Abstract
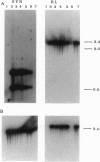
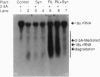
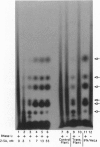
Resistance to virus infections in higher vertebrates is mediated in part through catalysis of RNA decay by the, interferon-regulated 2-5A system. A functional 2-5A system requires two enzymes, a 2-5A synthetase that produces 5'-phosphorylated, 2',5'-linked oligoadenylates (2-5A) in response to double-stranded RNA, and the 2-5A-dependent RNase L. We have coexpressed these human enzymes in transgenic tobacco plants by using a single plasmid containing the cDNAs for both human RNase L and a low molecular weight form of human 2-5A synthetase under control of different, constitutive promoters. Expression of the human cDNAs in the transgenic plants was demonstrated from Northern blots, by specific enzyme assays, and by immunodetection (for RNase L). Infection of leaves, detached or in planta, of the coexpressing transgenic plants by tobacco mosaic virus, alfalfa [correction of alfafa] mosaic virus, or tobacco etch virus resulted in necrotic lesions. In contrast, leaves expressing 2-5A synthetase or RNase L alone and leaves containing the plasmid vector alone produced typical systemic infections. While alfalfa mosaic virus produced lesions only in the inoculated leaves regardless of the concentration of virus in the inoculum, high, but not low, levels of tobacco etch virus inoculum resulted in escape of virus to uninoculated leaves. Nevertheless, there was a substantial reduction of tobacco etch virus yield as measured by ELISA assay in the coexpressing transgenic plants. These results indicate that expression of a mammalian 2-5A system in plants provides resistance to virus infections.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- A simple and general method for transferring genes into plants. Science. 1985 Mar 8;227(4691):1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cayley P. J., White R. F., Antoniw J. F., Walesby N. J., Kerr I. M. Distribution of the ppp(A2'p)nA-binding protein and interferon-related enzymes in animals, plants, and lower organisms. Biochem Biophys Res Commun. 1982 Oct 15;108(3):1243–1250. doi: 10.1016/0006-291x(82)92133-7. [DOI] [PubMed] [Google Scholar]
- Chebath J., Benech P., Revel M., Vigneron M. Constitutive expression of (2'-5') oligo A synthetase confers resistance to picornavirus infection. Nature. 1987 Dec 10;330(6148):587–588. doi: 10.1038/330587a0. [DOI] [PubMed] [Google Scholar]
- Clemens M. J., Williams B. R. Inhibition of cell-free protein synthesis by pppA2'p5'A2'p5'A: a novel oligonucleotide synthesized by interferon-treated L cell extracts. Cell. 1978 Mar;13(3):565–572. doi: 10.1016/0092-8674(78)90329-x. [DOI] [PubMed] [Google Scholar]
- Coccia E. M., Romeo G., Nissim A., Marziali G., Albertini R., Affabris E., Battistini A., Fiorucci G., Orsatti R., Rossi G. B. A full-length murine 2-5A synthetase cDNA transfected in NIH-3T3 cells impairs EMCV but not VSV replication. Virology. 1990 Nov;179(1):228–233. doi: 10.1016/0042-6822(90)90292-y. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr, Kerr I. M., Stark G. R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994 Jun 3;264(5164):1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- Devash Y., Biggs S., Sela I. Multiplication of tobacco mosaic virus in tobacco leaf disks is inhibited by (2'-5) oligoadenylate. Science. 1982 Jun 25;216(4553):1415–1416. doi: 10.1126/science.6178155. [DOI] [PubMed] [Google Scholar]
- Devash Y., Reichman M., Sela I., Reichenbach N. L., Suhadolnik R. J. Plant oligoadenylates: enzymatic synthesis, isolation, and biological activities. Biochemistry. 1985 Jan 29;24(3):593–599. doi: 10.1021/bi00324a008. [DOI] [PubMed] [Google Scholar]
- Dong B., Silverman R. H. 2-5A-dependent RNase molecules dimerize during activation by 2-5A. J Biol Chem. 1995 Feb 24;270(8):4133–4137. doi: 10.1074/jbc.270.8.4133. [DOI] [PubMed] [Google Scholar]
- Dong B., Xu L., Zhou A., Hassel B. A., Lee X., Torrence P. F., Silverman R. H. Intrinsic molecular activities of the interferon-induced 2-5A-dependent RNase. J Biol Chem. 1994 May 13;269(19):14153–14158. [PubMed] [Google Scholar]
- Falk B. W., Bruening G. Will transgenic crops generate new viruses and new diseases? Science. 1994 Mar 11;263(5152):1395–1396. doi: 10.1126/science.8179685. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Floyd-Smith G., Slattery E., Lengyel P. Interferon action: RNA cleavage pattern of a (2'-5')oligoadenylate--dependent endonuclease. Science. 1981 May 29;212(4498):1030–1032. doi: 10.1126/science.6165080. [DOI] [PubMed] [Google Scholar]
- Greene A. E., Allison R. F. Recombination between viral RNA and transgenic plant transcripts. Science. 1994 Mar 11;263(5152):1423–1425. doi: 10.1126/science.8128222. [DOI] [PubMed] [Google Scholar]
- Gribaudo G., Lembo D., Cavallo G., Landolfo S., Lengyel P. Interferon action: binding of viral RNA to the 40-kilodalton 2'-5'-oligoadenylate synthetase in interferon-treated HeLa cells infected with encephalomyocarditis virus. J Virol. 1991 Apr;65(4):1748–1757. doi: 10.1128/jvi.65.4.1748-1757.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassel B. A., Zhou A., Sotomayor C., Maran A., Silverman R. H. A dominant negative mutant of 2-5A-dependent RNase suppresses antiproliferative and antiviral effects of interferon. EMBO J. 1993 Aug;12(8):3297–3304. doi: 10.1002/j.1460-2075.1993.tb05999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovanessian A. G., Brown R. E., Kerr I. M. Synthesis of low molecular weight inhibitor of protein synthesis with enzyme from interferon-treated cells. Nature. 1977 Aug 11;268(5620):537–540. doi: 10.1038/268537a0. [DOI] [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E. pppA2'p5'A2'p5'A: an inhibitor of protein synthesis synthesized with an enzyme fraction from interferon-treated cells. Proc Natl Acad Sci U S A. 1978 Jan;75(1):256–260. doi: 10.1073/pnas.75.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Logemann J., Schell J., Willmitzer L. Improved method for the isolation of RNA from plant tissues. Anal Biochem. 1987 May 15;163(1):16–20. doi: 10.1016/0003-2697(87)90086-8. [DOI] [PubMed] [Google Scholar]
- Marié I., Hovanessian A. G. The 69-kDa 2-5A synthetase is composed of two homologous and adjacent functional domains. J Biol Chem. 1992 May 15;267(14):9933–9939. [PubMed] [Google Scholar]
- Mitra A., An G. Three distinct regulatory elements comprise the upstream promoter region of the nopaline synthase gene. Mol Gen Genet. 1989 Jan;215(2):294–299. doi: 10.1007/BF00339731. [DOI] [PubMed] [Google Scholar]
- Mitra A., Higgins D. W. The Chlorella virus adenine methyltransferase gene promoter is a strong promoter in plants. Plant Mol Biol. 1994 Oct;26(1):85–93. doi: 10.1007/BF00039522. [DOI] [PubMed] [Google Scholar]
- Rysiecki G., Gewert D. R., Williams B. R. Constitutive expression of a 2',5'-oligoadenylate synthetase cDNA results in increased antiviral activity and growth suppression. J Interferon Res. 1989 Dec;9(6):649–657. doi: 10.1089/jir.1989.9.649. [DOI] [PubMed] [Google Scholar]
- Sanders P. R., Winter J. A., Barnason A. R., Rogers S. G., Fraley R. T. Comparison of cauliflower mosaic virus 35S and nopaline synthase promoters in transgenic plants. Nucleic Acids Res. 1987 Feb 25;15(4):1543–1558. doi: 10.1093/nar/15.4.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman R. H., Cayley P. J., Knight M., Gilbert C. S., Kerr I. M. Control of the ppp(a2'p)nA system in HeLa cells. Effects of interferon and virus infection. Eur J Biochem. 1982 May;124(1):131–138. doi: 10.1111/j.1432-1033.1982.tb05915.x. [DOI] [PubMed] [Google Scholar]
- Silverman R. H. Functional analysis of 2-5A-dependent RNase and 2-5a using 2',5'-oligoadenylate-cellulose. Anal Biochem. 1985 Feb 1;144(2):450–460. doi: 10.1016/0003-2697(85)90141-1. [DOI] [PubMed] [Google Scholar]
- Slattery E., Ghosh N., Samanta H., Lengyel P. Interferon, double-stranded RNA, and RNA degradation: activation of an endonuclease by (2'-5')An. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4778–4782. doi: 10.1073/pnas.76.10.4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Truve E., Aaspôllu A., Honkanen J., Puska R., Mehto M., Hassi A., Teeri T. H., Kelve M., Seppänen P., Saarma M. Transgenic potato plants expressing mammalian 2'-5' oligoadenylate synthetase are protected from potato virus X infection under field conditions. Biotechnology (N Y) 1993 Sep;11(9):1048–1052. doi: 10.1038/nbt0993-1048. [DOI] [PubMed] [Google Scholar]
- Wells J. A., Swyryd E. A., Stark G. R. An improved method for purifying 2',5'-oligoadenylate synthetases. J Biol Chem. 1984 Jan 25;259(2):1363–1370. [PubMed] [Google Scholar]
- White K. A., Morris T. J. Recombination between defective tombusvirus RNAs generates functional hybrid genomes. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3642–3646. doi: 10.1073/pnas.91.9.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T. M. Strategies to protect crop plants against viruses: pathogen-derived resistance blossoms. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3134–3141. doi: 10.1073/pnas.90.8.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wreschner D. H., James T. C., Silverman R. H., Kerr I. M. Ribosomal RNA cleavage, nuclease activation and 2-5A(ppp(A2'p)nA) in interferon-treated cells. Nucleic Acids Res. 1981 Apr 10;9(7):1571–1581. doi: 10.1093/nar/9.7.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wreschner D. H., McCauley J. W., Skehel J. J., Kerr I. M. Interferon action--sequence specificity of the ppp(A2'p)nA-dependent ribonuclease. Nature. 1981 Jan 29;289(5796):414–417. doi: 10.1038/289414a0. [DOI] [PubMed] [Google Scholar]
- Zhou A., Hassel B. A., Silverman R. H. Expression cloning of 2-5A-dependent RNAase: a uniquely regulated mediator of interferon action. Cell. 1993 Mar 12;72(5):753–765. doi: 10.1016/0092-8674(93)90403-d. [DOI] [PubMed] [Google Scholar]