day. This dose was used in the monkeys which equaled 14 μg/10μl/kg/day administered daily in a food treat. After 1 month, DHT was suppressed 50%. Monkeys can metabolize drugs faster than humans; so the dutasteride dosage was doubled to 28 μg/20μl/kg/day for the remaining 2 months in an attempt to further reduce DHT. However, serum DHT was not further reduced with the higher dose suggesting that a maximally effective dose was achieved. With T+Dutasteride, it was expected that the brain was exposed to T and reduced DHT, but aromatase was intact and produced neuroE. In turn, there was reduced activation of AR and full activation of ER in a local manner (reduced AR & high ER).
Letrozole is a non-steroidal competitive inhibitor of aromatase. The pharmaceutical form (FemaraTR) is a tablet, which was pulverized with a mortar and pestle. Based upon the human dosage of 2.5 mg/98 mg tablet /day and an average human weight of 70 kg, letrozole was administered daily in a food treat at 35 μg/kg/day or 1.5 mg of tablet/kg/day. We had no way to determine brain aromatase activity in living macaques. In a pharmacokinetic study, plasma letrozole concentrations showed high interpatient variability (>10-fold) and were associated significantly with CYP2A6 genotypes, body mass index (BMI) and age (Desta et al., 2011). Therefore, due to faster drug metabolism and to insure adequate serum levels of letrozole across the 5 treated animals, the dosage was increased to 70 μg/kg/day at the same time the dutasteride dose was increased. No side effects were manifested to either drug. Blood samples were obtained routinely under ketamine sedation (ketamine HCl, 10mg/kg, s.c; Fort Dodge Laboratories, Fort Dodge, IA). Serum levels of T, DHT and estradiol (E) were measured prior to drug treatment, 1 or 2 months after treatment and 3 months after treatment, immediately prior to the fenfluramine challenge. With T+Letrozole, it was expected that the brain was exposed to T, DHT, and reduced neuroE from block of aromatase. Thus, we expected full activation of AR and reduced activation of ER to an unknown degree in a local manner (high AR & reduced ER).
After 3 months of treatment, the fenfluramine challenge was administered as described below. The animals were returned to their home pens and maintained on drug treatment until the results of the challenges were obtained. After obtaining the results of the fenfluramine challenges, the placebo- and T-treated groups were euthanized as recommended by the Panel on Euthanasia of the American Veterinary Association, and as described below. These groups could not be held over and repeated in year 2 due to per diem costs and housing constraints.
The T+Dutasteride and T+Letrozole groups had their implants removed and drug treatments discontinued at the same time as euthanasia of the other two groups. Several weeks after implant removal the animals were moved to small indoor/outdoor group housing. Attempts to house 2 of the bonded animals from Pen A with any of the other animals, singlely or in small groups, resulted in serious fighting. Japanese macaques are very antagonistic toward unfamiliar animals. Therefore, the 2 bonded animals were housed together and the remaining eight animals were housed together. These 10 animals rested until mid-October 2011 (7 months) in the hypogonadal state. The half-life of dutasteride is several weeks, which necessitated at least a six-month washout after initial treatment.
Two weeks prior to mating season, new treatments were initiated. We sought an aromatase inhibitor with verified inhibition in nonhuman primates. The noncompetitive steroidal aromatase inhibitor androsta-1,4,6-triene-3,17-dione (ATD) was chosen. ATD was shown to inhibit >80-90% of aromatase in macaque brain (Ellinwood, Hess, Roselli, Spies, & Resko, 1984). Two treatment groups were established (n=5/group). One group was treated with DHT + ATD. DHT is a nonaromatizable androgen providing only AR stimulation. Therefore, this group was established with significant androgen activity and 90% inhibition of aromatase, which in turn fully activated AR and nearly removed ER activation in a local manner (high AR & no ER).
The other group was treated with Flutamide + ATD (an androgen receptor antagonist plus ATD). The rationale for adding Flutamide to ATD was derived from the observation that ATD activated androgen receptors (AR) in castrated macaques (Resko, Connolly, Roselli, Abdelgadir, & Choate, 1993). Therefore, this group was established with no androgen activity and inhibition of most aromatase, which in turn significantly reduced activation of both AR and ER in a local manner (no AR & no ER).
Five animals received Flutamide 90-day pellets (5 pellets/animal; Innovation Research of America, Sarasota, FL), which were implanted subcutaneously on the left side of the periscapular region. The other five animals received DHT 90-day pellets (1 pellet/animal; Innovation Research of America) that were also implanted on the left side of the periscapular region. All animals received ~2 gms ATD powder (Changzhou Harvest Chemistry, Changzhou, Jiangsu, China) in 3 × 4 cm packets made from Silastic sheeting (0.01 in thick; AART, Inc., Reno, NV) and implanted subcutaneously on the right side of the periscapular region. The purity of the ATD was greater than 90% as determined with NMR (Dr. Andrew Placzek, Dept Physiology and Pharmacology, OHSU). Blood samples were obtained prior to treatment, after 1 or 2 months, and after 3 months of treatment for immunoassay of T, DHT and E. Behavioral observations were taken as described below. After 3 months of treatment, each animal received a fenfluramine challenge at which time the 3-month sample for steroid assays was obtained. At the end of the challenge and without waking up, the animals were taken to the necropsy suite and immediately euthanized as recommended by the Panel on Euthanasia of the American Veterinary Association, and as described below.
Two different aromatase inhibitors were used due to different mechanisms of action. Letrozole is a competitive inhibitor and thus obeys the laws of mass action. Letrozole is not a steroid. It will bind and unbind to the enzyme in competition with T and in a concentration dependent manner. On the other hand, ATD is a non-competitive inhibitor, also known as a ‘suicide’ inhibitor. ATD binds to aromatase and permantly inactivates it, or ‘kills’ the enzyme. ATD is a steroid. Since we could not determine active aromatase in the brain of living macaques, we employed both types of aromatase inhibitors before drawing conclusions.
Fenfluramine challenge
At the end of the 3-month treatment period, each animal received a fenfluramine challenge. On the day of challenge, the animal was directed to a transport cage, sedated with ketamine (100 mg) and transported to a surgical suite. The monkeys were placed on a temperature-regulated surgical table and connected to vital sign monitors under the supervision of the veterinary surgical staff. A catheter was inserted into the cephalic vein in the forearm and IV infusion of propofol for anesthesia was initiated at 160-200 mg/min/kg body weight and maintained throughout the duration of the experiment with a Harvard pump. The saphenous vein in the leg was catheterized for blood sampling and for administration of the fenfluramine with a short extension tube connected to a three-way stopcock. When the animals reached deep sedation/light anesthetic condition, they were allowed to stabilize for 1 hour. This allows all the ketamine to clear and prolactin to reach basal levels. Blood samples (0.5 ml) were obtained every 10 minutes for 1 hour to establish a baseline of hormone secretion. Then, fenfluramine (5 mg/kg in saline; Sigma, St. Louis, MO) was injected and blood samples were obtained for an additional 2 hours at 10-minute intervals. In year 1, surgical staff removed the catheters and the animals were awake within 5 minutes of termination of the propofol infusion. Animals were placed in an ICU cage and monitored for return of normal activities. After several days the animals were returned to group housing and readily accepted by cage mates. In year 2, the animals remained anesthetized and were immediately taken to necropsy. The blood samples were maintained on ice until the end of the experimental period and then centrifuged to obtain serum for immunoassay.
Behavioral observations
Table 2 details the ethogram of the behaviors coded in this study. Behaviors were organized into three behavioral classes, social behavior, non-social behavior, and events. Behaviors within the social and non-social behavioral class were mutually exclusive, but could co-occur with behaviors from the other class (e.g., an individual cannot be alone and touching another animal, but could be alone and stationary). Behaviors that naturally occurred in relatively short durations, such as scratches or threats, were classified as “events”. A trained observer utilized instantaneous focal sampling techniques (Easley, Coelho, & Taylor, 1989; Lehner, 1992) in which the social and non-social behavior of the subject was recorded at 15sec intervals for the 10 min. Because events had relatively short durations, we used all occurrence sampling to record these behaviors (i.e., the observer recorded the number of times the focal individual engaged in the behavior). For the purposes of this study, the analysis was focused on behaviors that might indicate anxiety (e.g., scratching, yawning, stereotypies) and social behaviors (e.g., any social interaction, grooming, aggressive behaviors).
Table 2.
Ethogram of the behaviors that were monitored during behavioral observations. Social and non-social behaviors were measured as percent of time, and events were measured as frequency (number per minute). Behaviors within the social or non-social behavioral class were mutually exclusive, but could co-occur with behavior from the other behavioral class. Astericks indicate behaviors unique to female introductions.
| Behavioral class | Behavior | Definition |
|---|---|---|
| Social behaviors | Groom | Focal individual is picking at hair and/or skin of another individual |
| Proximity | Focal individual is within arms length of another individual without touching |
|
| Touch | Focal is in physical contact with another individual | |
| Close* | Male is located within 1m of female during female introduction |
|
| Look* | Male is paying attention to female during female introduction |
|
| Non-social behaviors |
Consummatory | Handling and ingesting food and/or water |
| Locomotion | Movement – e.g., walk, run | |
| Stationary | Focal individual sitting quietly, not engaged in other behavior |
|
| Stereotypical | Repetitive behavior with no apparent purpose, such as pacing or circling |
|
| Toy play | Focal individual manipulates object (e.g., toys) other than food |
|
| Events | Aggression | Bite, hit, slap, threat, chase, yawn |
| Displace | Individual leaves promptly upon being approached | |
| Fear grimace | Focal individual bars teeth | |
| Lipsmack | Rapid movement of lips | |
| Scratch | Common usage | |
| Yawn | Common usage |
a. Behavior of males in home environment
In year 1, 10-minute focal observations were obtained on the subjects in their pens. Observations were taken 2-3 times per week before and after the animals started to receive treatment (see above) by a trained observer. Eight focal observations were obtained on each individual prior to the start of the treatment and 14 observations were obtained after treatment in the home environment. The order of observations was randomized, to ensure that animals had an equal probability of being observed in relationship to daily events such as morning feedings. All observations were taken in the morning, between 8 am and noon. The observer stood in the center of the room to take these observations. Before observations began, the observer spent at least 5 minutes standing in the room to allow the monkeys to acclimate to her presence. The monkeys quickly acclimated to the presence of the observer and took little notice of her presence. We averaged data across two conditions: baseline (i.e., before treatment; n=8 observations per individual) and post-treatment (i.e., one month after treatment began; n=14 observations per individual).
In year 2, we assessed behavior of the males in the second housing arrangement using 10 min focal observations as detailed above. Observations were taken 1-2 times per week after the animals received their implants for a total of 7-8 observations per individual in the home environment. The observer stayed approximately 1.5 meters from the outside portion of the enclosure. Before observations began, the observer spent at least 5 minutes standing outside to allow the monkeys to acclimate to her presence. If the focal individual went to the indoor area, the observer would count that as a “time out”. Because the behaviors of the animals within a group are highly dependent upon the others in the group, we only performed statistical analysis on the behavior of individuals from the group of 8 (Flutamide + ATD, n=4; DHT + ATD, n=4). This group also had a similar number of animals as the pens in year 1. The experience of the 2 monkeys housed separately due to fighting was very different from that of 8 monkeys housed together.
b. Response to females
Four female Japanese macaques were implanted subcutaneoulsy with Silastic capsules containing estradiol (4 cm) to maintain attractiveness and receptivity at all times. In order to assess the response of each male to a female without the influence of other group members, we ran a “female introduction” test. In this test, the male was sequestered into one compartment of his pen by himself, to avoid interactions by the other males. One of the four female Japanese macaques bearing estradiol implants (4 cm) was randomly selected and put into a cage and wheeled within 0.5 m from the front of the pen. The monkeys effectively had the potential for restricted tactile contact. Curtains were placed such that the females could not see the males in the other pens. The monkeys were videotaped remotely for 30 min to avoid confound of the observer’s presence. The videotapes were later scored using specialized software (The Observer, Noldus, the Netherlands). The amount of time the male spent close to the female (i.e., within 1 meter of the front of the females cage) and the amount of time the male spent attending to (i.e., looking at) the female were recorded. Any attempts to touch, present, lipsmack, or other affiliatative or aggressive behaviors (see ethogram) were also recorded. This female introduction was performed during treatment periods, two times on each individual and separated by at least 6 weeks. Only 1-2 males were tested on any given day, and males from the same group were never tested on the same day. These tests were performed in the afternoon, at least 2 hours after the home environment observations. We performed a modified version of this test in year 2. The day before the final fenfluramine challenge, the males were removed from their group and placed in a double cage in a separate room containing another conspecific with similar caging for visual contact. The morning prior to the challenge, a female Japanese macaque (one of the four original females) was put in a rolling cage, which was placed directly in front of the subject’s cage. The monkeys were videotaped for 30 min and the tapes were scored the same as for year 1. This test was only performed one time in year 2.
Measurement of TPH2 by western blot
To obtain a preliminary verification that T, or its metabolites DHT and E, act on serotonin at a cellular level, we examined the protein expression of TPH, the rate-limiting enzyme for serotonin synthesis in intact versus castrated males. The castrated males were agonadal for 1 month prior to being euthanized. The pontine midbrain was obtained, wrapped in foil, frozen in liquid nitrogen and stored at -80C until all of the midbrains were obtained. The midbrains were semi-thawed and dissected further to remove a small block of tissue containing the dorsal raphe area. The dorsal raphe microblock was homogenized and processed for western blot as described previously (Smith, Henderson, Abell, & Bethea, 2004). The samples loaded contained the same concentration of protein.
Aromatase activity ranking
To further compare the serotonin/prolactin response to fenfluramine and steroid hormone action, we speculatively ranked the aromatase activity, or the presumed production of neuroE, in each group on a scale of 0 (none) to 80 (extremely high activity). The ranking was based upon our best understanding of the mechanism of action of the aromatase inhibitors (Jordan & Brodie, 2007), the actual measurement of aromatase activity in the brain of macaques (Resko et al., 1993), the role of T as the substrate for aromatase activity (Reed & Ohno, 1976) and the role of T and DHT in the stimulation of aromatase gene expression (Roselli & Resko, 1989). The ranking is put forward only to provide visualization of a potential relationship and a hypothesis for further testing.
Assays
Assays for PRL, T, DHT and E were performed on serum using a Roche Diagnostics Cobas e411 automatic clinical platform assay instrument. Serum was subjected to liquid chromatography over Sephadex LH-20 to separate T and DHT prior to assay. Therefore, the DHT fraction contains >70% DHT and is compared to a DHT standard curve. The T fraction is compared to a T standard curve yielding specificity with an antiserum that recognizes both (Resko, Ellinwood, Pasztor, & Huhl, 1980). In addition, each assay has been validated for macaque plasma in comparison with traditional RIA. Within-assay coefficients of variation were less that 5% and between-assay coefficients of variation were less that 10%.
Euthanasia
Monkeys were euthanized at the end of the treatment periods for collection of the brain and other tissues according to procedures recommended by the Panel on Euthanasia of the American Veterinary Association. Each animal was either sedated with ketamine in a transport cage or maintained under sedation with propofol after the fenfluramine challenge. They were transported to the necropsy suite under sedation, given an overdose of pentobarbital (30 mg/kg, i.v., Hospira, Lake Forest, IL) and exsanguinated by severance of the descending aorta. The brain was perfused and removed for future study.
Statistical Analysis
Prolactin secretion was analyzed with a 2-way ANOVA, which only uses Bonferroni’s posthoc pairwise comparisons at each time point. The area under the prolactin secretion curves (AUC) was compared with 1-way ANOVA followed by Newman Keuls posthoc pairwise comparisons. A 2-way ANOVA was used to compare pre- and post-treatment behavioral values. Aggressive behaviors and yawning in rate/minute were also compared between the groups with 1-way ANOVA followed by Newman Keuls’s posthoc analysis. In addition, the t-test was used to compare combined pre-treatment behavioral values to each treatment group. Raw and log transformed scratching data were analyzed with 1-way ANOVA. The t-test was also applied to the log transformed scratching data due to limitations of the ANOVA. Although 10 animals from year 1 were carried over to year 2 with different treatments, we considered each treatment group independent, due to the long rest period between the protocols. Serum concentrations of T and DHT were analyzed with ANOVA followed by Newman Keul’s posthoc pairwise comparisons. The change in housing was mandated by the ONPRC Division of Comparative Medicine.
Results
Fenfluramine and serotonin-induced prolactin secretion
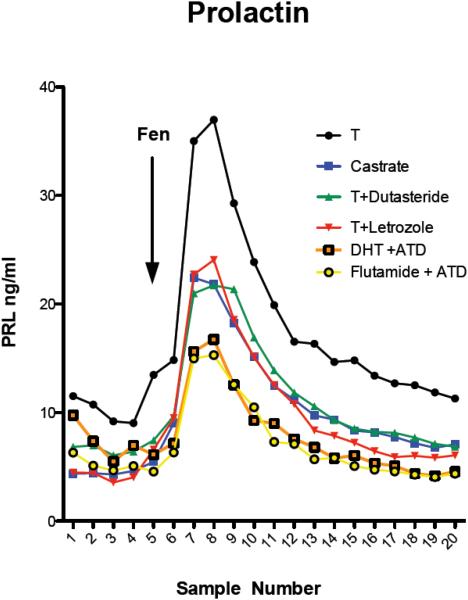
Prolactin secretion in response to fenfluramine administration is illustrated in Figure 1. With 2-way ANOVA, there was a significant difference across time (F[df19]=20.15, p=0.0001) and between the groups (F[df5]=30.86, p=0.0001). Between samples 7 through 10, the T-treated group was significantly higher than all of the other groups (Bonferroni’s p < 0.001 to p < 0.05). Of note, prolactin secretion was lowest in the Flutamide + ATD and DHT+ATD groups, an indication of very low serotonin availability. Both of these groups were predicted to have the lowest aromatase/neuroE activation. However, the DHT+ATD group was predicted to have high androgen activity and the Flutamide+ATD group was predicted to have no androgen activity. This prediction is supported by yawning behavior (below).
Figure 1.
Average prolactin secretion after fenfluramine administration to the 6 treatment groups. There was a significant difference across time and between treatment groups. Samples 7-10 of the T-treated group were significantly higher than all of the other groups. The standard error around the mean of each data point is not shown in this figure for clarity, but it is shown in the next figure for the area under the prolactin curve.
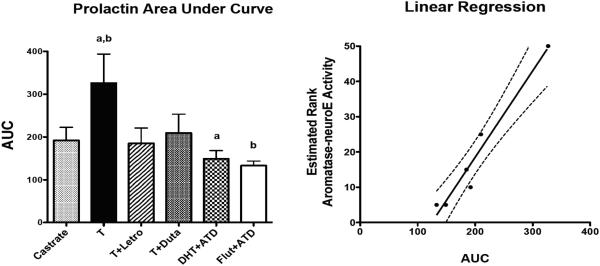
For further analysis, the area under the prolactin curve was obtained and subjected to statistical analysis. As illustrated in Figure 2-left, there was a significant difference between the groups in the AUC (F[df5]=2.901, p=0.035). The T-treated group was significantly higher than the DHT+ATD group and the Flutamide+ATD group by posthoc pairwise comparison (Newman Keul’s p < 0.05).
Figure 2.
The area under the prolactin curve (AUC) for each group and the regression analysis of the AUC values versus the estimated rank of aromatase/neuroE activity in each group is illustrated. Left.There was a significant difference between the groups in AUC. The prolactin AUC of the T-treated group was significantly higher than the DHT+ATD and the Flutamide+ATD groups. Right. There was a significant correlation between the AUC of prolactin secretion and the aromatase activity rankings (p < 0.0008; r2 = 0.95). There was no correlation between the AUC of prolactin secretion and the yawning/androgen activity scores (data not shown).
a, b Groups with the same letter are different with p < 0.05.
Linear regression analysis
Yawns, shown in Table 3, were used as an indication of androgenic activity in each group according to reports in the literature (Anderson, 2010; Deputte, Johnson, Hempel, & Scheffler, 1994; A. Troisi, Aureli, F., Schino, G., Rinaldi, F., DeAngelis, N., 1990). To determine if there was a correlation between fenfluramine-induced serotonin/prolactin secretion and either yawns/androgen activity or aromatase/neuroE activity, linear regression analysis was conducted. We found that there was no correlation between the AUC of prolactin/serotonin secretion and yawns/androgenic activity.
Table 3.
Concentrations of serum T, DHT and E prior to fenfluramine challenge and euthanasia. Also shown are the average area under the prolactin curve values, the theoretical ranking of aromatase activity, and yawning rate/minute X 100 as a direct indication of androgenic activity.
| Groups | ||||||
|---|---|---|---|---|---|---|
| Year 1 | Year 2 | |||||
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Castrate | T+Dutasteride | T+Letrozole | Testosterone | Flutamide+ATD | DHT+ATD | |
| Steroid concentrations | ||||||
| (mean±SEM) | ||||||
| Castrate 5 mo or Rested 7 mo | ||||||
| T (ng/ml) | 0.05±0.001 | 0.03±0.003 | 0.05±0.13 | 0.04±0.006 | 0.063±0.008 | 0.04±0.006 |
| DHT (ng/ml) | 0.11±0.02 | 0.15±0.01 | 0.34±0.16 | 0.08±0.009 | 0.178±0.007 | 0.24±0.053 |
| E (pg/ml) | <5 | <5 | <5 | <5 | <5 | <5 |
| 1 or 2 months Treatment | ||||||
| T (ng/ml) | <0.025 | 7.22±0.60 | 7.21±0.82 | 7.85±1.71 | 1.31±0.52 | 1.74±0.67 |
| DHT (ng/ml) | 0.22±0.15 | 0.93±0.46 | 1.94±0.39 | 2.07±0.79 | 0.31±0.05 | 19.37±3.69 |
| E (pg/ml) | <5 | <5 | <5 | <5 | <5 | <5 |
| 3 months Treatment | ||||||
| T (ng/ml) | 0.347±0.25 | 7.63±0.82 | 8.74±2.04 | 7.14±0.98 | 0.87±0.32 | 0.88±0.59 |
| DHT (ng/ml) | 0.15±0.03 | 0.96±0.15 | 2.31±0.48 | 2.93±0.83 | 0.49±0.049 | 20.52±5.32 |
| E (pg/ml) | <5 | <5 | <5 | <5 | <5 | <5 |
| AUC PRL (ng/ml) | 192±30.7 | 209±43.9 | 185±36.1 | 326±67.6 | 133±1 | 149±19.4 |
| Estimated Rank | ||||||
| neuroE production (0- 80) | 10 | 25 | 15 | 50 | 5 | 5 |
| Yawns X10E2/AR activity | 1 | 1.85 | 8.28 | 6.83 | 0.6 | 7.6 |
Aromatase activity/neuroE production was theoretically ranked using incomplete information, but according to our best understanding of the drugs administered. This is a risky, but potentially profitable endeavor to guide further experiments.
Table 3 shows the speculative ranking of aromatase activity/neuroE production for the individual groups. The ranking was based upon our best understanding of (a) the mechanism of action of the aromatase inhibitors (Jordan & Brodie, 2007), (b) actual aromatase inhibition by ATD measured in macaque brain (Resko et al., 1993), (c) the role of T as the substrate for aromatase activity (Reed & Ohno, 1976) and (d) the role of T and DHT in the stimulation of aromatase gene expression (Roselli & Resko, 1989).
The speculative rank order of aromatase activity was:
T> T+Dutasteride> T+Letrozole > Castrate > DHT+ATD = Flutamide+ATD
The justification for the ranking was as follows:
The T-treated group has unopposed aromatase activity, and it has a physiological concentration of T for sufficient substrate availability. In addition, T and its metabolite DHT, both stimulate aromatase gene expression. We reasoned that neuroE would be produced at normal physiological levels. Therefore, this group was given the highest rank of 50 since we do not know if aromatase activity can be driven higher.
T+Dutasteride was ranked second highest since there was no direct antagonism of aromatase and T provided sufficient substrate. However, DHT stimulates aromatase gene expression so the decrease in DHT may have played a small role in decreasing available aromatase and neuroE production.
The T+Letrozole group was ranked lower because Letrozole is a competitive inhibitor of aromatase. This means it competes with T for the enzyme-binding site. T was present for competition in physiological concentrations, and it provided substrate for any remaining aromatase activity. In addition, the presence of T and DHT would drive the expression of aromatase in the brain. We reasoned that in the balance of these factors, aromatase activity and neuroE would be present, but reduced.
The Castrate group was ranked slightly lower than the T+Letrozole group. T was absent to provide substrate; in addition, T and DHT were absent to drive aromatase gene expression. We reasoned that constitutively expressed aromatase could produce some neuroE de novo from cholesterol. In addition, we obtained data, shown below, that a significant amount of TPH protein remains after castration.
The Flutamide+ATD and DHT+ATD were ranked the lowest because ATD is an irreversible or non-competitive inhibitor of aromatase. ATD was previously shown to inactivate 90% of available aromatase in monkey brain (Resko et al., 1993). Although DHT provided stimulation of aromatase gene expression, ATD could immediately remove new aromatase from action. In addition, there was no substrate in either group since DHT cannot be aromatized and Flutamide is not a substrate. Flutamide was used because ATD was shown to bind to AR in castrated macaques (Resko et al., 1993); a mechanism that could have influenced aromatase gene expression. Therefore, with antagonism of AR and in the absence of any substrate, ATD would act to kill any active aromatase, which in turn would lead to the lowest production of neuroE amongst the groups.
As shown in Figure 2-right, there was a significant correlation between the AUC of prolactin secretion and the theoretical aromatase/neuroE rankings (p < 0.0008; r2 = 0.95). Even if only the rank order of activity was used, ie., 1, 2, 3, 4, and 5, there was still a significant correlation between the AUC of prolactin secretion and the posited rank order of aromatase activity (p < 0.013; r2 = 0.82). A more definitive measurement will be derived from future examination of aromatase gene and protein expression in the brains obtained.
Behavior
General
The male Japanese macaques adjusted to group housing and formed very stable groups in their respective pens with little aggressive behavior under pretreatment conditions. Observations for months 2 and 3 of treatment are reported. Grooming was consistent throughout the study. The treatment values compared to the pretreatment values are shown in the Supplemental Material, Figure D. With 2 way ANOVA, there was an overall significant increase in aggression (F[df1]=14.69, p=0.0004), yawning (F[df1]=17.85, p=0.001) and scratching (F[df1]=8.302, p=0.006) after treatment compared to individual pretreatment values (pre vs post). Pretreatment behaviors were not different between the treatment groups. Tension within the groups due to androgen treatments tended to affect the castrate placebo group. As noted in the Discussion, behavior in group-housed macaques is due to a complex interplay with other animals and environment.
Home environment observations, males only
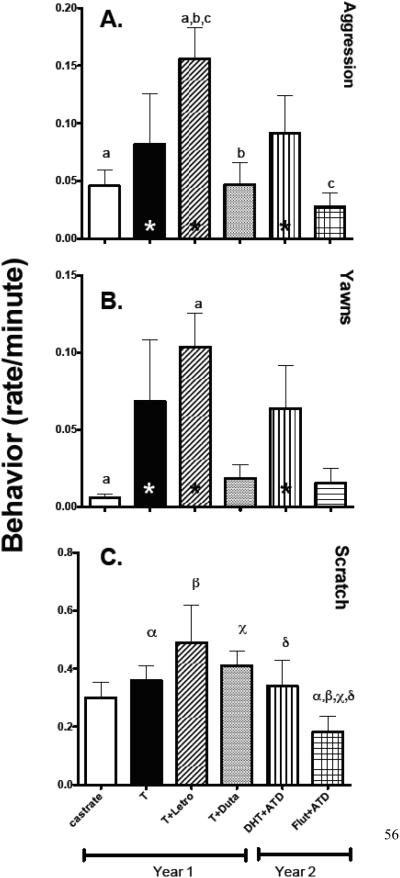
The rate/minute of aggressive behavior across treatment groups is illustrated in Figure 3A. There was a significant difference between the groups (F [df5]=3.383, p=0.02). The T+Letrozole treated group exhibited significantly more aggression than the animals treated with placebo, T+Dutasteride or Flutamide+ATD (Newman Keul’s post hoc p<0.05 each). It is important to note that there is no statistical difference in aggression between T-alone, T+Letrozole and DHT+ATD (astericks). Therefore, these groups were combined and compared to the other groups combined. There was a significant increase in aggression in combined T-alone, T+Letrozole and DHT+ATD compared to combined placebo, T+Dutasteride or Flutamide+ATD (t [df17] =2.941, p=0.009, with Welsh’s correction for uneven variences; F [df 13]=5.346, p=0.005). This indicates that there was an important elevation in aggression in T-alone, T+Letrozole and DHT+ATD groups, but the small group size and individual variance hindered the discrimination of ANOVA and Newman Keul’s post hoc analysis.
Figure 3.
Aggressive behavior during treatment periods is illustrated. There was a significant difference between the groups. Newman-Keul’s posthoc pairwise comparisons reported a significant difference between the T+Letrozole group and Castrated-Placebo, DHT+ATD, Flutamide+ATD treated groups. There was no statistical difference between T-alone, T+Letrozole or DHT+ATD treated groups. A combination of these groups was significantly higher compared to a combination of the remaining groups.
a, b, c Groups with the same letter are different with p < 0.05, Newman Keul’s posthoc analysis. * Groups are not different, p < 0.05, Newman Keul’s posthoc analysis.
Yawning during treatment periods is illustrated. There was a significant difference between the groups with a significant pairwise difference between the placebo and T+Letrozole-treated groups. However, there was a marked reduction trend in yawning in the T+Dutasteride and Flutamide+ATD groups as well. There was no statistical difference in yawning between T-alone, T+Letrozole or DHT+ATD treated groups. A combination of these groups was significantly higher compared to a combination of the remaining groups.
a Groups with the same letter are different with p < 0.05
* Groups are not different, p < 0.05 Newman-Keul’s posthoc analysis.
Scratching behavior during treatment periods is illustrated. There was no difference in scratching between the treatment groups with ANOVA when performed on the raw data as shown. However, the variance and gradual change across the groups hendered discrimination by the ANOVA. Therefore, a log transformation of the data was performed (see Supplemental Material). There was a near significant difference between the groups after log transformation (p=0.06). The posthoc tests will not compute with this probability, so the t-test was used. The t-test found that the steroid treated groups were significantly different from the log mean of Flut+ATD group. A further description of the test was placed in the Supplemental Material. α, β, χ, δ Groups are different with p < 0.05 after log transformation of the data.
Yawning for all groups is illustrated in Figure 3B. There was a significant difference between the groups (F[df5 ]=3.357, p=0.02). The T+Letrozole treated group exhibited significantly more yawning than the animals treated with placebo (Newman Keul’s post hoc p<0.05). Again, it is important to note that yawning was elevated and not statistically different between the T-alone, T+Letrozole and DHT+ATD groups (astericks). Therefore, these groups were combined and compared to the other groups combined. There was a significant increase in yawning in combined T-alone, T+Letrozole and DHT+ATD compared to combined placebo, T+Dutasteride or Flutamide+ATD (t [df17] =2.932, p= 0.009, with Welsh’s correction for uneven variences). This indicates that there was an important elevation in yawning in T-alone, T+Letrozole and DHT+ATD groups, but the small group size and individual variance hindered the discrimination of ANOVA and Newman Keul’s post hoc analysis. Yawning and aggression exhibited a similar pattern during treatment (r2= 0.63; p= 0.058).
Scratching and displacement behavior have been used as an index of anxiety in primate behavior and they are ameliorated by benzodiazepine treatment (Schino, Troisi, Perretta, & Monaco, 1991; A. Troisi, 2002). There was signigicant difference in scratching between pre- and post-treatment scratching by 2-way ANOVA (F [df1] = 8.32, p=0.005). In single group comparisons with the pre-treatment values of all groups combined, The T-alone, T+Letrozole and T+Dutasteride groups were significantly higher than the combined pretreatment values (t [df23]=2.349, p= 0.027; t [df23]=3.321, p=0.003 and t [df23]=3.372, p=0.002, respectively) whereas placebo, DHT+ATD, and Flutamide+ATD groups exhibited no difference. This pattern differs somewhat from the response pattern of aggression and yawning. The effect of treatment on scratching behavior is illustrated in Figure 3C. After the raw data (shown) was transformed to the log of the values (shown in Supplemental material), there was a trend toward difference between the groups (F [df5]=2.464, p=0.06), but posthoc tests would not compute with this probability. Nonetheless, a multiple t-test found a significant difference between the log mean of Flut +ATD group versus T (t [df4]=6.954, p=0.002), T+Letrozole (t [df4]=4.102, p=0.014), T+Dutasteride (t [df4]=9.573, p=0.0007), and DHT+ATD (t [df4]=3.761,p=0.019) groups, but not placebo. In addition, the T+Letrozole treated group showed a 281% increase in toy play over pre-treatment compared to 32, 58 and 82% in the placebo, T and T+Dutasteride (data not shown).
Response to female
There were no statistical differences in sexual or aggressive behavior between the treatments in the “female introduction” test. Of note however, during year 2, 0 out of 5 animals in the Flutamide+ATD group played with a toy while the female was present nearby, whereas 3 out of 5 animals in the DHT+ATD group played with a toy while the female was nearby (Chi square =5.63, df=1, p=0.018). The 3 out of 5 DHT+ATD-treated animals also lipsmacked at the female and 2 showed ‘display’ or dominance behavior toward her. We did not see these differences in year 1 female introduction tests.
Further correlations were conducted to determine if age or weight correlated with either dominance rank or aggressive score. There was a significant correlation between age and weight (p=0.0034) and a near significant correlation between weight and aggression (p=0.07). However, there was no correlation between age and rank, age and aggression or weight and rank.
Serum Hormone Concentrations Achieved
The serum concentrations of T, DHT and E throughout the protocol are shown in Table 3. Serum T, DHT and E were at the level of assay detection in all of the animals after castration or rest. After initiation of treatments, there was a significant difference in serum T and DHT between the groups at each time point (average F of both [df5]=15.84, p < 0.0001). The values for T and DHT in the testosterone treated group are in the physiological range for male macaques (Eaton & Resko, 1974; Rostal et al., 1986). Increasing the dose of Dutasteride and Letrozole after 1 month did not significantly change serum concentrations of T, DHT or E at 3 months.
In the second year, after 7 months of rest without any treatment (n=10 animals), the serum concentrations of T, DHT and E returned to castrate levels. Treatment with Flutamide +ATD had no significant effect on serum hormone concentrations. Treatment with DHT + ADT significantly elevated serum DHT concentrations. It may be noted that serum E was undetectable in all groups throughout the entire study. Thus, this measurement was useless in determining efficacy of the aromatase inhibitors. The serum was subjected to chromatography before assay, and the assay that was used is considered more sensitive and specific than E assays used in previous publications examining male macaques (personal communication, Director, Endocrine Technologies Support Core, ONPRC). The lack of detectable E in serum may be unique to male Japanese macaques.
Measurement of TPH2 by western blot
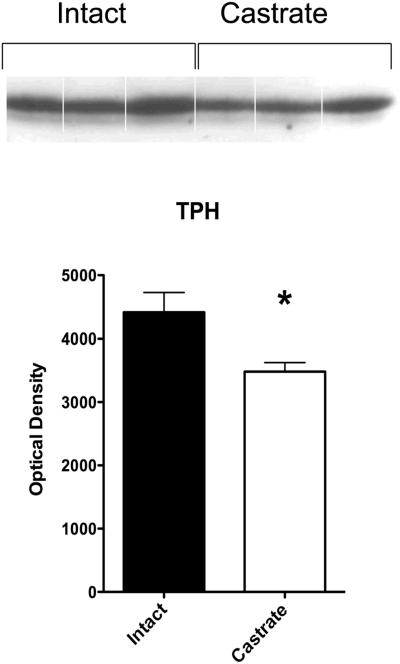
TPH was significantly higher in the intact group compared to the castrate group (t [4]=2.71, p< 0.05). Although the castrate and intact groups exhibit different concentrations of TPH/mg protein, a considerable amount of TPH remained in the castrate group to support serotonin production. This is important to note because the castrate group showed a moderate serotonin/prolactin response to fenfluramine. The results are illustrated in Figure 4.
Figure 4.
Illustration of the Western blot and optical density (average ±sem) of TPH in the raphe region of intact and castrated male Japanese macaques (n=3/group). TPH was significantly higher in the intact group compared to the castrate group.
* Groups are different t-test, p = 0.05.
Supplemental Material and Investigations
Animal grouping and mechanisms of drug administration are shown in Supplement Figure A. Win-loss observations for rank and the distribution of ranks among the pens and treatment groups are illustrated in Supplement Table A. The expression of genes that would govern the responses of the animals in this study was examined in the dorsal raphe of a representative male and the RT-PCR amplicons are illustrated in Supplement Figure B. The primers for the RT-PCR, based on human or monkey sequences, were obtained from Invitrogen Life Technologies and are shown in Supplement Table B. The log transformaton of the scratching data is shown in Supplement Figure C. A histogram illustrating the pre- and post-treatment behavior values is shown in Supplement Figure D.
Discussion
The expression of aggressive behavior, both in and out of appropriate social context, is of importance to forensic psychiatrists, parents, the government, the justice system, the military, care givers of mentally disabled people and to victims of assault. Indeed, much of human social structure has been established to contain aggression. Previous studies in humans and animal models, showed significant reductions in serotonin or its metabolite, 5HIAA, that correlated with elevated levels of aggression and androgens (Bonson, Johnson, Fiorella, Rabin, & Winter, 1994; G. L. Brown et al., 1982; Coccaro et al., 1997; Higley, Mehlman, et al., 1996; McGinnis, 2004; Stanley et al., 2000). Conversely, castration has been used for centuries to reduce androgens and aggression, but castration also reduced serotonin (Foresta et al., 1987; Long, Youngblood, & Kizer, 1983). Thus, a reduction of serotonin was present both in situations of elevated androgens and in the absence of androgens. We investigated the role of androgens and aromatase activity in the regulation of serotonin and behavior in male Japanese macaques. Previous studies found ERβ in serotonin neurons of female macaques (Gundlah et al., 2001), but no AR in serotonin neurons of rodents (Sheng et al., 2004). These data suggested that aromatase activity and the production of neuroE might play a pivotal role in the regulation of serotonin with metabolism of T.
A fenfluramine challenge with serum prolactin measurement was used to determine global serotonin availability. Fenfluramine is a potent serotonin releaser and it also blocks the re-uptake of serotonin (Rothman & Baumann, 2002). Therefore, synaptic serotonin is elevated promptly upon administration of fenfluramine; and the resultant concentration depends on the available serotonin. Prolactin secretion from the pituitary reflects the serotonin released. This pharmacological tool has enabled scientists to access relative serotonin ‘stores’ in animals and humans, and to determine whether one group of individuals has more endogenous serotonin than another group (Abel & Cleare, 1999; Bethea et al., 2005; Cleare et al., 1998). However, serotonin function is very complicated with homeostatic regulation of release and regulation of auto- and postsynaptic receptors all of which have widespread distribution throughout the brain and periphery. Therefore, it is important to remember that any or all of these mechanisms may be involved in fenfluramine-induced prolactin secretion, and the measurement of prolactin as a proxy for endogenous serotonin is indicative rather than absolute.
We found that fenfluramine-induced serotonin/prolactin was lowest in male macaques when aromatase activity and production of neuroE was blocked by ATD. It did not matter whether androgenic activity was high or low. This suggests that endogenous serotonin production may not be directly regulated by androgens, but rather regulated by neuroE from aromatase activity. T is metabolized to E and it increases aromatase activity and gene expression (Resko, Pereyra-Martinez, Stadelman, & Roselli, 2000; Roselli & Resko, 1989). Thus, it follows that neuroE and activation of ER will be elevated in the presence of a physiological concentration of T. However, T and DHT will still activate AR. We observed a reduction in serotonin/prolactin when Letrozole was added to T (reduces aromatase activity/neuroE). A further reduction in serotonin was observed with the greatest reduction in neuroE by administration of ATD, a non-competitive aromatase inhibitor, in the presence or absence of a nonmetabolizable androgen, DHT. DHT does not provide substrate for aromatase and DHT+ATD was used to provide an extreme example of androgen activity > aromatase activity. We do not know what would happen if T was greatly elevated without aromatase block, such as might be found in XYY individuals. There may be a ceiling for aromatase activity, which could be exceeded by excess androgens.
To move from a completely subjective comparison of treatments and serotonin availability, we calculated the area under the prolactin curve and put forward a theoretical ranking of the aromatase activity in order to perform a linear regression analysis. The ranking was based upon our best understanding of the different factors that impact aromatase activity, but which is still incomplete. Yawns were used as the best indication of androgen activity based upon previous studies. Yawning has been considered as a kind of low level threat in Old World macaques, and yawning is observed in tense situations in adult males who engage in ‘canine contests’ (Anderson, 2010). Moreover, yawning can be induced by exogenous androgens and blocked with flutamide (Deputte et al., 1994; Graves & Wallen, 2006). Yawning may be an indication of anxiety as well (Schino et al., 1991; A. Troisi, 2002; A. Troisi et al., 1991). Based upon our estimated ranking of aromatase/neuroE and yawns/androgenic activity, we suggest that endogenous serotonin correlates better with aromatase/neuroE activity than with androgenic activity within the dose range of androgens administered. We do not have representation of aromatase hyperactivation in the absence of androgens at this time.
Prolactin secretion was similar between T+Letrozole, T+Dutasteride and placebo suggesting that serotonin availability was similar as well. However, the pathways to similar serotonin were probably different. Similar serotonin between T+Letrozole and placebo is understandable since Letrozole blocks aromatase activity and the placebo group lacks substrate. However, the results from the T+Dutasteride group are a puzzle in that serum DHT was reduced. Aromatase activity should be present and T provides substrate. Aromatase expression was stimulated by DHT in male rats (Roselli & Resko, 1984) so perhaps the decrease in DHT reduced aromatase expression resulting in a reduction of global serotonin equal to placebo and T+Letrozole. Alternatively, dutasteride is an azasteroid and may have antagonist activity at either aromatase or ER, which in turn would reduce serotonin.
The behavior of the males was monitored by continuous video recordings (to look for fighting) and by focal observations during the treatment periods. It is important to keep in mind that behavioral data tend to be more variable than physiological data. In particular, behavioral data from monkeys living in a group is variable because the behavior of the individual depends not only on his own behavioral propensities, but also the behavior of the others in the group. First, there were no major fights resulting in injury during the study. However, it should be noted that the housing was different in year 1 and year 2; and the environmental differences may impact comparison of aggression across these 5 groups. In addition, behavioral studies are very sensitive to sample size, which is small in this study. Nonetheless, several notable observations and correlations were obtained.
The T alone-, T+Letrozole- and DHT+ATD-treated groups had the highest induction of aggression and the highest yawning regardless of serotonin availability. Consideration of pre- and post-treatment behavior showed aggression and yawning were significantly increased over pretreatment values only in T-alone, T+Letrozole and DHT+ATD treatment; thus, we reasoned that unopposed androgens, ie, not treated with dutasteride, increased aggression and yawning, but androgens did not uniformly decrease serotonin availability. That is, serotonin/prolactin was highest in T alone, next highest in T+Letrozole and lowest in DHT+ATD. This data challenges the claim that high levels of T increase aggression by reducing serotonin. Together, the data suggest that another neural system, driven by androgens, may play a pivotal role for aggression to increase in males. Previous investigations suggest that the noradrenergic (NE) system may be stimulated in aggressive, hyper-androgenic states (Chichinadze, Domianidze, Matitaishvili, Chichinadze, & Lazarashvili, 2010; Coccaro et al., 1991; Gerra et al., 1996; Haller, Makara, & Kruk, 1998; Marino, Bourdelat-Parks, Cameron Liles, & Weinshenker, 2005). Moreover, stimulation of the NE neurons of the locus ceruleus has been strongly implicated in anxiety and vigilance, and serotonin innervation of the LC inhibits NE (Aston-Jones, Akaoka, Charlety, & Chouvet, 1991; Aston-Jones, Chiang, & Alexinsky, 1991).
A reduction in serotonin may augment aggression, but it does not appear to be sufficient as demonstrated by the Flutamide+ADT group. This group had the lowest serotonin response to fenfluramine, but little aggression. This notion is consistent with previous observations in rodents. McGinnis and colleagues found that in rats, reducing serotonin with PCPA did not increase aggression. T alone increased aggression; and T+PCPA caused a significant decrease in attack latency particularly following provocation (Keleta, Lumia, Anderson, & McGinnis, 2007; Kubala, McGinnis, Anderson, & Lumia, 2008). In monkeys, T alone increased aggression with the highest serotonin response to fenfluramine. Decreasing aromatase activity with either letrozole or ATD decreased the serotonin response, but aggression was not significantly different from T alone.
In nonhuman primates, displacement behavior appears in situations characterized by social tension and is likely to reflect increased autonomic arousal (A. Troisi, 2002). Moreover, increased sensitivity to the environment has been noted in aggressive individuals (McGinnis et al., 2002). Scratching is considered a displacement behavior, and scratching increased in the unopposed androgen-treated groups. Another displacement behavior, yawning, was also increased by unapposed androgen administration. Finally, 3 members of the DHT+ATD group exhibited increased toy play in the presence of the female in year 2. There was no correlation between scratching or yawning and serotonin.
At this point we do not know which ER isoform is stimulating serotonin. In females, we showed that ERβ is exclusively expressed in serotonin neurons (Gundlah et al., 2001). Female macaques appear to lack ERα in this region (Vanderhorst, Terasawa, & Ralston, 2009). However, our preliminary examination of gene expression indicates that the male exhibited robust expression of ERα, ERβ and AR in the raphe region (Supplemental material). For now, our reference to ER includes both receptors. We also do not know the neuronal origin of aromatase in the raphe region. These questions will be resolved in future examination of gene expression in the brains of the macaques from this study.
Nonetheless, these data and the accompanying literature lead to the question of how ER and AR regulate serotonin, aggression or yawning. In female macaques, E acting through ERβ in serotonin neurons stimulates TPH2 gene and protein expression, which would increase serotonin production (Gundlah et al., 2001; Sanchez, Reddy, Centeno, Henderson, & Bethea, 2005). Although not indicated by rodent studies, it remains possible that serotonin neurons in male macaques contain ER and AR. Chambon and colleagues (Meyer et al., 1989) hypothesized that nuclear steroid receptors require common proteins that could constitute a limiting factor when competition, or squelching, takes place. Squelching, in turn may be rate limiting of steroid-receptor-mediated gene transcription (Arias et al., 1994; Torchia et al., 1997). Continuing this line of reasoning, ER and AR could compete for the same co-activator, such as SRC1 (Charlier, Ball, & Balthazart, 2005; Johnson & O'Malley, 2012). However, we think this is unlikely. Rather, it seems that aromatase/neuroE acts as the thermostat for serotonin production and androgen does something else that leads to aggression, yawning and scratching. In this scenario, ER and AR could be in different neurons and this suggestion is supported by the rodent data (Sheng et al., 2004). For example, ER may localize within serotonin neurons and AR may be stimulatory for NE neurons in the locus ceruleus (Aston-Jones, Chiang, et al., 1991). Excess NE transmission also plays a role in anxiety and hypervigilence (Aston-Jones, Chiang, et al., 1991), which is consistent with the notion that androgens increase NE and displacement or anxiety behaviors in macaques.
In summary, we observed that fenfluramine-induced serotonin/prolactin was lowest when aromatase was maximally inhibited and denied substrate, but it did not matter if androgen activity was high or low. Serotonin correlated best with theoretical aromatase activity and neural conversion of T to E, which in turn would promote neural ER activation. Aggression did not correlate with serotonin, which is consistent with the weak efficiacy of SSRIs in the treatment of severe forms of pathological aggression (Carrillo et al., 2009; Fava, 1997; Goedhard et al., 2006; Pappadopulos et al., 2006). However, aggression did correlate with yawning, which is an indication of androgenic activity. Both aggression and yawning were elevated with T alone, T+Letrozole and DHT+ATD in the presence of high, medium and low serotonin, respectively. These data raise a number of compelling questions regarding the mechanism of androgen-regulated aggression and whether there are different neural etiologies of aggression across the spectrum of human psychopathology. Further studies are underway to examine the expression of potential candidate genes in the male macaques.
Supplementary Material
Acknowledgements
We are very grateful to Kevin Muller for training the animals, administering drugs and monitoring the health and well-being of the animals. We thank the Primate Genetics Program at the Oregon National Primate Research Center for calculations of the relatedness of our animals. We are especially grateful to Dr. Jay Welch and the technicians of the Division of Animal Resources (DAR) for the management and care of our animals. We thank the Surgery and Pathology Sections of DAR for their expertise and handling of our needed surgeries and necropsies. Thank you to Kenny Phu for helping with the fenfluramine assays and to Andrew T. Placzek, Ph.D., post-doctoral research associate, laboratory of Dr. Thomas Scanlan, Department of Physiology and Pharmacology, OHSU, for determining the purity of the ATD by NMR.
Supported by NIH grants: MH86542 to CLB and 8P51OD011092 for the operation of ONPRC
Footnotes
Disclosure
Dr. Cynthia L Bethea has nothing to disclose.
Dr. Arubala P Reddy has nothing to disclose.
Ms. Nicola Robertson has nothing to disclose.
Dr. Kristine Colemen has nothing to disclose.
Literature Cited
- Abel KM, Cleare AJ. Peripheral hormonal responses to D-fenfluramine as a probe of central serotonergic function in humans. Psychopharmacology. 1999;142:68–72. doi: 10.1007/s002130050863. [DOI] [PubMed] [Google Scholar]
- Anderson JR. Non-human primates: a comparative developmental perspective on yawning. Front Neurol Neurosci. 2010;28:63–76. doi: 10.1159/000307082. [DOI] [PubMed] [Google Scholar]
- Arias J, Alberts AS, Brindle P, Claret FX, Smeal T, Karin M, Feramisco J, Montminy M. Activation of cAMP and mitogen responsive genes relies on a common nuclear factor. Nature. 1994;370(6486):226–229. doi: 10.1038/370226a0. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Akaoka H, Charlety P, Chouvet G. Serotonin selectively attenuates glutamate-evoked activation of noradrenergic locus coeruleus neurons. J Neurosci. 1991;11(3):760–769. doi: 10.1523/JNEUROSCI.11-03-00760.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Chiang C, Alexinsky T. Discharge of noradrenergic locus coeruleus neurons in behaving rats and monkeys suggests a role in vigilance. Progress in Brain Research. 1991;88:501–520. doi: 10.1016/s0079-6123(08)63830-3. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Pau FK, Fox S, Hess DL, Berga SL, Cameron JL. Sensitivity to stress-induced reproductive dysfunction linked to activity of the serotonin system. Fertil Steril. 2005;83(1):148–155. doi: 10.1016/j.fertnstert.2004.06.051. [DOI] [PubMed] [Google Scholar]
- Bonson KR, Johnson RG, Fiorella D, Rabin RA, Winter JC. Serotonergic control of androgen-induced dominance. Pharm Biochem Behav. 1994;49(2):313–322. doi: 10.1016/0091-3057(94)90427-8. [DOI] [PubMed] [Google Scholar]
- Brown GL, Ebert MH, Goyer PF, Jimerson DC, Klein WJ, Bunney WE, Goodwin FK. Aggression, suicide, and serotonin: relationships to CSF amine metabolites. Am J Psychiatry. 1982;139(6):741–746. doi: 10.1176/ajp.139.6.741. [DOI] [PubMed] [Google Scholar]
- Brown GL, LInnoila MI. CSF serotonin metabolite (5HIAA) studies in depression, impulsivity and violence. J Clin Psychiatry. 1990;51:31–41. [PubMed] [Google Scholar]
- Carrillo M, Ricci LA, Coppersmith GA, Melloni RH., Jr. The effect of increased serotonergic neurotransmission on aggression: a critical meta-analytical review of preclinical studies. Psychopharmacology (Berl) 2009;205(3):349–368. doi: 10.1007/s00213-009-1543-2. [DOI] [PubMed] [Google Scholar]
- Charlier TD, Ball GF, Balthazart J. Inhibition of steroid receptor coactivator-1 blocks estrogen and androgen action on male sex behavior and associated brain plasticity. J Neurosci. 2005;25(4):906–913. doi: 10.1523/JNEUROSCI.3533-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichinadze KN, Domianidze TR, Matitaishvili T, Chichinadze NK, Lazarashvili AG. Possible relation of plasma testosterone level to aggressive behavior of male prisoners. Bull Exp Biol Med. 2010;149(1):7–9. doi: 10.1007/s10517-010-0861-z. [DOI] [PubMed] [Google Scholar]
- Clark AS, Henderson LP. Behavioral and physiological responses to anabolic-androgenic steroids. Neurosci Biobehav Rev. 2003;27(5):413–436. doi: 10.1016/s0149-7634(03)00064-2. [DOI] [PubMed] [Google Scholar]
- Cleare AJ, Murray RM, O'Keane V. Assessment of sertonergic function in major depression using d-fenfluramine: relation to clinical variables and antidepressant response. Biol Psychiatry. 1998;44:555–561. doi: 10.1016/s0006-3223(98)00018-3. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Kavoussi RJ, Cooper TB, Hauger RL. Central serotonin activity and aggression: inverse relationship with prolactin response to d-fenfluramine, but not CSF 5-HIAA concentration, in human subjects. Am J Psychiatry. 1997;154(10):1430–1435. doi: 10.1176/ajp.154.10.1430. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Lawrence T, Trestman R, Gabriel S, Klar HM, Siever LJ. Growth hormone responses to intravenous clonidine challenge correlate with behavioral irritability in psychiatric patients and healthy volunteers. Psychiatry Res. 1991;39(2):129–139. doi: 10.1016/0165-1781(91)90082-z. [DOI] [PubMed] [Google Scholar]
- Dabbs JM, Jr., Frady RL, Carr TS, Besch NF. Saliva testosterone and criminal violence in young adult prison inmates. Psychosom Med. 1987;49(2):174–182. doi: 10.1097/00006842-198703000-00007. [DOI] [PubMed] [Google Scholar]
- Deputte BL, Johnson J, Hempel M, Scheffler G. Behavioral effects of an antiandrogen in adult male rhesus macaques (Macaca mulatta) Hormones and Behavior. 1994;28(2):155–164. doi: 10.1006/hbeh.1994.1013. [DOI] [PubMed] [Google Scholar]
- Desta Z, Kreutz Y, Nguyen AT, Li L, Skaar T, Kamdem LK, Henry NL, Hayes DF, Storniolo AM, Stearns V, Hoffmann E, Tyndale RF, Flockhart DA. Plasma letrozole concentrations in postmenopausal women with breast cancer are associated with CYP2A6 genetic variants, body mass index, and age. Clin Pharmacol Ther. 2011;90(5):693–700. doi: 10.1038/clpt.2011.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easley SP, Coelho AM, Jr., Taylor LL. Allogrooming, partner choice, and dominance in male anubis baboons. Am J Phys Anthropol. 1989;80(3):353–368. doi: 10.1002/ajpa.1330800309. [DOI] [PubMed] [Google Scholar]
- Eaton GG, Resko JA. Plasma testosterone and male dominance in a Japanese macaque (Macaca fuscata) troop compared with repeated measures of testosterone in laboratory males. Hormones and Behavior. 1974;5(3):251–259. doi: 10.1016/0018-506x(74)90033-6. [DOI] [PubMed] [Google Scholar]
- Eaton GG, Worlein JM, Glick BB. Sex differences in Japanese macaques (Macaca fuscata): effects of prenatal testosterone on juvenile social behavior. Hormones and Behavior. 1990;24(2):270–283. doi: 10.1016/0018-506x(90)90009-m. [DOI] [PubMed] [Google Scholar]
- Ellinwood WE, Hess DL, Roselli CE, Spies HG, Resko JA. Inhibition of aromatization stimulates luteinizing hormone and testosterone secretion in adult male rhesus monkeys. J Clin Endocrinol Metabol. 1984;59(6):1088–1096. doi: 10.1210/jcem-59-6-1088. [DOI] [PubMed] [Google Scholar]
- Fava M. Psychopharmacologic treatment of pathologic aggression. Psychiatr Clin North Am. 1997;20(2):427–451. doi: 10.1016/s0193-953x(05)70321-x. [DOI] [PubMed] [Google Scholar]
- Foresta C, Indino M, Scandellari C. Role of gonadal steroids in the serotoninergic control of prolactin secretion in men. Clin Endocrinol (Oxford) 1987;26(5):601–607. doi: 10.1111/j.1365-2265.1987.tb00816.x. [DOI] [PubMed] [Google Scholar]
- Gerra G, Avanzini P, Zaimovic A, Fertonani G, Caccavari R, Delsignore R, Gardini F, Talarico E, Lecchini R, Maestri D, Brambilla F. Neurotransmitter and endocrine modulation of aggressive behavior and its components in normal humans. Behav Brain Res. 1996;81(1-2):19–24. doi: 10.1016/s0166-4328(96)00038-1. [DOI] [PubMed] [Google Scholar]
- Giltay EJ, Tishova YA, Mskhalaya GJ, Gooren LJ, Saad F, Kalinchenko SY. Effects of testosterone supplementation on depressive symptoms and sexual dysfunction in hypogonadal men with the metabolic syndrome. J Sex Med. 2010;7(7):2572–2582. doi: 10.1111/j.1743-6109.2010.01859.x. [DOI] [PubMed] [Google Scholar]
- Goedhard LE, Stolker JJ, Heerdink ER, Nijman HL, Olivier B, Egberts TC. Pharmacotherapy for the treatment of aggressive behavior in general adult psychiatry: A systematic review. J Clin Psychiatry. 2006;67(7):1013–1024. doi: 10.4088/jcp.v67n0702. [DOI] [PubMed] [Google Scholar]
- Graves FC, Wallen K. Androgen-induced yawning in rhesus monkey females is reversed with a nonsteroidal anti-androgen. Hormones and Behavior. 2006;49(2):233–236. doi: 10.1016/j.yhbeh.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Gundlah C, Lu NZ, Mirkes SJ, Bethea CL. Estrogen receptor beta (ERb) mRNA and protein in serotonin neurons of macaques. Mol Brain Res. 2001;91:14–22. doi: 10.1016/s0169-328x(01)00108-5. [DOI] [PubMed] [Google Scholar]
- Hall RC, Chapman MJ. Psychiatric complications of anabolic steroid abuse. Psychosomatics. 2005;46(4):285–290. doi: 10.1176/appi.psy.46.4.285. [DOI] [PubMed] [Google Scholar]
- Hendricks T, Francis N, Fyodorov D, Deneris ES. The ETS domain factor Pet-1 is an early and precise marker of central serotonin neurons and interacts with a conserved element in serotonergic genes. J Neurosci. 1999;19(23):10348–10356. doi: 10.1523/JNEUROSCI.19-23-10348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J, Makara GB, Kruk MR. Catecholaminergic involvement in the control of aggression: hormones, the peripheral sympathetic, and central noradrenergic systems. Neurosci Biobehav Rev. 1998;22(1):85–97. doi: 10.1016/s0149-7634(97)00023-7. [DOI] [PubMed] [Google Scholar]
- Hendricks T, Francis N, Fyodorov D, Deneris ES. The ETS domain factor Pet-1 is an early and precise marker of central serotonin neurons and interacts with a conserved element in serotonergic genes. J Neurosci. 1999;19(23):10348–10356. doi: 10.1523/JNEUROSCI.19-23-10348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley JD, King ST, Jr., Hasert MF, Champoux M, Suomi SJ, Linnoila M. Stability of interindividual differences in serotonin function and its relationship to severe aggression and competent social behavior in rhesus macaque females. Neuropsychopharmacology. 1996;14(1):67–76. doi: 10.1016/S0893-133X(96)80060-1. [DOI] [PubMed] [Google Scholar]
- Higley JD, Mehlman PT, Poland RE, Taub DM, Vickers J, Suomi SJ, Linnoila M. CSF testosterone and 5-HIAA correlate with different types of aggressive behaviors. Biol Psychiatry. 1996;40(11):1067–1082. doi: 10.1016/S0006-3223(95)00675-3. [DOI] [PubMed] [Google Scholar]
- Johnson AB, O'Malley BW. Steroid receptor coactivators 1, 2, and 3: critical regulators of nuclear receptor activity and steroid receptor modulator (SRM)-based cancer therapy. Mol Cell Endocrinol. 2012;348(2):430–439. doi: 10.1016/j.mce.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan VC, Brodie AM. Development and evolution of therapies targeted to the estrogen receptor for the treatment and prevention of breast cancer. Steroids. 2007;72(1):7–25. doi: 10.1016/j.steroids.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keleta YB, Lumia AR, Anderson GM, McGinnis MY. Behavioral effects of pubertal anabolic androgenic steroid exposure in male rats with low serotonin. Brain Res. 2007;1132(1):129–138. doi: 10.1016/j.brainres.2006.10.097. [DOI] [PubMed] [Google Scholar]
- Kreuz LE, Rose RM. Assessment of aggressive behavior and plasma testosterone in a young criminal population. Psychosom Med. 1972;34(4):321–332. doi: 10.1097/00006842-197207000-00006. [DOI] [PubMed] [Google Scholar]
- Kubala KH, McGinnis MY, Anderson GM, Lumia AR. The effects of an anabolic androgenic steroid and low serotonin on social and non-social behaviors in male rats. Brain Res. 2008;1232:21–29. doi: 10.1016/j.brainres.2008.07.065. [DOI] [PubMed] [Google Scholar]
- Lehner PN. Sampling methods in behavior research. Poult Sci. 1992;71(4):643–649. doi: 10.3382/ps.0710643. [DOI] [PubMed] [Google Scholar]
- Long JB, Youngblood WW, Kizer JS. Effects of castration and adrenalectomy on in vitro rates of tryptophan hydroxylation and levels of serotonin in microdissected brain nuclei of adult male rats. Brain Res. 1983;277(2):289–297. doi: 10.1016/0006-8993(83)90936-8. [DOI] [PubMed] [Google Scholar]
- Marino MD, Bourdelat-Parks BN, Cameron Liles L, Weinshenker D. Genetic reduction of noradrenergic function alters social memory and reduces aggression in mice. Behav Brain Res. 2005;161(2):197–203. doi: 10.1016/j.bbr.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Maruhashi T. An ecological study of troop fissions of Japanese monkeys (Macaca fuscata yaku) on Yakushima Island, Japan. Primates. 1982;23:317–337. [Google Scholar]
- McGinnis MY. Anabolic androgenic steroids and aggression: studies using animal models. Ann New York Acad Sci. 2004;1036:399–415. doi: 10.1196/annals.1330.024. [DOI] [PubMed] [Google Scholar]
- McGinnis MY, Lumia AR, Breuer ME, Possidente B. Physical provocation potentiates aggression in male rats receiving anabolic androgenic steroids. Hormones and Behavior. 2002;41(1):101–110. doi: 10.1006/hbeh.2001.1742. [DOI] [PubMed] [Google Scholar]
- Melloni RH, Jr., Ferris CF. Adolescent anabolic steroid use and aggressive behavior in golden hamsters. Ann New York Acad Sci. 1996;794:372–375. doi: 10.1111/j.1749-6632.1996.tb32546.x. [DOI] [PubMed] [Google Scholar]
- Meyer ME, Gronemeyer H, Turcotte B, Bocquel MT, Tasset D, Chambon P. Steroid hormone receptors compete for factors that mediate their enhancer function. Cell. 1989;57(3):433–442. doi: 10.1016/0092-8674(89)90918-5. [DOI] [PubMed] [Google Scholar]
- O'Keane V, Dinan TG. Prolactin and cortisol responses to d-fenfluramine in major depression: evidence for diminished responsivity of central serotonergic function. Am J Psychiatry. 1991;148:1009–1015. doi: 10.1176/ajp.148.8.1009. [DOI] [PubMed] [Google Scholar]
- Pappadopulos E, Woolston S, Chait A, Perkins M, Connor DF, Jensen PS. Pharmacotherapy of aggression in children and adolescents: efficacy and effect size. J Can Acad Child Adolesc Psychiatry. 2006;15(1):27–39. [PMC free article] [PubMed] [Google Scholar]
- Ponholzer A, Madersbacher S, Rauchenwald M, Jungwirth S, Fischer P, Tragl KH. Serum androgen levels and their association to depression and Alzheimer dementia in a cohort of 75-year-old men over 5 years: results of the VITA study. Int J Impot Res. 2009;21(3):187–191. doi: 10.1038/ijir.2009.10. [DOI] [PubMed] [Google Scholar]
- Popova NK. From genes to aggressive behavior: the role of serotonergic system. Bioessays. 2006;28(5):495–503. doi: 10.1002/bies.20412. [DOI] [PubMed] [Google Scholar]
- Reed KC, Ohno S. Kinetic properties of human placental aromatase. Application of an assay measuring 3H2O release from 1beta,2beta-3H-androgens. J Biol Chem. 1976;251(6):1625–1631. [PubMed] [Google Scholar]
- Resko JA, Connolly PB, Roselli CE, Abdelgadir SE, Choate JV. Differential effects of aromatase inhibition on luteinizing hormone secretion in intact and castrated male cynomolgus macaques. J Clin Endocrinol Metabol. 1993;77(6):1529–1534. doi: 10.1210/jcem.77.6.8263136. [DOI] [PubMed] [Google Scholar]
- Resko JA, Ellinwood WE, Pasztor LM, Huhl AE. Sex steroids in the umbilical circulation of fetal rhesus monkeys from the time of gonadal differentiation. J Clin Endocrinol Metabol. 1980;50(5):900–905. doi: 10.1210/jcem-50-5-900. [DOI] [PubMed] [Google Scholar]
- Resko JA, Pereyra-Martinez AC, Stadelman HL, Roselli CE. Region-specific regulation of cytochrome P450 aromatase messenger ribonucleic acid by androgen in brains of male rhesus monkeys. Biol Reprod. 2000;62(6):1818–1822. doi: 10.1095/biolreprod62.6.1818. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Resko JA. Androgens regulate brain aromatase activity in adult male rats through a receptor mechanism. Endocrinology. 1984;114(6):2183–2189. doi: 10.1210/endo-114-6-2183. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Resko JA. Testosterone regulates aromatase activity in discrete brain areas of male rhesus macaques. Biol Reprod. 1989;40(5):929–934. doi: 10.1095/biolreprod40.5.929. [DOI] [PubMed] [Google Scholar]
- Rostal DC, Glick BB, Eaton GG, Resko JA. Seasonality of adult male Japanese macaques (Macaca fuscata): androgens and behavior in a confined troop. Hormones and Behavior. 1986;20(4):452–462. doi: 10.1016/0018-506x(86)90007-3. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Serotonin releasing agents. Neurochemical, therapeutic and adverse effects. Pharm Biochem Behav. 2002;71:825–836. doi: 10.1016/s0091-3057(01)00669-4. [DOI] [PubMed] [Google Scholar]
- Sanchez RL, Reddy AP, Centeno ML, Henderson JA, Bethea CL. A second tryptophan hydroxylase isoform, TPH-2 mRNA, is increased by ovarian steroids in the raphe region of macaques. Brain Res: Mol Brain Res. 2005;135(1-2):194–203. doi: 10.1016/j.molbrainres.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Schino G, Troisi A, Perretta G, Monaco V. Measuring anxiety in nonhuman primates: effect of lorazepam on macaque scratching. Pharm Biochem Behav. 1991;38(4):889–891. doi: 10.1016/0091-3057(91)90258-4. [DOI] [PubMed] [Google Scholar]
- Sheng Z, Kawano J, Yanai A, Fujinaga R, tanaka M, Watanabe Y, Shinoda K. Expression of estrogen receptors (a, b) and androgen receptor in serotonin neurons of the rat and mouse dorsal raphe nuclei; sex and species differences. Neurosci Res. 2004;49:185–196. doi: 10.1016/j.neures.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Smith LJ, Henderson JA, Abell CW, Bethea CL. Effects of ovarian steroids and raloxifene on proteins that synthesize, transport and degrade serotonin in the raphe region of macaques. Neuropsychopharmacology. 2004;29:2035–2045. doi: 10.1038/sj.npp.1300510. [DOI] [PubMed] [Google Scholar]
- Stanley B, Molcho A, Stanley M, Winchel R, Gameroff MJ, Parsons B, Mann JJ. Association of aggressive behavior with altered serotonergic function in patients who are not suicidal. Am J Psychiatry. 2000;157(4):609–614. doi: 10.1176/appi.ajp.157.4.609. [DOI] [PubMed] [Google Scholar]
- Torchia J, Rose DW, Inostroza J, Kamei Y, Westin S, Glass CK, Rosenfeld MG. The transcriptional co-activator p/CIP binds CBP and mediates nuclear-receptor function. Nature. 1997;387(6634):677–684. doi: 10.1038/42652. [DOI] [PubMed] [Google Scholar]
- Troisi A. Displacement activities as a behavioral measure of stress in nonhuman primates and human subjects. Stress. 2002;5(1):47–54. doi: 10.1080/102538902900012378. [DOI] [PubMed] [Google Scholar]
- Troisi A, Aureli F, Schino G, Rinaldi F, DeAngelis N. The influence of age, sex, and rank on yawning behavior in two species of macaques (Macaca fascicularis and M. fuscata) Ethology. 1990;86:303–310. [Google Scholar]
- Troisi A, Schino G, D'Antoni M, Pandolfi N, Aureli F, D'Amato FR. Scratching as a behavioral index of anxiety in macaque mothers. Behav Neural Biol. 1991;56(3):307–313. doi: 10.1016/0163-1047(91)90469-7. [DOI] [PubMed] [Google Scholar]
- Vanderhorst VG, Terasawa E, Ralston HJ. Estrogen receptor-alpha immunoreactive neurons in the brainstem and spinal cord of the female rhesus monkey: species-specific characteristics. Neuroscience. (3rd) 2009;158(2):798–810. doi: 10.1016/j.neuroscience.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virkkunen M, Goldman D, Nielsen DA, Linnoila M. Low brain serotonin turnover rate (low CSF 5-HIAA) and impulsive violence. J Psychiatry Neurosci. 1995;20:271–275. [PMC free article] [PubMed] [Google Scholar]
- Wilson AP, Vessey SH. Behavior of free-ranging castrated rhesus monkeys. Folia Primatol (Basel) 1968;9(1):1–14. doi: 10.1159/000155164. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ma W, Barker JL, Rubinow DR. Sex differences in expression of serotonin receptors (subtypes 1A and 2A) in rat brain: a possible role of testosterone. Neuroscience. 1999;94:251–259. doi: 10.1016/s0306-4522(99)00234-1. [DOI] [PubMed] [Google Scholar]
- Zumpe D, Bonsall RW, Michael RP. Effects of the nonsteroidal aromatase inhibitor, fadrozole, on the sexual behavior of male cynomolgus monkeys (Macaca fascicularis) Hormones and Behavior. 1993;27(2):200–215. doi: 10.1006/hbeh.1993.1015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.