Abstract
We present a series of hydrogel nanoparticles (nanogels) incorporating either acyclic or cyclic metal chelates as crosslinkers. These crosslinkers are used to formulate polyacrylamide-based nanogels (diameter 50 to 85 nm) yielding contrast agents with enhanced relaxivities (up to 6-fold greater than Dotarem®), because this nanogel structure slows the chelator's tumbling frequency and allows fast water exchange. Importantly, these nanogels also stabilize Gd3+ within the chelator thermodynamically and kinetically against metal displacement through transmetallation, which should reduce toxicity associated with release of free Gd3+. This chelation stability suggests that the chelate crosslinker strategy may prove useful for other applications of metal-chelating nanoparticles in medicine, including other imaging modalities and radiotherapy.
Introduction
Nanogels, nanoscale hydrogel particles formed by chemically or physically crosslinked polymer chains, have attracted much attention in recent years for their high drug-carrying capacity, environmental responsiveness, and stability in aqueous media. 1-2 While they have been employed in several studies to deliver small therapeutic agents 3-4 and biomacromolecules, 5-6 their advantages for use as biomedical imaging agents, such as water access throughout the particle 7 and protection of the payload have not been thoroughly explored.
Metals have many applications in medicine, including as contrast agents in multiple biomedical imaging modalities (PET, MRI), and as radiotherapeutics. Incorporating metals into nanogels can impart properties useful in medicine. However, no versatile and reproducible chemistry yet exists to incorporate metals into nanogels in a stable manner that is of clinical relevance. Clinical development of high-contrast magnetic resonance imaging (MRI) agents is an important area of investigation that relies on Gd3+ chelates. Gd based MRI agents could allow tissue- or disease-specific imaging using this accurate diagnostic imaging modality capable of providing submillimeter spatial resolution and unrivalled soft tissue contrast. 8-10 Previously employed strategies combining Gd3+ chelates with nanogels include the encapsulation of small contrast agent molecules inside an electrostatically crosslinked polymer 11 or the post polymerization functionalization of nanogels with contrast agents, 12 both of which enhance relaxivity by slowing the chelates’ molecular tumbling rate. 14, 15 These designs follow the example of numerous other groups that attach Gd3+ chelates to high molecular weight macromolecules, including dendrimers, 13-16 polymers, 17-19 micelles 20-21 and metallostars 22 or bind them to micro- 23 or nanoparticles 24-25 or proteins through covalent 26-30 or non-covalent interactions. 31-33By using gadolinium complexes directly as crosslinkers, the whole nanoparticle incorporates contrast agents, in contrast to post-functionalized nanoparticles or gadolinium-based inorganic nanoparticles, in which only surface gadolinium can relax water protons. This strategy also allows us to keep the nanogel surface available for further functionalization, for example with targeting agents. We also hypothesized that incorporating the contrast agent into a macromolecular network as a crosslinker would further increase rigidity and thus relaxivity. Importantly, metal chelating crosslinkers can be used with a variety of metals, such as radioisotopes for positron emission tomography or cancer radiotherapy. 34
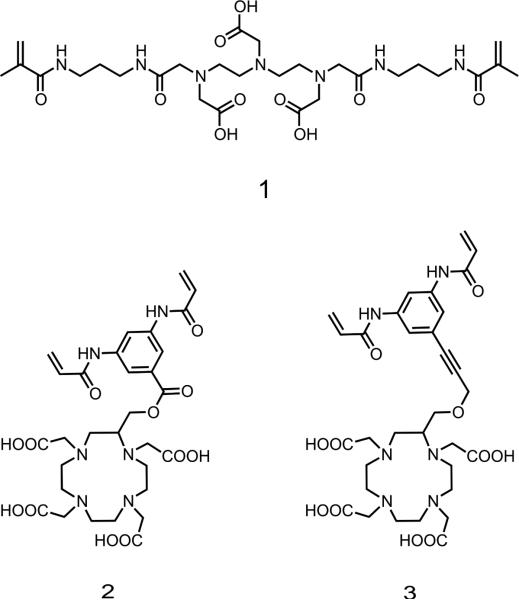
The design of the target crosslinker 1 (Figure 1) was based on a diethylenetriaminepentacetatic acid (DTPA) and was first selected because of its ease of synthesis. The second crosslinker structures were selected for their potential for greater safety in vivo; it has been reported that cyclic structures are more stable than DTPA, 35 better protecting and binding the central Gd3+. A cyclic crosslinker would thus decrease toxicity, which can increase the risk of nephrogenic systemic fibrosis (NSF) via transmetallation with endogenous ions such as zinc or copper and resulting release of free Gd3+. Thus we decided to design two other crosslinkers based on the 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) chelating agent. The design of these macrocyclic crosslinkers 2 and 3 (Figure 1) was based on a DOTA unit bearing a bisacrylamide moiety. The DOTA macrocycle is C-substituted in order to avoid any interference with the coordination site of the ligand. This choice is based on previous observations that chelates with a hydration number greater than one are relatively unstable; q = 1 for all commercial agents. Therefore, though other designs allow a greater hydration number, which yields higher relaxivity, 36-39 we chose C substitution to minimize dechelation. Furthermore, incorporating the chelate into a nanogel both enhances relaxivity by limiting movement and improves Gd3+ chelating stability by reducing access of other metal ions. To the best of our knowledge, this work is the first example of nanogels incorporating DOTA chelating agents as crosslinkers.
Figure 1.
Chemical structures of the target DTPA and DOTA-based crosslinkers 1, 2 and 3.
Herein we report the development of model polyacrylamide (PAA)-based nanogels incorporating DTPA- and DOTA-based crosslinkers. Given the size of these nanogels (<100 nm), which should allow prolonged circulation, these chelators should be useful in the development of biodegradable nanoparticles for a number of biomedical applications involving metals.
Results and discussion
Synthesis of the crosslinkers
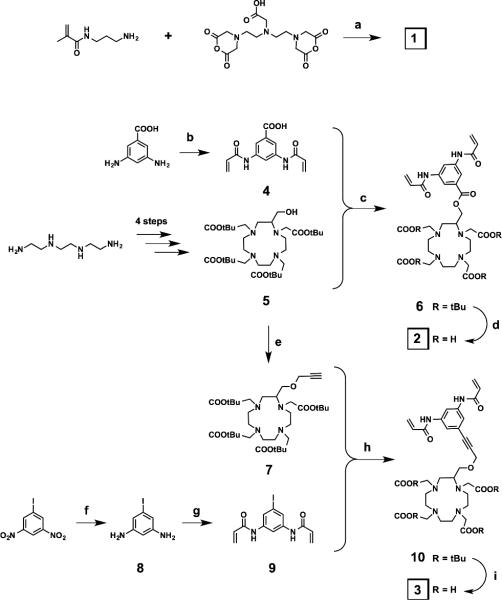
Crosslinker 1 was obtained in a one-step synthesis by reacting commercially available DTPA-bisanhydride with N-(3-aminopropyl) methacrylamide hydrochloride in the presence of triethylamine. The adopted strategy for the synthesis of the two DOTA-based crosslinkers 2 and 3 was convergent (Scheme 1). The C-substituted protected DOTA derivatives 5 and 7 were prepared in 4 and 5 steps, respectively, according to a procedure reported in the literature. 40 In parallel, the bisacrylamide derivatives 4 and 9 were synthesized in one or two steps from the commercial 3,5-diaminobenzoic acid and 3,5-dinitroiodobenzene, respectively. The latter was first reduced with tin chloride to give the 3,5-diaminoiodobenzene 8. The two diamino compounds were acrylated by using acryloyl chloride under Schotten-Baumann conditions (two-phase solvent system) to give 4 and 9. These bisacrylamide derivatives were then coupled with the protected DOTA derivatives 5 and 7 via coupling reactions: an ester coupling reaction to form the protected crosslinker 6, and a Sonogashira cross-coupling reaction to form the protected crosslinker 10. Finally, the two target crosslinkers 2 and 3 were obtained after a TFA deprotection of the tert-butyl ester functions of 6 and 10.
Scheme 1.
Synthesis of crosslinkers 1, 2 and 3. a) Et3N, r.t., 24 h, 61%; b) acryloyl chloride, K2CO3, EtOAc/H2O, 0 °C to r.t., 30 min, 73%; c) DCC, DMAP, DCM, 0 °C to r.t., 48 h, 59%; d) TFA/DCM 1:1, r.t., 16 h, 100%; e) propargyl bromide, tetrabutylammonium iodide, CsOH, DCM, 0 °C to r.t., 1 h, 75%; f) SnCl2.(H2O)2, 70 °C, 30 min, 100%; g) acryloyl chloride, EtOAc/H2O, 0 °C to r.t., 10 min, 100%; h) Pd(PPh3)2Cl2, CuI, Et3N, THF, 50 °C, 48 h, 59%; i) TFA, r.t., 4 h, 45%.
Nanogel Preparation
The crosslinkers 1, 2 and 3 were chelated using GdCl3 and heating at 40 °C at pH 6. Excess gadolinium was removed by adding the chelating resin Chelex-100 and filtering the solution. The gadolinium complexes 1Gd(III), 2Gd(III) and 3Gd(III) were then used to prepare polyacrylamide-based nanogels PAA/1Gd(III), PAA/2Gd(III) and PAA/3Gd(III) through an inverse emulsion process using ammonium persulfate (APS) as the initiator and by adding tetramethylethylenediamine (TMEDA) to control the radical polymerization rate (Figure 2).
Figure 2.
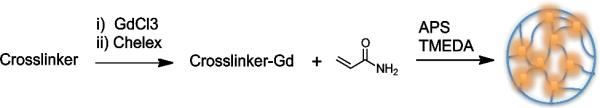
Preparation of nanogels through inverse emulsion process.
Nanogel characterization
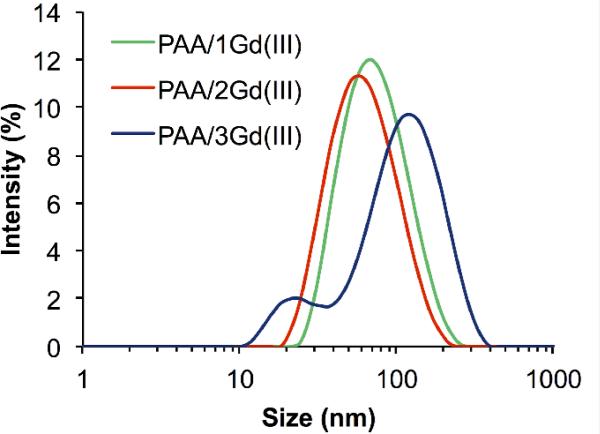
The nanogels obtained were characterized by dynamic light scattering (DLS) in order to evaluate their hydrodynamic volume and size distribution (Figure 3). PAA/1Gd(III) had an average size of 65 nm with a polydispersity index (PDI) of 0.16, PAA/2Gd(III) and PAA/3Gd(III) were on average 54 nm and 85 nm in diameter with PDI values of 0.25 and 0.39, respectively.
Figure 3.
Size distribution of PAA/1Gd(III), PAA/2Gd(III) and PAA/3Gd(III) nanogels as measured by dynamic light scattering.
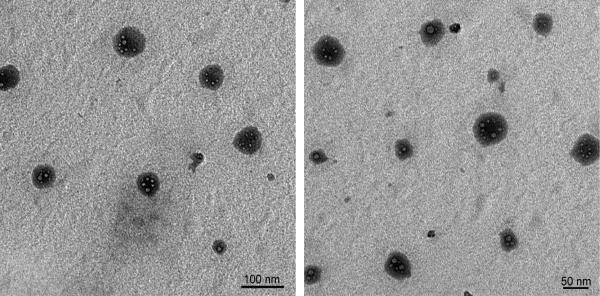
Nanogels were then visualized (Figure 4) by transmission electron microscopy (TEM). Spherical nanogel particles with smaller sizes were observed, which is in accordance with the hydrodynamic sizes measured by DLS. Spherical voids seen on the TEM microphotographs are most likely generated by water escaping from the nanogel upon placement under vacuum and irradiation by the electron beam..
Figure 4.
TEM microphotographs of polyacrylamide nanogels with Gd3+-chelating crosslinkers PAA/2Gd(III)..
To evaluate the effect of nanogels on cell viability, murine macrophages were incubated with PAA/1Gd(III), PAA/2Gd(III) and PAA/3Gd(III) at various concentrations, and cytotoxicity was measured by MTT assay (Figure S1). From the MTT assay results, it appears that PAA/1Gd(III), PAA/2Gd(III) and PAA/3Gd(III) are well tolerated by the cells at concentrations up to 100 μg/ml.
Relaxivity studies
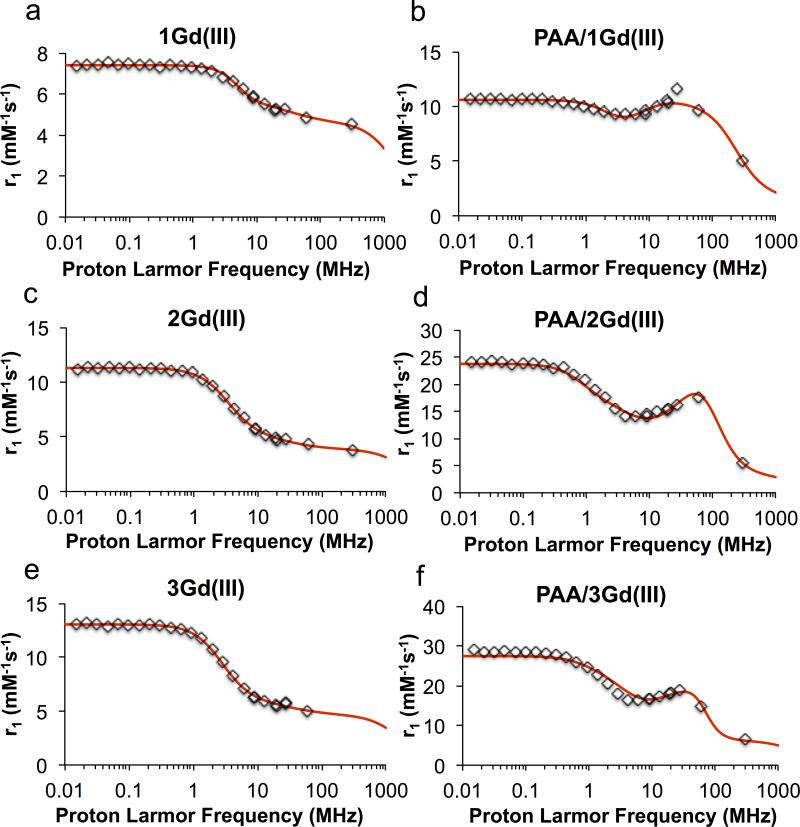
In order to test our central hypothesis, the relaxivity values of all Gd3+ complexes (crosslinkers alone and nanogels incorporating crosslinkers 1, 2, and 3) were calculated. T1 was measured at 60 MHz (1.41 T), 37 °C in PBS buffer and exact concentrations of Gd3+ were measured by inductively coupled plasma atomic emission spectroscopy (ICP-AES). Under these conditions, the clinically used contrast agents Magnevist® (DTPA-Gd3+ complex) and Dotarem® (DOTA-Gd3+ complex) had relaxivities of 3.6 ± 0.3 mM−1s−1 and 2.9 ± 0.3 mM−1s−1, respectively. Proton NMRD profiles were also recorded at 37 °C and analyzed using the classical inner sphere and outer sphere theories (Figure 5). As expected from its larger molecular weight, the complexed crosslinker 1Gd(III) has a larger relaxivity than Magnevist®. The larger value of the fitted water residence time agrees with previous results showing that replacement of carboxylic groups with amides reduces the water exchange rate. 1, 41 The incorporation of the complexed crosslinker 1Gd(III) into nanogels resulted in an increase of relaxivity from 4.8 ± 0.2 mM−1s−1 for the crosslinker alone to 9.7 ± 0.5 mM−1s−1 for the corresponding PAA nanogel at 60 MHz and 37°C. This corresponds to a 2-fold improvement in relaxivity over Magnevist® after incorporation of the DTPA as a crosslinker. The decrease in mobility of the Gd complex in the nanogels was further confirmed by the NMRD profiles showing a “hump” between 10 and 100 MHz. The r1 increase is lower than expected for PAA/1Gd(III) due to the slow water exchange rate resulting from the presence of the amide chelating groups.
Figure 5.
NMRD profiles of the chelated crosslinkers a) 1Gd(III), c) 2Gd(III) e) 3Gd(III) and their corresponding PAA nanogels b) PAA/1Gd(III), d) PAA/2Gd(III) and f) PAA/3Gd(III). The relaxivity measurements were performed at 37 °C. Data points shown in rhombus and fitted curve in solid line.
As expected, since the substitution of the DOTA macrocycle would slow molecular tumbling, our complexed crosslinkers 2Gd(III) and 3Gd(III) exhibited higher relaxivities than Dotarem®, of 4.3 ± 0.2 mM−1s−1 and 5.0 ± 0.3 mM−1s−1, respectively, at 60 MHz. The slightly higher relaxivity of 3Gd(III) compared to 2Gd(III) could be explained by its higher rotational correlation time τR (Table S1) corresponding to a slightly slower tumbling frequency. As anticipated, the NMRD profile shows that the τM, τSO and τV values are quite similar for both complexes and comparable to those of Gd-DOTA (Table S1). The relaxivity of nanogels incorporating 2Gd(III) and 3Gd(III) was greater than that of the complexed crosslinkers alone, at 17.6 ± 0.9 mM−1s−1 and 14.8 ± 0.7 mM−1s−1, respectively. This 3 to 4-fold increase in relaxivity can be explained by the relative rigidity of the system after crosslinking. These r1 values are 5.1 to 6.1-fold improvements over Dotarem®. While these enhancements are limited by the slower water exchange rate in the nanogels, the faster water exchange rate as compared to PAA/1Gd(III) results in a faster relaxation rate of these two DOTA-based nanogels.
Based on the density of our nanogels (1.016 g/mL; see ESI), the Gd concentration, and the average nanoparticle volume (calculated from the nanoparticle size measured by DLS), we calculated the number of Gd3+ per particle to be 134. This value allowed us to obtain the relaxivity per particle, which was 2352 mM−1.s−1 in the case of PAA/2Gd(III).
Although the tumbling frequency of the crosslinker in the nanogel is reduced because the attachment of the bismethacrylamide or bisacrylamide linkers restricts each chelate's rotation, the motions of the linkers are still rapid. This internal flexibility becomes the limiting factor for further reduction in tumbling frequency and increase in relaxivity.42 This also explains the similar r1 among nanogels with different crosslinking densities (see Figure S2). These nanogels have an overall denser structure at a higher crosslinking density; however, locally, the flexibility of the linkers remains high. As a result, increasing crosslinking density has no significant effect on r1.
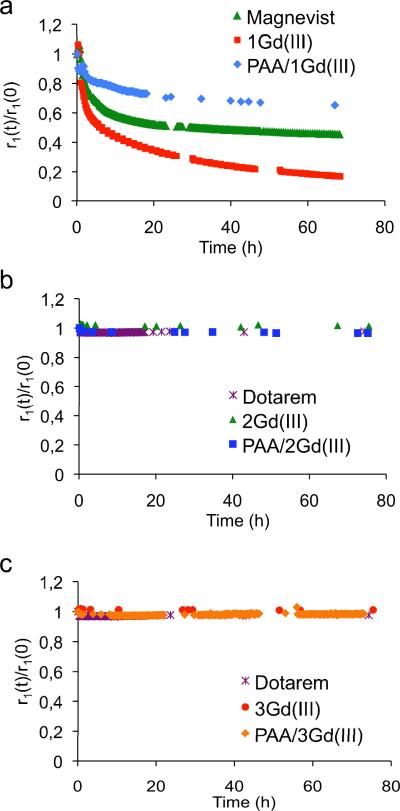
To be clinically translatable, an MRI contrast agent should minimize release of toxic Gd, which occurs mainly through transmetallation of Gd with endogenous metal ions. 7, 43-44 This released Gd3+ can accumulate in the body, sometimes causing nephrogenic systemic fibrosis, especially in patients with reduced kidney function. 43-44 Among the endogenous ions, Zn2+ is most likely to displace Gd3+ because of its relatively high concentration in vivo, high affinity to metal chelates, and similar radius to that of Gd3+. The stability of all the chelated crosslinkers and their corresponding nanogels toward transmetallation was evaluated by monitoring their paramagnetic longitudinal relaxation rate 1/T1 over time in the presence of other metal ions (Figure 6). Specifically, we followed a method described in the literature,44-45 incubating our complexes at a Gd3+ concentration of 2.5 mM with 2.5 mM zinc chloride in phosphate buffer ([PO43-] = 25 mM) at 37 °C. The concentration of Zn2+ used is at least twenty times greater than in plasma; this high concentration makes any difference in transmetallation apparent more rapidly. Upon transmetallation of Gd3+ with Zn2+, free Gd3+ ions are released and an insoluble Gd(PO4) complex is formed, preventing rechelation of Gd3+ to DOTA. As a control, complexes were incubated without zinc chloride.
Figure 6.
Stability of the Gd3+ complexes of a) DTPA-based crosslinker 1 and its corresponding PAA nanogel, b) DOTA-based crosslinker 2 and its corresponding PAA nanogel, and c) DOTA-based crosslinker 3 and its corresponding PAA nanogel. Experiments were carried out in phosphate buffer with 2.5 mM ZnCl2 at 37 °C and pH 7.4. Control experiments were carried out in the absence of ZnCl2 (see Figure S3).
For all three DTPA-based contrast agents, r1 decreases over time, indicating transmetallation with Zn2+. However, the transmetallation in the nanogel is much slower than that of Magnevist® and 1Gd(III), taking three to five times as long to decrease to 80% of initial r1, respectively. This indicates that our nanogel PAA/1Gd(III) has a slower rate of transmetallation than the other two chelates. After 66 h, the nanogel's r1 decreased by 23%, compared to ~40% and ~80% decreases for Magnevist® and 1Gd(III), respectively, suggesting that PAA/1Gd(III) transmetallates to a lesser extent. This stability likely results from greater structural rigidity35 and reduced Zn2+ accessibility to chelating sites. In a control experiment without Zn2+, r1 did not change appreciably for any of the three contrast agents (see Fig. S3). These results suggest that our nanogel, compared to Magnevist® or 1Gd(III), is more inert against dechelation and is potentially a safer MRI contrast agent.
DOTA-based crosslinkers 2 and 3 and their corresponding nanogels all appear highly stable, with 2Gd(III) and 3Gd(III) losing less than 5% of Gd3+ over three days. The nanogels’ relaxation rate did not decrease at all and instead increased slightly, likely because of evaporation of buffer during the three days of incubation. These results confirm that the incorporation of the crosslinkers into nanogels leads to enhanced stability, which is maximal in the case of the DOTA-based nanogels.
We also examined the contrast provided by all our Gd(III) complexes at 7 T of 1Gd(III), 2Gd(III), 3Gd(III) and their corresponding nanogels PAA/1Gd(III), PAA/2Gd(III), PAA/3Gd(III) by phantom imaging, with Magnevist® and Dotarem® as controls (see Figure S4). At identical gadolinium concentrations, better contrast was obtained with the nanogels compared to either chelated crosslinkers alone or the clinical contrast agents. Therefore, by using such systems, smaller doses of gadolinium would provide similar contrast to that obtained with clinically used contrast agents.
Conclusions
In this article, we have shown that incorporation of gadolinium chelate crosslinkers into nanogels leads to a significant (3 to 4-fold) increase in relaxivity. The relaxivities of the complexed DTPA crosslinker 1Gd(III) and DOTA crosslinkers 2Gd(III) and 3Gd(III) are 4.8 mM−1s−1, 4.3 mM−1s−1, and 5.0 mM−1s−1, respectively, at 60 MHz and 37 °C. When formulated into nanogels, these values rise to 9.7 mM−1s−1, 17.6 mM−1s−1, and 14.8 mM−1s−1, respectively. Importantly, DTPA-based nanogels are thermodynamically and kinetically more inert against transmetallation than Magnevist®, suggesting that crosslinker chelates may represent an important approach towards stable metal-chelating biomedical agents. Moreover, we have reported here the first example of nanogels incorporating a DOTA-based contrast agent as a crosslinker itself. This strategy yields a high relaxivity agent with better contrast than commercial agents and maximal stability toward transmetallation. We are now working on the incorporation of our crosslinkers into nanogels with a biodegradable polymer backbone to allow breakdown into biocompatible small molecules to facilitate clearance. We also plan to use different metals in these chelating crosslinkers to yield nanogels for theranostics combining different imaging modalities such as MRI (Gd3+) and PET (64Cu) with radiotherapy (177Lu).
Supplementary Material
Acknowledgments
We thank Prof. R. Muller for his help with NMRD measurements. The authors also thank V. A. Nguyen Huu for his help with cell culture and Dr. M. L. Viger for his help with TEM imaging. MC thanks Dr. J. Sankaranarayanan. NMR data was acquired at the UCSD Skaggs School of Pharmacy and Pharmaceutical Sciences NMR Facility. The authors gratefully acknowledge the assistance of staff at the Center for Functional MRI in phantom imaging. LVE and SL thank the Fonds de la Recherche Scientifique (F.N.R.S.) and the ARC, PAI and ENCITE programs for their financial help. This research was made possible by the NIH New Innovator Award (DP 2OD006499), NSF grant 1006081 and KACST (through the KACST-UCSD Center of Excellence in Nanomedicine).
Abbreviations
- DCM
dichloromethane
- DCC
N,N′-Dicyclohexylcarbodiimide
- DLS
Dynamic Light Scattering
- DMAP
4-Dimethylaminopyridine
- DMF
dimethylformamide
- GBCA
gadolinium-based contrast agents
- HR ES-MS
high resolution electrospray mass spectrometry
- LC-MS
Liquid Chromatography-Mass Spectrometry
- MeOH
Methanol
- NMR
nuclear magnetic resonance
- ES-MS
electrospray mass spectrometry
- NMRD
Nuclear Magnetic Relaxation Dispersion
- PAA
polyacrylamide
- PTA
Phosphotungstic Acid
- TEA
triethylamine
- TEM
Transmission Electronic Microscopy
- TLC
thin layer chromatography
Footnotes
Electronic Supplementary Information (ESI) available: Synthesis procedures. Nanogels preparation. MTT assay. MRI Phantoms. Transmetallation, relaxivity and Dynamic Light Scattering (DLS) measurements. See DOI: 10.1039/b000000x/
References
- 1.Lemieux P, Vinogradov SV, Gebhart CL, Guerin N, Paradis G, Nguyen HK, Ochietti B, Suzdaltseva YG, Bartakova EV, Bronich TK, St-Pierre Y, Alakhov VY, Kabanov AV. J Drug Target. 2000;8:91–105. doi: 10.3109/10611860008996855. [DOI] [PubMed] [Google Scholar]
- 2.Kabanov AV, Vinogradov SV. Angew Chem Int Edit. 2009;48:5418–5429. doi: 10.1002/anie.200900441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bronich TK, Vinogradov SV, Kabanov AV. Nano Lett. 2001;1:535–540. [Google Scholar]
- 4.Missirlis D, Kawamura R, Tirelli N, Hubbell JA. Eur J Pharm Sci. 2006;29:120–129. doi: 10.1016/j.ejps.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Kwon YJ, Standley SM, Goh SL, Frechet JMJ. J Control Release. 2005;105:199–212. doi: 10.1016/j.jconrel.2005.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Standley SM, Mende I, Goh SL, Kwon YJ, Beaudette TT, Engleman EG, Frechet JMJ. Bioconjugate Chem. 2007;18:77–83. doi: 10.1021/bc060165i. [DOI] [PubMed] [Google Scholar]
- 7.Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Chem Rev. 1999;99:2293–2352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 8.Mulder WJM, Strijkers GJ, Griffioen AW, van Bloois L, Molema G, Storm G, Koning GA, Nicolay K. Bioconjugate Chem. 2004;15:799–806. doi: 10.1021/bc049949r. [DOI] [PubMed] [Google Scholar]
- 9.Josephson L, Tung CH, Moore A, Weissleder R. Bioconjugate Chem. 1999;10:186–191. doi: 10.1021/bc980125h. [DOI] [PubMed] [Google Scholar]
- 10.Mazooz G, Mehlman T, Lai TS, Greenberg CS, Dewhirst MW, Neeman M. Cancer Res. 2005;65:1369–1375. doi: 10.1158/0008-5472.CAN-04-2269. [DOI] [PubMed] [Google Scholar]
- 11.Courant T, Roullin VG, Cadiou C, Callewaert M, Andry MC, Portefaix C, Hoeffel C, de Goltstein MC, Port M, Laurent S, Vander Elst L, Muller R, Molinari M, Chuburu F. Angew Chem Int Edit. 2012;51:9119–9122. doi: 10.1002/anie.201203190. [DOI] [PubMed] [Google Scholar]
- 12.Soleimani A, Martinez F, Economopoulos V, Foster PJ, Scholl TJ, Gillies ER. J Mater Chem B. 2013;1:1027–1034. doi: 10.1039/c2tb00352j. [DOI] [PubMed] [Google Scholar]
- 13.Jaszberenyi Z, Moriggi L, Schmidt P, Weidensteiner C, Kneuer R, Merbach AE, Helm L, Toth E. J Biol Inorg Chem. 2007;12:406–420. doi: 10.1007/s00775-006-0197-3. [DOI] [PubMed] [Google Scholar]
- 14.Nicolle GM, Toth E, Schmitt-Willich H, Raduchel B, Merbach AE. Chem-Eur J. 2002;8:1040–1048. doi: 10.1002/1521-3765(20020301)8:5<1040::aid-chem1040>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 15.Rudovsky J, Hermann P, Botta M, Aime S, Lukes I. Chem Commun. 2005:2390–2392. doi: 10.1039/b418712a. [DOI] [PubMed] [Google Scholar]
- 16.Floyd WC, Klemm PJ, Smiles DE, Kohlgruber AC, Pierre VC, Mynar JL, Frechet JMJ, Raymond KN. J Am Chem Soc. 2011;133:2390–2393. doi: 10.1021/ja110582e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schopf E, Sankaranarayanan J, Chan M, Mattrey R, Almutairi A. Mol Pharmaceut. 2012;9:1911–1918. doi: 10.1021/mp2005998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lebduskova P, Kotek J, Hermann P, Vander Elst L, Muller RN, Lukes I, Peters JA. Bioconjugate Chem. 2004;15:881–889. doi: 10.1021/bc049966g. [DOI] [PubMed] [Google Scholar]
- 19.Corsi DM, Vander Elst L, Muller RN, van Bekkum H, Peters JA. Chem-Eur J. 2001;7:1383–1389. doi: 10.1002/1521-3765(20010401)7:7<1383::aid-chem1383>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 20.Kielar F, Tei L, Terreno E, Botta M. J Am Chem Soc. 2010;132:7836–+. doi: 10.1021/ja101518v. [DOI] [PubMed] [Google Scholar]
- 21.Zhang GD, Zhang R, Wen XX, Li L, Li C. Biomacromolecules. 2008;9:36–42. doi: 10.1021/bm700713p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verwilst P, Eliseeva SV, Vander Elst L, Burtea C, Laurent S, Petoud S, Muller RN, Parac-Vogt TN, De Borggraeve WM. Inorg Chem. 2012;51:6405–6411. doi: 10.1021/ic300717m. [DOI] [PubMed] [Google Scholar]
- 23.Gebhart CL, Sriadibhatla S, Vinogradov S, Lemieux P, Alakhov V, Kabanov AV. Bioconjugate Chem. 2002;13:937–944. doi: 10.1021/bc025504w. [DOI] [PubMed] [Google Scholar]
- 24.Lux F, Mignot A, Mowat P, Louis C, Dufort S, Bernhard C, Denat F, Boschetti F, Brunet C, Antoine R, Dugourd P, Laurent S, Vander Elst L, Muller R, Sancey L, Josserand V, Coll JL, Stupar V, Barbier E, Remy C, Broisat A, Ghezzi C, Le Duc G, Roux S, Perriat P, Tillement O. Angew Chem Int Edit. 2011;50:12299–12303. doi: 10.1002/anie.201104104. [DOI] [PubMed] [Google Scholar]
- 25.Morlieras J, Chezal JM, Miot-Noirault E, Roux A, Heinrich-Balard L, Cohen R, Tarrit S, Truillet C, Mignot A, Hachani R, Kryza D, Antoine R, Dugourd P, Perriat P, Janier M, Sancey L, Lux F, Tillement O. Nanoscale. 2013;5:1603–1615. doi: 10.1039/c2nr33457g. [DOI] [PubMed] [Google Scholar]
- 26.Anderson EA, Isaacman S, Peabody DS, Wang EY, Canary JW, Kirshenbaum K. Nano Lett. 2006;6:1160–1164. doi: 10.1021/nl060378g. [DOI] [PubMed] [Google Scholar]
- 27.Prasuhn DE, Yeh RM, Obenaus A, Manchester M, Finn MG. Chem Commun. 2007:1269–1271. doi: 10.1039/b615084e. [DOI] [PubMed] [Google Scholar]
- 28.Nicolle GM, Toth E, Eisenwiener KP, Macke HR, Merbach AE. J Biol Inorg Chem. 2002;7:757–769. doi: 10.1007/s00775-002-0353-3. [DOI] [PubMed] [Google Scholar]
- 29.Allen M, Bulte JWM, Liepold L, Basu G, Zywicke HA, Frank JA, Young M, Douglas T. Magn Reson Med. 2005;54:807–812. doi: 10.1002/mrm.20614. [DOI] [PubMed] [Google Scholar]
- 30.Garimella PD, Datta A, Romanini DW, Raymond KN, Francis MB. J Am Chem Soc. 2011;133:14704–14709. doi: 10.1021/ja204516p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caravan P, Cloutier NJ, Greenfield MT, McDermid SA, Dunham SU, Bulte JWM, Amedio JC, Looby RJ, Supkowski RM, Horrocks WD, McMurry TJ, Lauffer RB. J Am Chem Soc. 2002;124:3152–3162. doi: 10.1021/ja017168k. [DOI] [PubMed] [Google Scholar]
- 32.Caravan P, Parigi G, Chasse JM, Cloutier NJ, Ellison JJ, Lauffer RB, Luchinat C, McDermid SA, Spiller M, McMurry TJ. Inorg Chem. 2007;46:6632–6639. doi: 10.1021/ic700686k. [DOI] [PubMed] [Google Scholar]
- 33.Henoumont C, Henrotte V, Laurent S, Vander Elst L, Muller RN. J Inorg Biochem. 2008;102:721–730. doi: 10.1016/j.jinorgbio.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 34.Guo ZJ, Sadler PJ. Angew Chem Int Edit. 1999;38:1513–1531. [Google Scholar]
- 35.Port M, Idee JM, Medina C, Robic C, Sabatou M, Corot C. Biometals. 2008;21:469–490. doi: 10.1007/s10534-008-9135-x. [DOI] [PubMed] [Google Scholar]
- 36.Manus LM, Strauch RC, Hung AH, Eckermann AL, Meade TJ. Anal Chem. 2012;84:6278–6287. doi: 10.1021/ac300527z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Urbanczyk-Pearson LM, Femia FJ, Smith J, Parigi G, Duimstra JA, Eckermann AL, Luchinat C, Meade TJ. Inorg Chem. 2008;47:56–68. doi: 10.1021/ic700888w. [DOI] [PubMed] [Google Scholar]
- 38.Bonnet CS, Buron F, Caille F, Shade CM, Drahos B, Pellegatti L, Zhang J, Villette S, Helm L, Pichon C, Suzenet F, Petoud S, Toth E. Chem-Eur J. 2012;18:1419–1431. doi: 10.1002/chem.201102310. [DOI] [PubMed] [Google Scholar]
- 39.Caille F, Bonnet CS, Buron F, Villette S, Helm L, Petoud S, Suzenet F, Toth E. Inorg Chem. 2012;51:2522–2532. doi: 10.1021/ic202446e. [DOI] [PubMed] [Google Scholar]
- 40.Ochietti B, Lemieux P, Kabanov AV, Vinogradov S, St-Pierre Y, Alakhov V. Gene Ther. 2002;9:939–945. doi: 10.1038/sj.gt.3301716. [DOI] [PubMed] [Google Scholar]
- 41.Powell DH, NiDhubhghaill OM, Pubanz D, Helm L, Lebedev YS, Schlaepfer W, Merbach AE. J Am Chem Soc. 1996;118:9333–9346. [Google Scholar]
- 42.Toth E, Helm L, Merbach AE. Top Curr Chem. 2002;221:61–101. [Google Scholar]
- 43.Aime S, Caravan P. J Magn Reson Imaging. 2009;30:1259–1267. doi: 10.1002/jmri.21969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robic C, Catoen S, Goltstein DM, Idée J, Port M. Biometals. 2011;24:759–768. doi: 10.1007/s10534-011-9422-9. [DOI] [PubMed] [Google Scholar]
- 45.Laurent S, Vander Elst L, Henoumont C, Muller RN. Contrast Media Mol I. 2010;5:305–308. doi: 10.1002/cmmi.388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.