Abstract
Brain–derived neurotrophic factor (BDNF) is critically involved in synaptic plasticity and neurotransmission. Our lab has previously found that BDNF activation of TrkB is required for fear memory formation and that GABAA receptor subunits and the GABAA clustering protein gephyrin are dynamically regulated during fear memory consolidation. We hypothesize that TrkB-dependent internalization of GABAA receptors may partially underlie a transient period of amygdala hyperactivation during fear memory consolidation. We have previously reported that BDNF modulates GABAA receptor α1 subunit sequestration in cultured hippocampal and amygdala neurons by differential phosphorylation pathways. At present, no studies have investigated the regulation of gephyrin and GABAA receptor α1 subunits following BDNF activation in amygdala. In this study, we confirm the association of GABAA receptor α1 and γ2 subunits with gephyrin on mouse amygdala neurons by coimmunoprecipitation and immunocytochemistry. We then demonstrate that rapid BDNF treatment, as well as suppression of gephyrin protein levels on amygdala neurons, induced sequestration of surface α1 subunits. Further, we find that rapid exposure of BDNF to primary amygdala cultures produced decreases in gephyrin levels, whereas longer exposure resulted in an eventual increase. While total α1 subunit levels remained unchanged, gephyrin was downregulated in whole cell homogenates, but enhanced in complexes with GABAA receptors. Our data with anisomycin suggest that BDNF may rapidly induce gephyrin protein degradation, with subsequent gephyrin synthesis occurring. Together, these findings suggest that gephyrin may be a key factor in BDNF-dependent GABAA receptor regulation in amygdala. This work may inform future studies aimed at elucidating the pathways connecting BDNF, GABAA systems, gephyrin, and their role in underlying amygdala-dependent learning.
Keywords: Amygdala, Fear, GABA, Memory, Consolidation, Gephyrin
Introduction
The activation of GABAA receptors (GABAARs) mediates the majority of fast inhibitory neurotransmission in the CNS. These receptors are pentameric structures predominantly comprised of alpha (α) and beta (β) subunits, but must also contain either gamma (γ) or delta (δ) subunits. Among these combinations, at least 16 GABAAR subtypes have been identified; the most abundant subtype in brain is composed of α1β2γ2 subunits, representing over half of all GABAARs (McKernan and Whiting, 1996; Gao and Fritschy, 1994; Sperk et al., 1997; Olsen and Sieghart, 2009). In some brain regions, including the amygdala, α1-containing subtypes (GABAARα1) are present on both pyramidal cells and parvalbumin-positive interneurons (Freund and Gulyas, 1997; McDonald and Mascagni, 2004; Muller et al., 2007). Such receptors play a role in both reinforcing and negative feedback as well as tonic inhibition, in addition to mediating the synchronized rhythmic activity of pyramidal cells important for proper functioning (Mann et al., 2005; Wu et al., 2012).
GABAARs undergo dynamic changes on the neuronal cell surface. Their trafficking to and from the synapse is regulated by activation of several cell-signaling pathways, which have profound effects on both GABAAR function and the efficacy of GABAAR-mediated synaptic inhibition. Past studies have demonstrated that intracellular signaling pathways activated by brain-derived neurotrophic factor (BDNF) influence GABAergic transmission. For example, Brunig et al. (2001) found a decrease in miniature inhibitory postsynaptic current (mIPSC) amplitude after a 5-minute application of BDNF in hippocampal neurons. In cerebellar granule cells, BDNF application induces the internalization of GABAAR β2/3 subunits and a depression of GABA-induced currents (Cheng and Yeh, 2003). Additionally, we have previously reported that BDNF application to cultured hippocampus and amygdala neurons induced the rapid internalization of GABAAR α1 subunits (Mou et al., 2010). However, the mechanism by which GABAARs are regulated by BDNF signaling is unknown. The current literature suggests that BDNF-induced changes in GABAergic transmission may differ across brain regions and cell types (Jovanovic et al., 2004; Cheng and Yeh, 2005; Palma et al., 2005). Several factors could be underlying the variability reported across studies, including the type of neurons studied (Cheng and Yeh, 2005), duration of BDNF application (Henneberger et al., 2005), and the maturation of cells (Baldelli et al., 2002; Mizoguchi et al., 2003; Yamada et al., 2002). Yet the steps between BDNF induced TrkB activation and changes in GABAAR function remain unclear.
Previous work in our lab has demonstrated that gephyrin, a clustering protein of GABAAR, is dynamically regulated along with GABAAR following emotional learning. For example, we have demonstrated that gephyrin protein levels and GABAAR surface expression in the amygdala were decreased in parallel after fear acquisition (Chhatwal et al., 2005), and that fear conditioning is both BDNF- and TrkB-dependent (Rattiner et al., 2004a,b, 2005; Choi et al., 2010). In another study, we reported that gephyrin gene expression was significantly downregulated in the amygdala during consolidation, after fear acquisition (Ressler et al., 2002). We further showed that fear acquisition induced a down-regulation of mRNA markers related to GABAergic function within the amygdala, whereas fear extinction upregulated gephyrin (Heldt and Ressler, 2007). However, the mechanism of learning-dependent, rapid GABAAR downregulation and alteration of gephyrin levels is unknown.
A growing literature suggests a role for gephyrin in the formation and/or stabilization of GABAAR clusters. Gephyrin anti-sense oligonucleotides have been shown to destabilize postsynaptic GABAAR clusters in treated neuronal cultures (Essrich et al., 1998). Cultured hippocampal neurons from gephyrin knockout mice failed to express clusters of GABAARs containing γ2 and α2 subunits in one study (Kneussel et al., 1999). However, another study reported that GABAAR α2 and γ2 subunits did cluster at synapses in hippocampal cultures from gephyrin knockout mice, indicating gephyrin-independent GABAergic synapses (Levi, et al., 2004). Additionally, the removal of gephyrin by gene targeting or RNA expression interference dramatically alters GABAA R clustering (Yu et al., 2007). More recently, GABAAR α1 subunits have been shown to be directly associated with gephyrin at inhibitory synapses in cultured rat hippocampal neurons (Mukherjee et al., 2011). Importantly, inhibiting gephyrin expression significantly decreases the number of GABAAR clusters at the cell surface, while having no effect on the total number of surface GABAARs expressed (Jacob, et al., 2005). However, to date there have been few studies examining the interactions between surface GABAARs and gephyrin on amygdala neurons, particularly the α1-containing GABAARs. In the present study, we investigated the role of gephyrin in the BDNF-mediated decrease of surface GABAARα1 in cultured amygdala neurons.
Methods
Amygdala neuronal cell culture
All procedures involving animal use were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Primary cultures of postnatal amygdala neurons were performed as described previously (Mou et al., 2011). Briefly, C57BL/6J mice (postnatal 14 days) were decapitated, and the amygdala were punched from brain slides and immersed in ice-cold dissection buffer consisting of Hibernate-A medium (BrainBits, Springfield, IL, USA), B27 supplement (Invitrogen, Carlsbad, CA, USA), 2 mM Glutamax (Invitrogen), and gentamycin (Invitrogen) (12 μg/ml). Then the amygdala tissues were sliced and enzymatically digested with papain (Worthington, Lakewood, NJ, USA) in Hibernate-A medium at 32°C for 30 min. Cells were dissociated by triturating with pasteur pipettes. Neurons were purified in the density gradient media including Hibernate-A and OptiPrep (Sigma, St. Louis, MO, USA) by centrifugation. Neurons were then transferred into a new tube. After being washed with dissection buffer, neuronal cells were plated onto Poly-D-Lysine (Sigma) coated plates at the density of 2.5×105 cells/cm2 in culture media consisting of Neurobasal A medium (Invitrogen) with 2% B27 supplement, 2 mM glutamax and gentamycin (5 μg/ml). Thereafter, the cultures were kept in a humidified incubator at 37°C and 5% CO2, and media were changed every 5 days until used for experiments. Cells were used for the experiments in this study after 2 weeks in vitro. The neuronal culture viability was tested by adding 4% Trypan Blue solution (Mediatech Inc., Herndon, VA, USA) onto cultures and >99% viability was assured before experiments.
BDNF peptide and antibodies
Recombinant human BDNF was purchased from Cell Sciences (Canton, MA, USA) and reconstituted in sterile PBS as 100mg/ml stock. The aliquots of stock were stored at −30°C and final concentration of application on neurons was 100ng/ml.
The solution of anisomycin (Sigma) was made in sterile water as a stock of 10mM and stored at −30°C in small aliquots. The final working concentration on neurons is 10μM.
The following antibodies were used in the described experiments: polyclonal rabbit antisera against α1-GABAAR subunits (epitope region: N-terminus, Millipore, Temecula, CA, USA); polyclonal rabbit antisera against γ2-GABAAR subunits (Affinity BioReagents, Rockford, IL, USA); monoclonal mouse antibody against gephyrin (BD Transduction Laboratories, San Jose, CA, USA; Synaptic Systems, Goettingen, Germany); goat anti-rabbit IgG conjugated with Alexa Fluor 488 (Invitrogen); goat anti-rabbit IgG conjugated with Alexa Fluor 568 (Invitrogen); Donkey anti-mouse IgG conjugated with Alexa Fluor 568 (Invitrogen); peroxidase-conjugated horse anti-mouse secondary (Vector Laboratories, Burlingame, CA, USA); peroxidase-conjugated goat anti-rabbit secondary (Vector Laboratories).
Neuronal Transfection
Before starting transfection, the culture media in cell chambers were changed with half fresh. 1μl of Lipofectamine2000 (Invitrogen) was diluted with 50μl culture media, and 0.5μg DNA (either pLV-siGephyrin, a gephyrin siRNA containing GFP, or pLV-siRNA control) diluted in another 50μl culture media. After 5 minutes of incubation at room temperature, the Lipofectamine and DNA mixtures were combined into one vial. Following 20 minutes of incubation at room temperature, the DNA-lipofectamine mixture in 100μl culture media was added into the cell chamber (area 1.8cm2). After 3 hours in the cell incubator, the media in the cell chamber was changed to regular. The cells were used for experiments 48 hours post-transfection. The cells that received lipofectamine without DNA also served as controls for transfection and ICC experiments.
Immunocytochemistry and analysis of Immunofluorescence
Antibody feeding protocol
The surface GABAAR α1 subunits were tagged in living cultured amygdala neurons with the primary antibody against α1-GABAAR subunits. The tagged α1 subunits were allowed to undergo endocytosis at 37 °C before fixation of cells. First, half of the culture media was changed with fresh media, and the cultures were incubated with rabbit antibody against α1-GABAAR subunits (diluted 1:200) for 30 min at 37°C. After washing three times with dissection buffer, culture media was returned to cells with half fresh media. Cells were then treated with BDNF for 10 minutes, 20 minutes, or 1 hour. Treatments were stopped by removing media and rinsing cells three times with dissection buffer. To label the surface α1 subunits, cells were then incubated with goat anti-rabbit IgG conjugated with Alexa Fluor 568 or Alexa Fluor 488 (1:2000) diluted in culture media for 20 min in incubator. Cells were then rinsed with ice-cold PBS on ice and fixed with methanol at −20°C for 20 min. After washing with PBS, cells were ready for microscopy or for further gephyrin staining.
Immunocytochemistry (ICC) of total GABAAR α1 subunits and gephyrin or of gephyrin following surface α1 staining
Following removal of the culture media, the cells were fixed in 4% paraformedehyde (Sigma) in 1X PBS at room temperature for 20 minutes. After being washed five times with 1X PBS, cells were incubated with blocking buffer (1% BSA, 3% normal goat serum and 0.01% Triton X-100 (Sigma) in 1X PBS) at room temperature for 1 hour with gentle shaking. All subsequent antibodies were diluted in the blocking buffer. Rabbit anti- GABAAR α1 (1:500), mouse anti-gephyrin (1:500), or a combination, was added onto cells and incubated overnight at 4°C. Following washing with 1X PBS, the cells were incubated with either goat anti-rabbit IgG conjugated with Alexa Fluor 488 (1:2000) or donkey anti-mouse conjugated with Alexa Fluor 568 (1:2000), or a combination, at room temperature for 2 hours. After final washing with 1X PBS, the cells were ready for microscopy. Cells without primary antibodies or secondary antibodies added were used as controls.
Analysis of immunofluorescence
Immunofluorescence images were visualized and captured using Nikon eclipse TE300 microscope with a high resolution digital camera (Nikon, Melville, NY, USA) and using Olympus confocal microscope FV1000. The relative immnofluorescence intensity was analyzed by using software of NIS-Elements BR2.30 (Nikon). Measured immunofluorescence intensity levels were subtracted from non-fluorescence background. The maximum intensities were normalized to controls. For quantitative analysis in each experiment, imaged cells were chosen based on the presence of fluorescence signals, and randomly selected neurites including ones close or remote to soma or soma from 20 neurons were measured. Student’s t-test was applied at p <0.05 to determine significance. ImageJ was used for quantitative analysis of confocal microscopy images. The ICC experiment was done independently at least three times.
Surface biotinylation and isolation of surface proteins
BDNF or vehicle was added into indicated wells for 20 minutes, then the media was aspirated and the cells were washed with ice-cold 1X PBS on ice. The cell surface protein isolation kit (Thermo scientific, Rockford, IL, USA) was used for the cell surface biotinylation process. Briefly, 1 mg biotin was applied onto cells in each well of 6-well plates. The cell lysates were obtained by sonicating cells in lysis buffer containing proteinase inhibitors (Roche diagnostics, Indianapolis, IN, USA) and supernatants were collected. The biotin-labeled protein was isolated and the surface protein was eluted from the biotin in SDS-PAGE sample buffer.
Immunoprecipitation and immunoblot analysis
The above surface proteins were applied to further immunoprecipitation (IP) experiments. 100μg protein per sample was used after being adjusted to equal with buffer. In parallel experiments, cells were treated with BDNF for 20 minutes in indicated wells. Then the cells were harvested with RIPA buffer (50mM Tris–HCl, pH 8, 150mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% SDS) containing protease inhibitors (protease inhibitor cocktail tablet, Roche diagnostics). The protein solutions were collected after cell lysis and centrifugation. 260μg protein per sample was used for IP. Then, each sample was divided into two equal parts. Mouse anti-gephyrin (1:100) was added into first half, and rabbit anti-GABAAR α1 (1:100) or γ2 (1:100) into the second. The reactions were incubated overnight at 4°C. The antigen-antibody complex was added into Protein A/G agarose (Thermo Scientific) and incubated for 2 hours at room temperature with gentle mixing. The immune complex was eluted with electrophoresis loading buffer and evaluated by SDS-PAGE. After SDS-PAGE, the blot of IP with anti-gephyrin was incubated with rabbit anti-GABAAR α1 or γ2, and vice versa. The dilution of antibodies was 1:500. Finally, immunocomplexes were visualized by using a peroxidase-conjugated goat anti-rabbit or horse anti-mouse secondary (1:2000). Super Signal west pico chemiluminescent substrate (Thermo Scientific) was used for detecting horse radish peroxidase. Bands were quantified by Fluorchem Sp (Alpha Innotech, San Leandro, CA, USA). For each IP experiment, 20μl of protein solution was always analyzed along with IP samples on SDS-PAGE as a control of IP. The experiment was performed three times independently.
Construct of LV-siGephyrin
Viral vectors containing siGephyrin were derived from the HIV-based lentivirus backbone pLV-CMV–GFP–U3Nhe, and were designed, tested, and produced as recently described for siGAD67 (Heldt et al., 2012). Each small interfering RNA-expressing viral vector (LV-siRNA) was created by subcloning a H1-siRNA coding cassette into the Nhe1 restriction enzyme site of the pLV-CMV–GFP–U3Nhe backbone as described (Tiscornia et al., 2003). The sense and antisense sequences targeting Gephyrin were 5′-ACAUGGCGACCGAGGGAAU and 5′-AUUCCCUCGGUCGCCAUGU, respectively. Active viral particles were produced by co-transfecting these lentiviral packaging constructs with plasmids coding for delta8.9 and VSV-G into HEK-293 T cells following standard methods (Tiscornia et al., 2006; Jasnow et al., 2009; Heldt et al., 2012), using a titer of approaximately 1×109 infectious particles per ml. The pLV-siRNA control construct was designed to have no complementarity to gephyrin, with the following sequence: 5′-UGGCAGUGACUAGCUGGUUGU.
Results
GABAAR α1 and γ2 subunits and gephyrin are associated in cultured mouse amygdala neurons
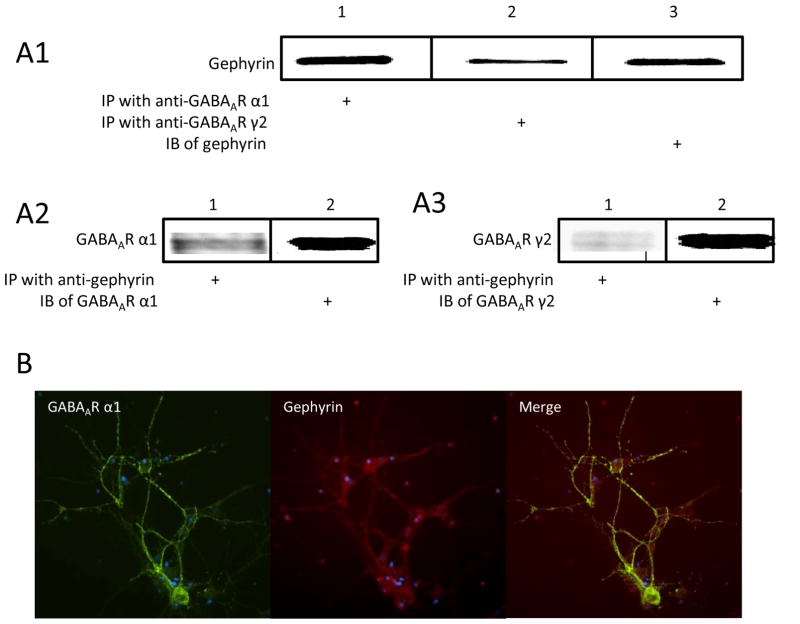
In this study, we first wanted to confirm that GABAARs are co-localized with gephyrin in cultured mouse amygdala neurons. By using co-IP, protein samples from primary amygdala neuronal cultures were precipitated with antibodies either against GABAAR α1 or γ2, and then immunoblotted with anti-gephyrin antibody. Our data clearly indicated that GABAAR α1 or γ2 subunits were associated with gephyrin (Figure 1A1). The protein samples were also immunoprecipitated with the antibody against gephyrin and then blotted with either anti-α1 or γ2 on western blot. The bands of α1 and γ2 subunits were clearly observed as expected (Figure 1A2 and 1A3). We next performed ICC with amygdala neurons by staining for the presence of GABAAR α1 subunits (green fluorescent secondary) and gephyrin (red fluorescent secondary), and observed their co-localization on neuronal soma and dendrites (Figure 1B).
Figure 1. GABAAR α1 and γ2 subunits were associated with gephyrin on cultured mouse amygdala neurons.
Panel A shows results of co-IP of gephyrin and GABAAR α1 and γ2 subunits. A1 shows gephyrin protein from samples immunoprecipitated with either anti-α1 or anti-γ2. A2 shows α1 subunits from samples immunoprecipitated with gephyrin antibody. A3 represents γ2 subunits from samples immunoprecipitated with anti-gephyrin. Panel B illustrates the green-fluorescent labeled GABAAR α1 subunits and red fluorescent labeled gephyrin and the colocalization of both.
Decreased gephyrin expression with siRNA decreases surface GABAAR α1 on cultured amygdala neurons
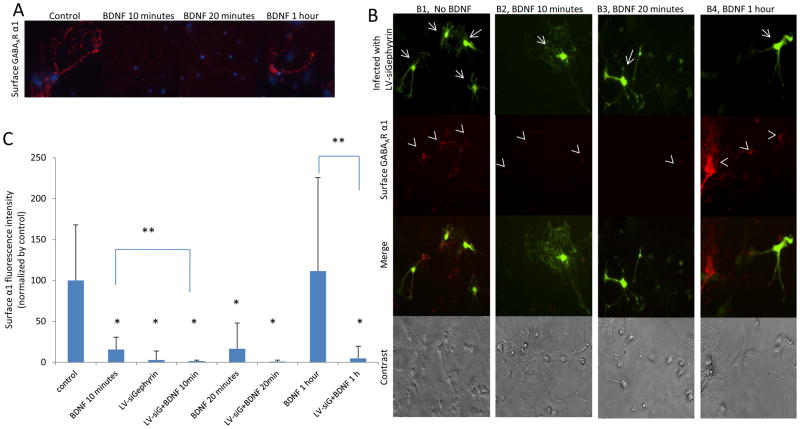
Previously, we have shown that rapid BDNF treatment on amygdala neurons induced the sequestration of surface GABAAR α1 subunits (Mou et al. 2010). Here, we first replicated this effect using ICC. We observed that 10 or 20 minutes, but not 1 hour, of BDNF treatment on neurons resulted in a decrease of surface GABAAR α1 subunits (Figure 2A). This reduction was more than 80%, when compared with the control (Figure 2C).
Figure 2. Decreased gephyrin expression with siRNA decreases surface GABAAR α1 on cultured amygdala neurons.
Panel A shows red-fluorescent surface GABAAR α1 subunits. The surface α1 subunits are decreased in neurons treated with BDNF for 10 or 20 minutes. Panel B shows single- and double-label ICC. GFP expression indicates neurons infected by LV-siGephyrin (arrows) and the surface GABAAR α1 is labeled with red fluorescence (arrowheads). B1 represents neurons infected with LV-siGephyrin and without BDNF treatment. The surface GABAAR α1 subunits were decreased only in infected neurons. B2 and B3 are neurons treated with BDNF for 10 or 20 minutes, respectively, and also infected with LV-siGephyrin. The surface α1 was decreased in infected neurons and non-infected neurons. B4 presents neurons treated with BDNF for 1 hour and infected with LV-siGephyrin. The surface α1 decreases only on infected neurons. Panel C illustrates the quantified surface α1 fluorescence intensity of Panel A and B. (LV-siG stands for LV-siGephyrin). (* P ≤ 0.01 comparing with the control. ** p ≤ 0.05 comparing between groups).
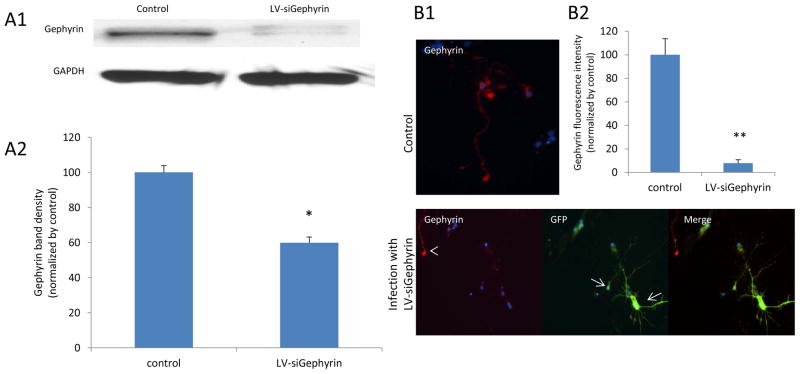
Published studies demonstrated that GABAAR α1 subunits binding directly to gephyrin could facilitate the receptor clustering at inhibitory synapses (Tretter et al., 2008, Mukherjee et al., 2011; Tretter et al., 2011). We have previously found that activation of TrkB by BDNF was required for fear memory formation and, separately, that GABAAR subunits and gephyrin were dynamically regulated during fear memory consolidation (Chhatwal et al. 2005; Heldt and Ressler 2007). Therefore, we hypothesized that the BDNF-induced surface GABAARs changes could be mediated by a change in gephyrin expression. To test this, we created a lentivirus construct harboring siGephyrin (LV-siGephyrin). To validate the construct, we applied it to our primary amygdala neuron cultures. The cells were infected for 3 days, then harvested and protein homogenates were probed for gephyrin via Western blot. The LV-siGephyrin was verified to be able to inhibit the gephyrin expression (Figure 3A1) up to 40% comparing with the control (Figure 3A2). We also tested LV-siGephyrin action by ICC. Gephyrin expression was significantly inhibited (Figure 3B1) in infected neurons up to 90%, comparing to non-infected control neurons (Figure 3B2). Next, we used this construct to further assess whether blocking gephyrin expression had any effect on surface GABAAR α1 subunits by ICC (Figure 2B). Infection with LV-siGephyrin is indicated by GFP expression (Top panels of Figure 2B1, 2B2, 2B3 and 2B4), and surface α1 is labeled with red-fluorescent secondary antibody. In the no-BDNF treatment group that received LV-siGephryin (Figure 2B1), surface α1 subunits were also greatly decreased in infected neurons more than 90% compared to control (non-infected, no-BDNF) neurons (Figure 2C). The effect of blocking gephyrin expression on the surface α1 subunits is same as the effect of 10 or 20 minutes BDNF treatment on neurons (Figure 2A). Application of BDNF for 10 minutes (Figure 2B2) or 20 minutes (Figure 2B3) in LV-siGephyrin-infected cells decreased surface α1 levels not only on infected neurons but also on non-infected ones by more than 95% (Figure 2C). In LV-siGephyrin infected neurons treated with BDNF for 1 hour (Figure 2B4), LV-siGephyrin only lowered surface α1 subunit levels in infected neurons that had GFP expression (Figure 2C). Moreover, neurons infected with LV-siGephyrin that were treated with BDNF for 10 minutes (Figure 2B2) showed a further, significant reduction in surface α1 subunit levels, compared to BDNF-treated neurons lacking siGephyrin (Figure 2A and 2C). Overall, suppression of gephyrin expression had similar depressive effects as rapid BDNF treatment on the surface level of GABAAR α1 subunits.
Figure 3. Infection by LV-siGephyrin inhibits gephyrin expression on cultured mouse amygdala neurons.
Panel A1 is a western blot result showing gephyrin expression on neurons infected with LV-siGephyrin compared to non-infected control neurons. The gephyrin expression in infected cells was significantly decreased, compared to the control, * p<0.002 (A2). Panel B is an ICC result, demonstrating decreased gephyrin on infected, GFP expressing neurons, (arrows in B1) compared to non-infected neurons (arrowhead). B2 represents the quantified fluorescence intensity of gephyrin from B1. (** p = 0.001, compared to control).
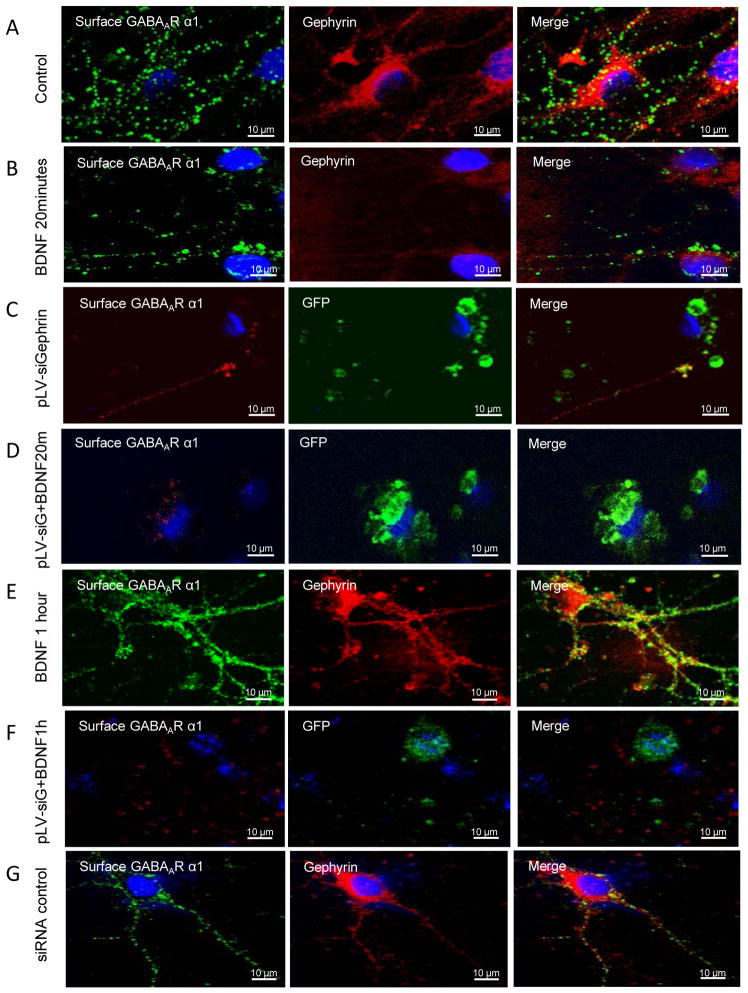
We performed several additional control experiments to confirm the above results. First, when similar experiments were performed with a control (scrambled) siRNA that has no complementarity to gephyrin (Figure 4), no effects were seen, demonstrating that it was the specificity of the siGephryin, and not the constructs in general, providing these findings. Additionally, co-localization studies were performed using confocal microscopy, to confirm that the gephyrin and GABAAR α1 signals were located on synaptic puncta (Figure 5).
Figure 4. Confocal microscopy verifies that surface GABAAR α1 subunits are decreased by gephyrin siRNA on amygdala neurons.
Panel A is an example of control group, showing green-fluorescent α1 and red-fluorescent gephyrin, and merged picture. Panel B represents neurons treated with BDNF for 20 minutes. Both α1 and gephyrin puncta are decreased compared with Panel A control. Panel C shows cells transfected with pLV-siGephyrin, a gephyrin siRNA with GFP expression. The red-fluorescent α1 puncta are decreased. Panel D shows cells transfected with pLV-siGephyrin and treated with BDNF for 20 minutes. As in Panel C, the red-fluorescent α1puncta are greatly lowered. Panel E shows cells treated with BDNF for 1 hour, a time at which both gephyrin and α1puncta are markedly increased. Panel F shows cells transfected with pLV-siGephyrin and treated with BDNF for 1 hour. The red-fluorescent α1 subunits are largely decreased comparing with α1 in Panel E. Panel G is an example of cells transfected with pLV-siRNA control. The α1 and gephyrin expression is same as the control Panel A. (pLV-siG stands for pLV-siGephyrin).
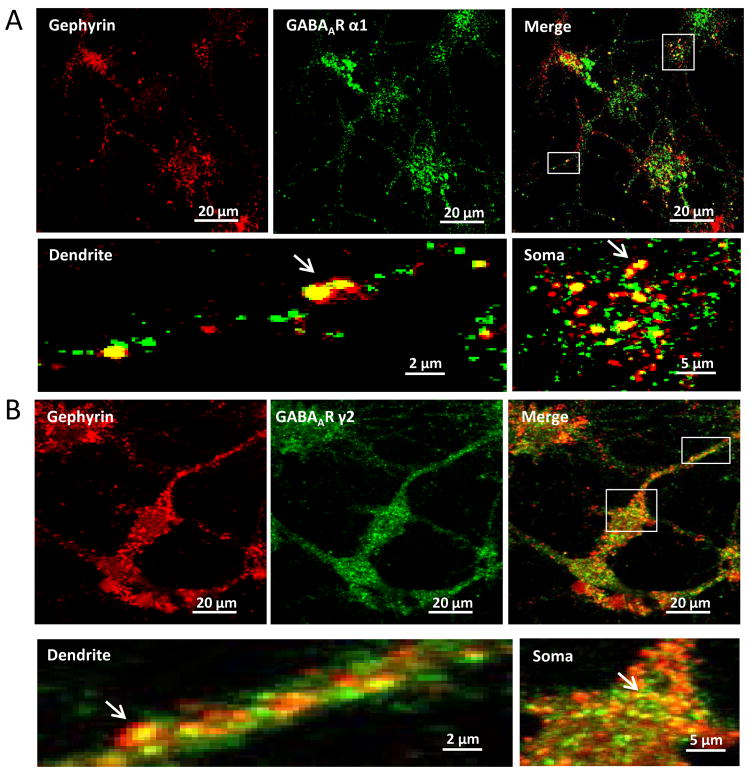
Figure 5. Confocal microscopy shows co-localization of gephyrin and GABAAR α1 or γ2 subunits on cultured mouse amygdala neurons.
Panel A shows red-fluorescent gephyrin and green-fluorescent total α1 subunits on dendrites and somata. The merged picture emphasizes the co-localization of both. The pictures of dendrite and soma correspond to the areas in the merged picture, showing details of co-localization of puncta pointed by arrows. Panal B represents red-fluorescent gephyrin and green-fluorescent total γ2 subunits. The pictures of dendrite and soma are the framed areas in the merged picture. The arrows point to the co-localized puncta.
BDNF treatment leads to biphasic changes in gephyrin levels in cultured amygdala neurons
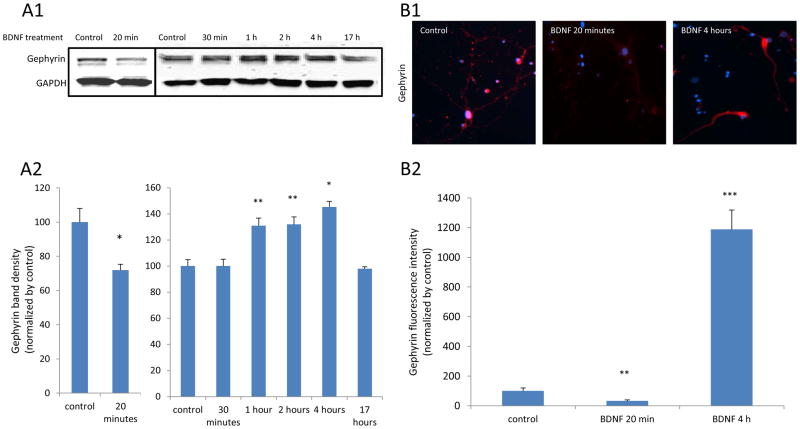
Next, we sought to determine whether BDNF application on amygdala neurons directly induced any changes on gephyrin. Cultured amygdala neurons were treated with BDNF for 20 minutes, 30 minutes, 1 hour, 2 hours, 4 hours and 17 hours, respectively. Then cells were harvested and an antibody against gephyrin was added into samples of protein homogenates for western blot. We observed that the level of gephyrin protein was significantly decreased after 20 minutes of BDNF treatment, but was similar to baseline levels at the 30-minute timepoint. Then, we saw an increase in gephyrin expression after 1 hour of BDNF exposure, which was maintained at 4 hours exposure to BDNF. However, gephyrin levels returned back to control levels after overnight BDNF exposure (Figure 6A1 and 6A2). In a parallel set of ICC experiments, gephyrin expression was decreased more than 60% after 20 minutes BDNF treatment while greatly increased on 4 hours BDNF treatment (Figure 6B1 and 6B2). The agreement of these data supports the hypothesis that BDNF treatment can directly alter gephyrin levels in neurons.
Figure 6. BDNF treatment leads to biphasic changes in gephyrin levels on cultured amygdala neurons.
Panel A1 is a result of a western blot for gephyrin from neurons treated with BDNF for 20 and 30 minutes and 1, 2, 4 or 17 Hours, respectively. A2 shows quantified western blot data demonstrating that gephyrin levels are decreased with 20 minutes of BDNF treatment. In a separate experiment, we showed that 1, 2 and 4 hours of BDNF treatment increased the gephyrin. (* P < 0.05 and ** p < 0.01, compared to control). Panel B1 shows ICC results of red fluorescent-labeled gephyrin on neurons exposed to BDNF for 20 minutes or 4 hours. B2 represents the quantified Gephyrin fluorescence intensities from ICC. (** p < 0.01 and *** p < 0.004, compared to control).
Decreased GABAAR α1 subunits on cell membranes were associated with gephyrin changes induced by rapid BDNF treatment
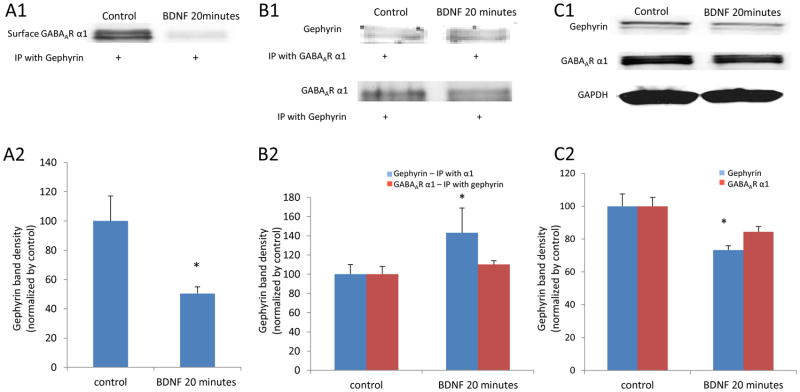
After we confirmed that gephyrin was also decreased by rapid BDNF treatment on neurons, we wanted to test whether GABAAR α1 subunits previously located on the surface (identified with surface biotinylation) were associated with gephyrin after BDNF treatment, which presumably leads to receptor internalization. To answer the question, we performed a co-immunoprecipitation on protein samples obtained from cultured amygdala neurons treated with BDNF. After treatment, cells were incubated with biotin, and the biotinynated proteins were isolated. After releasing the proteins from the biotin, samples were immunoprecipitated with the anti-gephyrin antibody, and then immunoblotted with an anti-GABAAR α1 antibody. We found that the surface α1 subunits internalized after a 20-minute BDNF treatment were indeed associated with gephyrin on cell membranes (Figure 7A1 and 7A2).
Figure 7. Decreased GABAAR α1 subunits on cell membranes were associated with gephyrin changes induced by rapid BDNF treatment.
Panel A1 is a western blot result of the surface GABAAR α1 subunits from biotinylated protein samples and then co-immunoprecipitated with an antibody against gephyrin. The surface GABAAR α1 specifically bound to gephyrin was significantly decreased by 20 minutes of BDNF treatment. (* p < 0.05, compared to control) (A2). Panel B1 shows co-IP results of gephyrin and GABAAR α1 from neurons treated with BDNF for 20 minutes and compared to untreated control neurons. B2 shows that gephyrin bound to GABAAR α1 was increased with BDNF treatment (* p < 0.05, compared to control). Panel C1 is a western blot result of gephyrin and total GABAAR α1 subunits. There is no change in total GABAAR α1 while gephyrin was significantly decreased (C2). (* p < 0.05, compared to control).
We also performed a separate set of Co-IP experiments with whole cell homogenates to determine whether gephyrin levels were altered in complex with GABAARs. We found that gephyrin was significantly enhanced by 20 minutes of BDNF treatment on neurons compared to control, while GABAAR α1 subunits remained unchanged (Figure 7B1 and 7B2). Also, we did western blot experiments on gephyrin and GABAAR α1 subunits on whole cell homogenates. We observed that gephyrin was significantly decreased following 20 minutes of BDNF treatment, while there was no change in total GABAAR α1 subunit levels (Figure 7C1 and 7C2). Thus, we conclude that BDNF treatment induced rapid sequestration of surface GABAARs through dynamic regulation of gephyrin levels in amygdala neurons. This said, it is also possible that in some cases GABAAR regulation is the initial target of BDNF treatment, which then results in secondary alterations in gephyrin levels.
Protein synthesis is not involved in the initial gephyrin decrease but is required for its later rebound
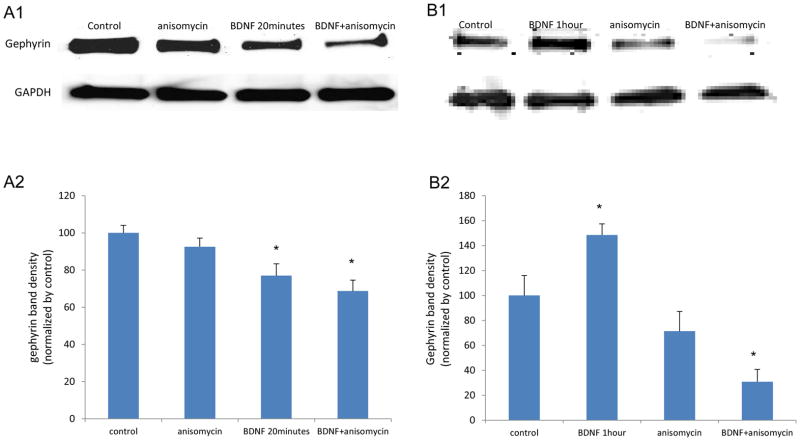
We wished to determine if protein synthesis was involved in BDNF-dependent gephryin modulation. Our data (Figure 8) suggest that the processes of protein synthesis and degradation activated by BDNF may be involved in this modulation. At 20 minutes after BDNF treatment, the application of a protein synthesis inhibitor, anisomycin, further decreased gephyrin (or doesn’t prevent its decrease) (Figure 8A1 and 8A2). However, the longer BDNF treatment does enhance gephyrin synthesis as inhibition of protein synthesis by anisomycin blocks the increased gephyrin levels at 1 hour (Figure 8B1 and 8B2). These data suggest that BDNF may rapidly induce gephyrin protein degradation, with subsequent gephyrin synthesis occuring, perhaps in a homeostatic fashion.
Figure 8. In cultured mouse amygdala neurons, anisomycin further decreases gephyrin after 20 minutes of BDNF treatment and blocks the enhanced gephyrin induced by 1 hour of BDNF treatment.
Panel A1 represents the western blot result on gephyrin from neurons treated with 20 minutes of BDNF either with or without application of anisomycin (a protein synthesis inhibitor). GAPDH is the protein loading control. Anisomycin stimulation lowers the dereased gephyrin (Panel A2) (* p < 0.05 compared to control). Panel B is a result of gephyrin western blot data on neurons in 1 hour BDNF with or without anisomycin. Anisomycin application blocks the increased gephyrin. (* p < 0.05 compared to control).
Discussion
Work from our lab has begun to investigate the molecular mechanism behind the neuronal adaptation of GABAergic inhibition influenced by signal pathways initiated by BDNF. We reported previously that on cultured hippocampal and amygdala neurons, rapid BDNF application (minutes) induces sequestration of surface GABAARs (Mou et al., 2011). In earlier studies, our lab demonstrated that after fear acquisition, gephyrin gene expression was greatly downregulated (Ressler et al., 2002) and surface GABAARs and gephyrin protein levels were decreased in amygdala in parallel (Chhatwal et al., 2005). Building on this work, we conducted the current study and found that BDNF application on cultured amygdala neurons induces a change in gephyrin expression which leads to a decrease of surface GABAAR α1.
The association of gephyrin and GABAARs in the cultured amygdala neurons
In the present study, we first confirmed that GABAARs and gephyrin are associated on cultured amygdala neurons. There has been some disagreement in the literature as to whether the association of these two proteins takes place. Kneussel et al. (1999) reported that in brain sections and cultured hippocampal neurons derived from gephrin knock-out mice, synaptic GABAAR clusters containing either the γ2 or the α2 subunit were absent, suggesting gephyrin may be directly involved with the localization of these receptors. However, Levi et al. (2004) reported that gephyrin does not contribute to insertion or stabilization of α2- or γ2-containing GABAARs in cell membranes, and found GABAAR clusters at synapses in hippocampal neurons to be gephyrin-independent. Our data by co-IP and ICC suggests that gephyrin and GABAARs are indeed colocalized with each other in cultured amygdala neurons, in agreement with the Kneussel study.
Surface GABAAR α1 subunits are decreased by reduced gephyrin which is a result of rapid BDNF application on amygdala neurons
Further, this study demonstrates that the association of gephyrin and GABAARs has functional consequences. Our data suggest that knocking down gephyrin expression by siRNA decreases the population of surface GABAARs. Small interfering RNA of gephyrin has long been used to study GABAAR clustering and stability (Yu et al., 2007). We applied this technique to the present study and found that lowered gephyrin expression via siRNA decreases surface levels of GABAAR α1 on cultured amygdala neurons, the same effect we observed previously with rapid BDNF treatment on neurons (Mou et al., 2011). Our co-IP of surface GABAARα1 likewise indicates that the reduction of surface α1 is gephyrin-related. Postsynaptic GABAARs are critical for effective synaptic inhibition. Their trafficking to and from the cell surface is targeted for regulation at multiple steps. These include receptor localization, stability, insertion and diffusion into membranes, receptor removal, and degradation (Jacob et al., 2008). At least 40% of the total cell surface population of GABAARs is capable of being dynamically internalized (Kittler et al., 2004). To date, the number of published studies on surface GABAARs is limited, and studies specifically on surface GABAARα1 on cultured amygdala neurons are few. To our knowledge, the present study is the first to demonstrate a loss of surface GABAAR α1 subunits induced by gephyrin decrease in response to rapid BDNF application on cultured amygdala neurons. These changes are accompanied by no change in total GABAAR α1 and a decrease of total gephyrin. Our data suggest that BDNF-dependent gephyrin downregulation has a consequential effect on the presentation and localization of surface GABAARs.
BDNF application induces biphasic expression changes of gephyrin
We found that BDNF dynamically regulates gephyrin protein levels over time in cultured amygala neurons. While 20 minutes of BDNF exposure leads to a decrease in gephyrin levels, 1–4 hours of BDNF leads to an increase in gephyrin. Focusing on the more rapid BDNF effect, we found that while total gephyrin is decreased, a greater proportion of gephyrin is present in the complexes with GABAARs than at baseline. This may serve as a compensatory or homeostatic mechanism to recruit receptors back to the cell membrane. Indeed, it was recently reported that gephyrin plays a major role as a cytoskeletal structure in transporting GABAARs to and from the synapses (Tretter et al., 2012). However, the mechanism underlying how BDNF regulates gephyrin is unclear. Our data with anisomycin suggest that BDNF may rapidly induce gephyrin protein degradation, with subsequent gephyrin synthesis occuring, perhaps in a homeostatic fashion. We are continuing to study signaling pathways and the dynamic changes in gephyrin induced by BDNF activation, with the goal of identifying underlying mechanisms.
BDNF is a critical mediator of short-term neuronal plasticity (Lessmann et al., 1994; Berninger and Poo., 1996) and plays a diverse role in GABAergic inhibition (Brunig et al. 2001; Cheng and Yeh, 2003). Our lab and others have shown that BDNF is required for amygdala-related fear learning (Rattiner et al., 2004; Mahan et al., 2012). The BDNF-TrkB signaling cascade activates multiple intracellular responses, including the Ras/ERK pathway, the PI3-K pathway, and phospholipase C-gamma (Carvalho et al., 2008). The rapid effects of BDNF on neuronal plasticity are partly mediated by protein phosphorylation downstream of TrkB receptor activation (Santos et al., 2010). Currently, there are no published studies showing the effect of activation of BDNF-TrkB on gephyrin. Elucidating the signaling pathways between BDNF-TrkB and gephyrin, and determining the role of these pathways in fear memory consolidation, are goals for future study.
Acknowledgments
This work was supported through NIH (DA019624, MH096764(KJR)) as well as the Burroughs Wellcome Fund. Support was received from the NIH/National Center for Research Resources base grant P51RR000165 to Yerkes National Primate Research Center.
Footnotes
Disclosures: The authors have no financial or other potential conflictual relationships to disclose related to the work described in this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baldelli P, Novara M, Carabelli V, Hernandez-Guijo JM, Carbone E. BDNF up-regulates evoked GABAergic transmission in developing hippocampus by potentiating presynaptic N- and P/Qtype Ca2_ channels signalling. Eur J Neurosci. 2002;16:2297–2310. doi: 10.1046/j.1460-9568.2002.02313.x. [DOI] [PubMed] [Google Scholar]
- Berninger B, Poo M. Fast actions of neurotrophic factors. Curr Opin Neurobiol. 1996;6:324–330. doi: 10.1016/s0959-4388(96)80115-2. [DOI] [PubMed] [Google Scholar]
- Brunig I, Penschuck S, Berninger B, Benson J, Fritschy JM. BDNF reduces miniature inhibitory postsynaptic currents by rapid downregulation of GABA(A) receptor surface expression. Eur J Neurosci. 2001;13:1320–1328. doi: 10.1046/j.0953-816x.2001.01506.x. [DOI] [PubMed] [Google Scholar]
- Carvalho AL, Caldeira MV, Santos SD, Duarte CB. Role of the brain derived neurotrophic factor at glutamatergic synapses. Br J Pharmacol. 2008;153(Suppl 1):S310–S324. doi: 10.1038/sj.bjp.0707509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, Yeh HH. Brain-derived neurotrophic factor attenuates mouse cerebellar granule cell GABA(A) receptor-mediated responses via postsynaptic mechanisms. J Physiol. 2003;548:711–721. doi: 10.1113/jphysiol.2002.037846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, Yeh HH. PLCgamma signaling underlies BDNF potentiation of Purkinje cell responses to GABA. J Neurosci Res. 2005;79:616–627. doi: 10.1002/jnr.20397. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Myers KM, Ressler KJ, Davis M. Regulation of gephyrin and GABAA receptor binding within the amygdala after fear acquisition and extinction. J Neurosci. 2005;25(2):502–506. doi: 10.1523/JNEUROSCI.3301-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DC, Maguschak KA, Ye K, Jang SW, Myers KM, Ressler KJ. Prelimbic cortical BDNF is required for memory of learned fear but not extinction or innate fear. Proc Natl Acad Sci. 2010;107(6):2675–2680. doi: 10.1073/pnas.0909359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B. Postsynaptic clustering of major GABAA receptor subtypes required the γ2 subunit and gephyrin. Nature neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- Freund TF, Gulyas AI. Inhibitory control of GABAergic interneurons in the hippocampus. Can J Physiol Pharmacol. 1997;75:479–487. [PubMed] [Google Scholar]
- Gao B, Fritschy JM. Selective allocation of GABAA receptors containing the alpha 1 subunit to neurochemically distinct subpopulations of rat hippocampal interneurons. Eur J Neurosci. 1994;6:837–853. doi: 10.1111/j.1460-9568.1994.tb00994.x. [DOI] [PubMed] [Google Scholar]
- Heldt SA, Ressler KJ. Training-induced changes in the expression of GABAA-associated genes in the amygdala after the acquisition and extinction of Pavlovian fear. Eur J Neurosci. 2007;26(12):3631–3644. doi: 10.1111/j.1460-9568.2007.05970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt SA, Ressler KJ. Amygdala-specific reduction of alpha1-GABAA receptors disrupts the anticonvulsant, locomotor, and sedative, but not anxiolytic, effects of benzodiazepines in mice. J Neurosci. 2010;30(21):7139–51. doi: 10.1523/JNEUROSCI.0693-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt SA, Mou L, Ressler KJ. In vivo knockdown of GAD67 in the amygdala disrupts fear extinction and the anxiolytic-like effect of diazepam in mice. Transl Psychiatry. 2012 Nov;13:2, e181. doi: 10.1038/tp.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneberger C, Kirischuk S, Grantyn R. Brain-derived neurotrophic factor modulates GABAergic synaptic transmission by enhancing presynaptic glutamic acid decarboxylase 65 levels, promoting asynchronous release and reducing the number of activated postsynaptic receptors. Neuroscience. 2005;135:749–763. doi: 10.1016/j.neuroscience.2005.06.044. [DOI] [PubMed] [Google Scholar]
- Jacob TC, Bogdanov YD, Magnus C, Saliba RS, Kittler JT, Haydon PG, Moss SJ. Gephyrin Regulates the Cell Surface Dynamics of Synaptic GABAA Receptors. J Neurosci. 2005;25(45):10469–10478. doi: 10.1523/JNEUROSCI.2267-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasnow AM, Rainnie DG, Maguschak KA, Chhatwal JP, Ressler KJ. Construction of cell-type specific promoter lentiviruses for optically guiding electrophysiological recordings and for targeted gene delivery. Methods Mol Biol. 2009;515:199–213. doi: 10.1007/978-1-59745-559-6_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic JN, Thomas P, Kittler JT, Smart TG, Moss SJ. Brain-derived neurotrophic factor modulates fast synaptic inhibition by regulating GABA(A) receptor phosphorylation, activity, and cellsurface stability. J Neurosci. 2004;24:522–530. doi: 10.1523/JNEUROSCI.3606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Thomas P, Tretter V, Bogdanov YD, Haucke V, Smart TG, Moss SJ. Huntingtin-associated protein 1 regulates inhibitory synaptic transmission by modulating gamma-aminobutyric acid type A receptor membrane trafficking. Proc Natl Acad Sci. 2004;101(34):12736–12741. doi: 10.1073/pnas.0401860101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneussel M, Brandstätter JH, Laube B, Stahl S, Müller U, Betz H. Loss of postsynaptic GABA(A) receptor clustering in gephyrin-deficient mice. J Neurosci. 1999;19(21):9289–97. doi: 10.1523/JNEUROSCI.19-21-09289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessmann V, Gottmann K, Heumann R. BDNF and NT-4/5 enhance glutamatergic synaptic transmission in cultured hippocampal neurons. Neuroreport. 1994;6:21–25. doi: 10.1097/00001756-199412300-00007. [DOI] [PubMed] [Google Scholar]
- Lévi S, Logan SM, Tovar KR, Craig AM. Gephyrin is critical for glycine receptor clustering but not for the formation of functional GABAergic synapses in hippocampal neurons. J Neurosci. 2004;24:207–217. doi: 10.1523/JNEUROSCI.1661-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahan AL, Mou L, Shah N, Hu JH, Worley P, Ressler KJ. Epigenetic modulation of homer1a transcription regulation in amygdala and hippocampus with Pavlovian fear conditioning. J Neurosci. 2012;32(13):4651–4659. doi: 10.1523/JNEUROSCI.3308-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann EO, Radcliffe CA, Paulsen O. Hippocampal gamma-frequency oscillations: from interneurones to pyramidal cells, and back. J Physiol. 2005;562:55–63. doi: 10.1113/jphysiol.2004.078758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Parvalbumin-containing interneurons in the basolateral amygdala express high levels of the alpha1 subunit of the GABAA receptor. J Comp Neurol. 2004;473:137–146. doi: 10.1002/cne.20101. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- Mizoguchi Y, Ishibashi H, Nabekura J. The action of BDNF on GABA(A) currents changes from potentiating to suppressing during maturation of rat hippocampal CA1 pyramidal neurons. J Physiol. 2003;548:703–709. doi: 10.1113/jphysiol.2003.038935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou L, Heldt S, Ressler K. Rapid brain-derived neurotrophic factor –dependent sequestration of amygdala and hippocampal GABAA receptors via different tyrosine receptor kinase B-mediated phosphorylation pathways. Neuroscience. 2011;176:72–85. doi: 10.1016/j.neuroscience.2010.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee J, Kretschmannova K, Gouzer G, Maric HM, Ramsden S, Tretter V, Harvey K, Davies PA, Triller A, Schindelin H, Moss SJ. The residence time of GABAARs at inhibitory synapses is determined by direct binding of the receptor α1 subunit to gephyrin. J Neurosci. 2011;31(41):14677–14687. doi: 10.1523/JNEUROSCI.2001-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Postsynaptic targets of somatostatin-containing interneurons in the rat basolateral amygdala. J Comp Neurol. 2007;500:513–529. doi: 10.1002/cne.21185. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma E, Torchia G, Limatola C, Trettel F, Arcella A, Cantore G, Di Gennaro G, Manfredi M, Esposito V, Quarato PP, Miledi R, Eusebi F. BDNF modulates GABAA receptors microtransplanted from the human epileptic brain to Xenopus oocytes. Proc Natl Acad Sci USA. 2005;102:1667–1672. doi: 10.1073/pnas.0409442102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Paschall GY, Zhou XL, Davis M. Regulation of synaptic plasticity genes during consolidation of fear learning. J Neurosci. 2002;22:7892–7902. doi: 10.1523/JNEUROSCI.22-18-07892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, French CT, Ressler KJ. Brain-derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala-dependent fear conditioning. J Neurosci. 2004a;24(20):4796–806. doi: 10.1523/JNEUROSCI.5654-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, Ressler KJ. Differential regulation of brain-derived neurotrophic factor transcripts during the consolidation of fear learning. Learn Mem. 2004b;11(6):727–731. doi: 10.1101/lm.83304. [DOI] [PubMed] [Google Scholar]
- Santos AR, Comprido D, Carlos B. Duarte Regulation of local translation at the synapse by BDNF Progress in Neurobiology. 2010;92:505–516. doi: 10.1016/j.pneurobio.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Sperk G, Schwarzer C, Tsunashima K, Fuchs K, Sieghart W. GABA(A) receptor subunits in the rat hippocampus I: immunocytochemical distribution of 13 subunits. Neuroscience. 1997;80:987–1000. doi: 10.1016/s0306-4522(97)00146-2. [DOI] [PubMed] [Google Scholar]
- Tiscornia G, Singer O, Ikawa M, Verma IM. A general method for gene knockdown in mice by using lentiviral vectors expressing small interfering RNA. Proc Natl Acad Sci USA. 2003;100:1844–1848. doi: 10.1073/pnas.0437912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiscornia G, Singer O, Verma IM. Production and purification of lentiviral vectors. Nature Protocols. 2006;1:241–245. doi: 10.1038/nprot.2006.37. [DOI] [PubMed] [Google Scholar]
- Tretter V, Jacob TC, Mukherjee J, Fritschy JM, Pangalos MN, Moss SJ. The clustering of GABA(A) receptor subtypes at inhibitory synapses is facilitated via the direct binding of receptor alpha 2 subunitstogephyrin. J Neurosci. 2008;28:1356–1365. doi: 10.1523/JNEUROSCI.5050-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V, Kerschner B, Milenkovic I, Ramsden SL, Ramerstorfer J, Saiepour L, Maric HM, Moss SJ, Schindelin H, Harvey RJ, Sieghart W, Harvey K. Molecular basis of the GABAA receptor alpha 3 subunit interaction with gephyrin. J Biol Chem. 2011;286:37702–37711. doi: 10.1074/jbc.M111.291336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V, Mukherjee J, Maric HM, Schindelin H, Sieghart W, Moss SJ. Gephyrin, the enigmatic organizer at GABAergic synapses. Front Cell Neurosci. 2012;6:23. doi: 10.3389/fncel.2012.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Wu Z, Ning G, Guo Y, Ali R, Macdonald RL, De Blas AL, Luscher B, Chen G. γ-Aminobutyric Acid Type A (GABAA) Receptor α Subunits Play a Direct Role in Synaptic Versus Extrasynaptic Targeting. J Biol Chem. 2012;287(33):27417–30. doi: 10.1074/jbc.M112.360461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada MK, Nakanishi K, Ohba S, Nakamura T, Ikegaya Y, Nishiyama N, Matsuki N. Brain-derived neurotrophic factor promotes the maturation of GABAergic mechanisms in cultured hippocampal neurons. J Neurosci. 2002;22:7580–7585. doi: 10.1523/JNEUROSCI.22-17-07580.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Jiang M, Miralles CP, Li RW, Chen G, DeBlas AL. Gephyrin clustering is required for the stability of GABAergic synapses. Mol Cel Neurosci. 2007;36:484–500. doi: 10.1016/j.mcn.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]