Abstract
Background
Cardiac catheterization is routinely used as a diagnostic tool in single ventricle patients with superior cavopulmonary connection (SCPC). This physiology presents inherent challenges in applying the Fick principle to estimate flow. We sought to quantitatively define the error in oximetry-derived flow parameters, using phase-contrast cardiac MRI (CMR) as a reference.
Methods and Results
Thirty patients with SCPC who underwent combined cardiac MRI and catheterization between July 2008 and June 2012 were retrospectively analyzed. Estimates of flow and resistance calculated using the Fick equation were compared to CMR measurements. Oximetry underestimated CMR-measured pulmonary blood flow (Qp) by an average of 1.1 L/min/m2, or 32% of the CMR value (p < .0001). Oximetry overestimated systemic blood flow (Qs) by an average of 0.5 L/min/m2, or 15% of the CMR value (p = .009). There was no correlation between the Qp:Qs ratio derived by Fick and that measured by CMR (ρc = 0.01). The error in Fick Qp correlated moderately with the measured systemic to pulmonary arterial collateral flow (r =0.39). The median total oxygen consumption calculated using combined CMR and oximetry data was 173 mL/min/m2, higher than the assumed values used to calculate flows by the Fick equation. The upper body circulation received on average 51% of systemic blood flow while conducting only 39% of total body metabolism.
Conclusions
Fick-derived estimates of flow are inherently unreliable in patients with superior cavopulmonary connections. Integrating flows measured by CMR and pressures measured by catheter will provide the best characterization of SCPC physiology.
Keywords: heart defects, congenital, catheterization, magnetic resonance imaging, collateral circulation, cavopulmonary anastomosis
Historically, cardiac catheterization has served as an important diagnostic and therapeutic tool in patients undergoing staged palliation of single ventricle heart disease. For those with a superior cavopulmonary connection (SCPC), catheterization may be indicated either to assess candidacy for Fontan completion or to investigate abnormal clinical findings such as hypoxemia or low cardiac output. Management decisions are then guided by the quantitative indicators of SCPC circuit function that result, most notably pulmonary (Qp) and systemic (Qs) blood flow estimates, Qp:Qs ratio, and pulmonary vascular resistance.
In the presence of intracardiac mixing, catheter-based estimates of flow and resistance are derived from the Fick principle. Admittedly, the SCPC is not an ideal Fick system for several reasons. Systemic to pulmonary arterial collaterals are nearly ubiquitous in these patients,1–4 and provide an additional source of pulmonary blood flow that cannot be reliably accounted for in conventional oximetry calculations. In addition, the systemic venous circulation is completely separated into superior (SVC) and inferior vena cava (IVC) circuits, meaning there is no true mixed venous blood available for sampling.
Oximetry is widely used despite these drawbacks, primarily due to the historical lack of an alternative method to measure flow in complex anatomic circuits. Without a gold standard modality capable of accounting for systemic to pulmonary arterial collaterals, it has been impossible to describe the degree of inaccuracy in the Fick calculations and therefore to understand whether the error is clinically relevant. Fortunately, recent developments in phase-contrast CMR techniques have provided new insight by allowing us to quantify collateral burden in a reliable fashion.1–8 The accuracy of CMR for simple flow assessment has been previously validated in both in vivo and in vitro studies, including in patients with congenital heart disease.9–14
The primary objective of this study was to compare Fick-derived estimates of pulmonary and systemic blood flow to direct measurements obtained using CMR. We hypothesized that Qp derived by Fick would underestimate the measured CMR values due to the inability to account for systemic to pulmonary arterial collateral flow. In addition, we suspected that oximetry-based calculations of Qs would be inaccurate compared to CMR measurements due to the lack of a true mixed venous saturation.
Methods
Patients
All patients with SCPC who had CMR quantification of systemic to pulmonary arterial collateral flow between July 2008 and June 2012 were reviewed. The subset that underwent combined CMR and catheterization (XMR) under the same general anesthetic were eligible for inclusion in this study. SCPC was defined as any operation that involved complete re-routing of the superior vena caval flow to the pulmonary arteries, including bidirectional Glenn anastomosis, bilateral bidirectional Glenn, or hemi-Fontan procedure (superior cavopulmonary anastomosis incorporating a portion of the right atrium). Patients with residual antegrade pulmonary blood flow and those with interrupted IVC (Kawashima-type procedures) were excluded. Patients with systemic vein to pulmonary vein collaterals visible by MRI, or those with pulmonary vein saturations less than 95% were also excluded. A retrospective review of the medical record was conducted to extract the demographic and clinical variables of interest. The study was approved by the institutional review board.
Cardiac MRI
All patients underwent CMR immediately prior to catheterization. It is our current practice to perform both procedures with the patient mechanically ventilated on room air, minimizing variability in physiologic parameters such as blood pressure and heart rate to the greatest extent possible. A minority (6/30) of patients whose studies were performed during our early experience did receive supplemental oxygen during the CMR portion.
Baseline CMR images were acquired on a 1.5-T MR scanner (Siemens Avanto). Localization of velocity mapping image planes was performed using multiplanar reformatting of a static balanced steady-state free precession axial stack gated to late diastole. Retrospectively gated, through-plane phase contrast cines (PC-MRI) were performed in the pulmonary arteries (PA), pulmonary veins (PV), vena cavae (SVC and IVC), and aorta. Right and left pulmonary artery measurements were obtained individually, and the RPA measurement was performed proximal to the origin of the right upper lobe PA. In patients with very proximal RPA branching, the right upper lobe PA was measured separately. Typical parameters for the phase encoded velocity mapping sequence for a typical R-R interval of 600 msec include a 220×165 mm field of view, 192×144 matrix, 3–4 mm slice thickness, TE of 2.82 msec, bandwidth of 501 Hz/px, TR of 34 msec, 25°flip angle, 14 measured phases, 24 calculated phases, 3 segments and 3 averages. Typical encoding velocities were 150 cm/sec for the aorta, 60 cm/sec for the SVC, IVC, RPA and LPA, and 80 cm/sec for the pulmonary veins. Acquisition times generally ranged from 55 to 110 seconds per velocity map, and 12–18 minutes overall. These parameters were chosen based on clinical experience and unpublished flow phantom experiments which suggest that spatial resolution is more critical than temporal resolution in resolving flow. This is particularly true with the low frequency venous flows which predominate the protocol.
PC-MRI measurements are highly dependent on adequate spatial resolution, which in general requires at least 16 voxels in the contoured lumen to achieve less than 10% error.15 For the pulmonary vein measurements, 23 were measured at a single confluence, and in 37 the upper and lower veins were measured separately. The minimum number of voxels in an individual upper or lower pulmonary vein was on average 30 (range 14 to 89). On average, the total number of voxels used to measure the pulmonary vein flow on one side was 59 (range 19 to 139). Only one vessel acquisition had fewer voxels (14) than 16. There was one patient in this cohort in which we had to measure a separate right upper lobe pulmonary artery branch, in which the number of voxels contoured was 16.
Qp was defined as total PV flow, and Qs was defined as the total caval (SVC + IVC) flow. Systemic to pulmonary arterial collateral flow can be calculated independently as the difference between PV and PA flow or the difference between aortic and caval flow. It is our convention to define it as the average of these two measures. The full details of this methodology have been outlined previously.2
CMR flow data were analyzed by a single study physician who was blinded to the results of the cardiac catheterization. We have previously reported excellent intra- and inter-observer reliability of velocity mapping measurements in a similar and partially overlapping cohort of SCPC patients.2 Given the importance of MRI QP measurements to this study, those data were re-analyzed to provide coefficients of agreement specific to the pulmonary vein measurement. Internal consistency of the PC-MRI technique was also assessed for this study cohort by comparing total venous return to the heart (IVC + PV) to measured aortic outflow.
Cardiac Catheterization
All oximetry data used in Fick calculations were obtained with the patient mechanically ventilated on room air. If interventions were performed during the catheterization, only the pre-intervention hemodynamic data were analyzed. Saturation measurements were abstracted from the chart and used to calculate systemic and pulmonary blood flow according to the conventional Fick equation. The lowest SVC or PA saturation was used as the mixed venous saturation. Most patients had multiple PV saturations measured, which were averaged to produce the final value. In two patients with no direct measurement, the PV saturation of 96% assumed at the time of the procedure was used in our calculations.
Total body oxygen consumption (VO2) was assumed for each patient according to published weight-based formulae (patient age < 3 yrs.) or age-based tables (> 3 yrs.).16, 17 Cath-derived PVR was calculated using the Fick Qp and the recorded pulmonary arterial and pulmonary venous atrial pressures. In the case of unequal left and right transpulmonary gradients, the higher gradient was used. It should be noted though that no patient in this cohort had more than a 1 mm Hg discrepancy between right and left transpulmonary gradients. An MRI-corrected total PVR was also calculated using left and right pulmonary venous flows and their respective transpulmonary gradients, according to the formula:
Where RT = total PVR, RR = right lung PVR, and RL = left lung PVR.
Metabolic Calculations
Although it is common practice to assume a total body oxygen consumption for the purposes of the Fick equation, the actual VO2 for each patient can be calculated using combined MRI and catheterization data. Upper body oxygen consumption can be defined as the difference between oxygen delivery to the SVC distribution and SVC oxygen return:
| [1] |
Where h = 13.6 * hgb (g/dL), and SSA and SSVC are systemic arterial and SVC saturations. QSVC is defined as the SVC flow measured just above the cavopulmonary anastomosis. In the case of bilateral SVCs, equation [1] is calculated separately for each SVC, and the results summed to produce the total upper body VO2. Likewise, the lower body oxygen consumption can be defined as the difference between oxygen delivery and return in the IVC distribution. Because we do not typically measure an IVC saturation, the IVC oxygen return must be calculated indirectly from the conservation of mass around the heart. In patients with SCPC, all oxygen enters the heart via the IVC and PVs (excluding a small amount of coronary sinus flow). This must be equal to oxygen leaving the heart through the aortic valve. IVC oxygen return is therefore the difference between aortic oxygen delivery and pulmonary venous oxygen return. The following can then be extrapolated:
| [2] |
Where SIVC and SPV are IVC and PV saturations and QAo is total aortic outflow measured just above the aortic and/or neo-aortic valves. The total body oxygen consumption is then defined as the sum of equations [1] and [2].
Statistical Considerations
Baseline demographic and clinical variables were summarized using standard descriptive statistics. Normally distributed variables were reported as mean ± standard deviation, and skewed variables were reported as median with range. The concordance correlation coefficient (ρc), as described by Lin et. al, was used to quantify agreement between CMR and catheterization measurements of flow, and to evaluate intra and inter-observer reliability.18 Means were compared using the paired Student t-test for normal variables or the Wilcoxon sign-rank test when variables had significant positive skew. Comparisons of pulmonary vascular resistance were performed after natural logarithmic transformation, which resulted in normally distributed data. All p-values reported are two-sided. Statistical significance was established a priori at p<0.05. Analyses were conducted with STATA, v. 12.0 (StataCorp, College Station, TX).
Results
Thirty patients met inclusion criteria and were included in the analyses. Baseline characteristics are outlined in Table 1. Patients were between 11months and 7 years of age at XMR (median 2.6 years), with a median of 23 months elapsed from the time of SCPC. The predominant anatomic diagnoses were hypoplastic left heart syndrome and unbalanced common atrioventricular canal defect, and the majority of patients (77%) had a systemic right ventricle. Approximately half of subjects had bidirectional Glenn anastomoses, 20% had hemi-Fontan, and 33% had bilateral cavopulmonary connections. One third of patients carried a diagnosis of heterotaxy syndrome.
Table 1.
Baseline Characteristics of Included Patients (n = 30)
| Age at XMR | 2.6y (11m – 7y) |
| BSA (m2) | 0.56 ± 0.12 |
| Sex | |
| Male | 16 (53%) |
| Female | 14 (47%) |
| Age at SCPC | 6.4m (3.6m – 2y) |
| Time from SCPC to XMR | 23m (3m – 6.8y) |
| Type of SCPC | |
| Bidirectional Glenn | 14 (47%) |
| Hemi-Fontan | 6 (20%) |
| Bilateral SCPC | 10 (33%) |
| Systemic Ventricle | |
| Right | 23 (77%) |
| Left | 5 (17%) |
| Both | 2 (6%) |
Data reported as mean ± SD, median (range), or count (% of total).
XMR indicates combined catheterization & cardiac magnetic resonance imaging; BSA, body surface area; SCPC, Superior cavopulmonary connection.
Hemodynamic and physiologic parameters recorded at cardiac catheterization are summarized in Table 2. The population was generally representative of single ventricle patients after SCPC, with mean systemic arterial saturation of 84 ± 5%, SVC pressure of 12.7 ± 2.8 mm Hg, and ventricular end-diastolic pressure of 7.5 ± 2.3 mm Hg.
Table 2.
Catheterization Parameters (n = 30)
| Systemic arterial saturation | 84 ± 5% |
| Mixed venous saturation | 63 ± 8% |
| SVC Pressure (mm Hg) | 12.7 ± 2.8 |
| PA Pressure (mm Hg) | 12.1 ± 2.6 |
| PV Atrial Pressure (mm Hg) | 7.4 ± 2.6 |
| Ventricular EDP (mm Hg) | 7.5 ± 2.3 |
| Hemoglobin (g/dL) | 15.5 (12.7 – 21.1) |
| aVO2 (mL/min/m2) | 160 (130–190) |
Data reported as mean ± SD, median (range), or count (% of total).
SVC indicates superior vena cava; PA, pulmonary artery; PV, pulmonary vein; EDP, end diastolic pressure; aVO2, assumed oxygen consumption.
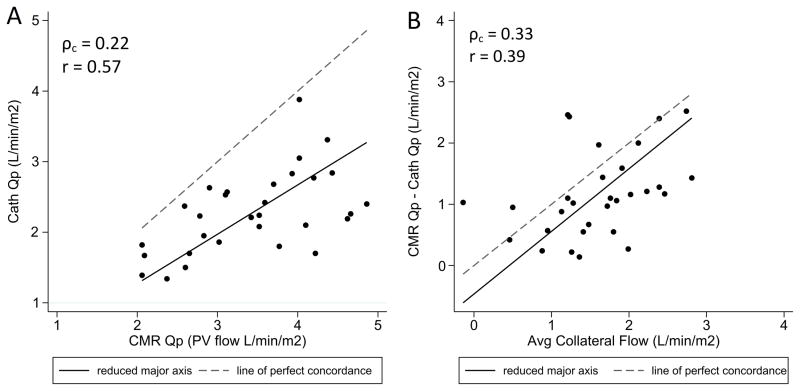
Table 3 compares conventional Fick-based calculations of flow and resistance with those obtained by CMR. There was a notable discrepancy between catheterization and CMR measurements of pulmonary blood flow, with cath Qp underestimating the CMR values by an average of 1.1 L/min/m2 (p < .0001), or 32% of the CMR value. The range of error across individual patients was wide, varying from 0.1 to 2.5 L/min/m2. Although oximetry and CMR did correlate linearly to some extent (Figure 1a), the substantial degree of systematic error led to poor overall agreement (ρc = 0.22). The absolute error in Fick Qp correlated modestly with systemic to pulmonary arterial collateral flow (r=0.39, Figure 1b), but only weak agreement (ρc = 0.33) was observed when comparing cath Qp to CMR pulmonary arterial flow. In this comparison, oximetry consistently overestimated the CMR-measured PA flow with a mean difference of 0.5 L/min/m2 (p = .0008).
Table 3.
Flow and Resistance Calculations - Oximetry vs. CMR
| Oximetry | MRI | p* | |
|---|---|---|---|
| Qp (L/min/m2) | 2.3 ± 0.6 | ||
| QPV | 3.4 ± 0.8 | < .0001 | |
| QPA | 1.8 ± 0.7 | .0002 | |
| Qs (L/min/m2) | 3.8 ± 1.0 | 3.3 ± 0.8 | .009 |
| Qp:Qs | 0.6 (0.4–0.8) | 1.1 (0.5–1.6) | < .0001 |
| PVR (iWU) | 2.0 (0.8–5.6) | 1.1 (0.6–3.7) | < .0001 |
Data reported as mean ± SD for normal variables, and median (range) for skewed variables.
Comparisons made by paired Student t or Wilcoxon sign-rank tests as appropriate.
Qp indicates pulmonary blood flow; Qs, systemic blood flow; QPA, pulmonary artery flow; QPV, pulmonary vein flow; iWU, indexed Wood units.
Figure 1.
Scatter plots comparing (A) pulmonary blood flow measured by oximetry vs. CMR and (B) error in pulmonary blood flow as measured by oximetry vs. CMR collateral flow. Reduced major axis best-fit lines and lines of identity are overlaid. Qp indicates pulmonary blood flow; PV, pulmonary vein; ρc, concordance correlation coefficient; r, Pearson’s linear correlation.
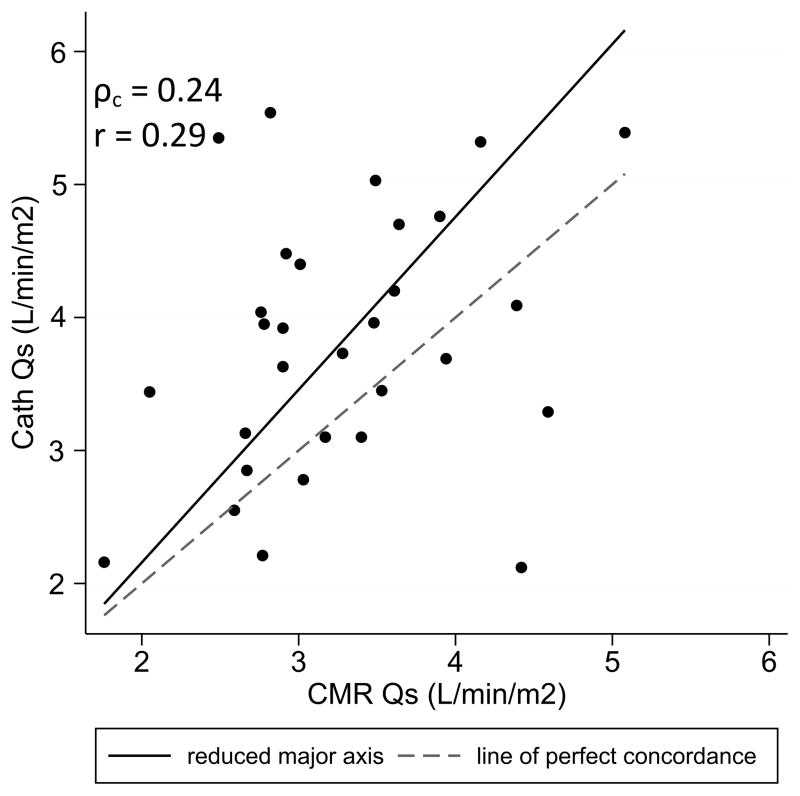
Examining systemic blood flow, the Fick Qs exceeded the CMR-derived total caval flow by an average of 0.5 L/min/m2 (p = .009), or 15% of the CMR value. Weak linear correlation and poor overall agreement (ρc = 0.24) were observed between the two measurement techniques (Figure 2). The catheterization-derived values overestimated the CMR measurements in most cases. To investigate possible sources for the inaccuracy in Qs, the total oxygen consumption for each patient was calculated as described above (Equations 1 and 2) and compared to the assumed values. The median calculated VO2 was found to be 173 mL/min/m2 compared to a median assumed VO2 of 160 mL/min/m2. On average, 39% of the total body metabolism occurred in the SVC distribution and 61% in the IVC distribution.
Figure 2.
Scatter plot comparing systemic blood flow measured by oximetry vs. CMR. Reduced major axis best-fit line and line of identity are overlaid. Qs indicates systemic blood flow; ρc, concordance correlation coefficient; r, Pearson’s linear correlation.
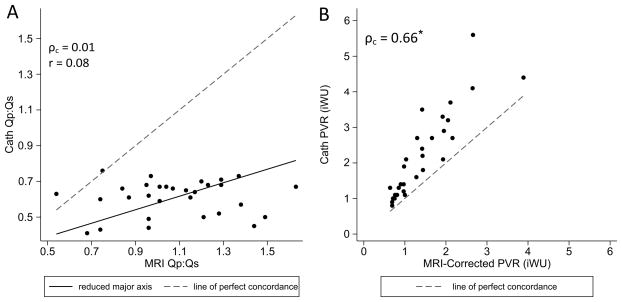
When the Qp:Qs ratio was calculated by oximetry, all values fell between 0.4 and 0.8. Furthermore, there was no correlation between cath Qp:Qs and that measured by CMR (Figure 3a). The Fick-based pulmonary vascular resistance was higher than the CMR-corrected value in all patients but one, with a mean difference of 0.6 iWU (p < .0001). Although there was a reasonable linear correlation between the two measurements of PVR (Figure 3b), the agreement was moderate at best (ρc = 0.66) because of the systematic error.
Figure 3.
Scatter plot comparing (A) Qp:Qs ratio and (B) Pulmonary vascular resistance as measured by oximetry vs. CMR. Reduced major axis best-fit lines and lines of identity are overlaid as indicated. Qp indicates pulmonary blood flow; Qs, systemic blood flow; PVRi, indexed pulmonary vascular resistance; ρc, concordance correlation coefficient; r, Pearson’s linear correlation. *Concordance analysis performed after natural logarithmic transformation.
Internal consistency of the PC-MRI measurements was excellent when comparing total venous return to the heart vs. aortic outflow. The mean difference between these two measures was 0.2 ± 0.4 L/min/m2, with excellent agreement (ρc = 0.87) and no systematic bias.
Discussion
The physiology of the SCPC presents inherent challenges in applying the Fick principle to estimate flow. To better define the clinical utility of oximetry in this population, we describe the nature and degree of its error using phase-contrast CMR as a reference. In this cohort of 30 patients, we found that oximetry inaccurately estimated systemic and pulmonary blood flow, Qp:Qs ratio, and pulmonary vascular resistance.
In these subjects, the Fick-derived Qp always underestimated CMR pulmonary vein flow, with a mean error of 1.1 L/min/m2 (range 0.1 to 2.5 L/min/m2). Although that average discrepancy is significant in and of itself, also important clinically is the wide range of the error across the cohort. For 7 of 30 patients, the Fick Qp was reasonably accurate, differing from the CMR-measured pulmonary blood flow by less than 20%. In 5 others, the error comprised more than 50% of the CMR value. This inconsistency can be explained physiologically by the varying contribution of systemic to pulmonary arterial collaterals to effective pulmonary blood flow. In patients with high absolute collateral burden or relatively higher systemic saturations, the ineffective collateral flow is greater and the Fick estimate of Qp should become more inaccurate. Our data do support this, as the error in cath Qp was found to correlate positively with absolute collateral flow. The substantial degree of scatter in that correlation (and the failure of Fick Qp to agree with CMR pulmonary arterial flow) can then be at least partially attributed to varying systemic saturation.
Given the nature of the error in Fick Qp, it was not surprising that oximetry-derived pulmonary vascular resistance overestimated the CMR-corrected values. There was a clear proportional error, with the discrepancy between the two modalities being most notable in patients with higher PVR. This could become clinically relevant if a patient was excluded from Fontan completion based on an erroneously high cath-derived resistance. The likelihood of that scenario could not be determined from this cohort, however, due to a lack of subjects with markedly elevated PVR.
Somewhat less intuitive than the error in Qp was the observation that Fick-derived Qs typically overestimated the CMR-measured total caval flow. Systemic to pulmonary arterial collaterals cannot be implicated in this case because they do not participate in systemic metabolism. The most likely sources of inaccuracy in calculated Qs would therefore be an erroneously high mixed venous saturation or a systematic overestimate of VO2.
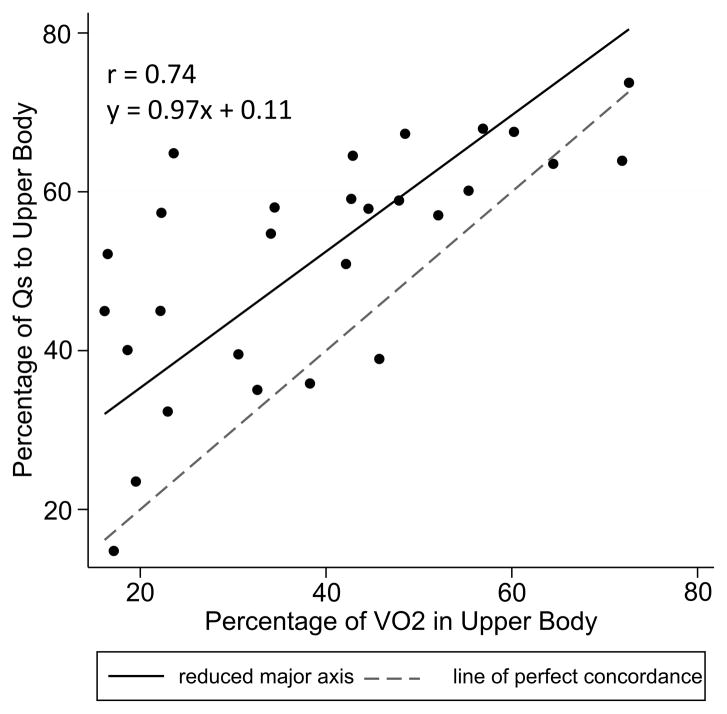
The conventional substitution of the SVC saturation for a true mixed venous sample is physiologically valid only if the SVC and IVC saturations are equal. In the typical biventricular circulation, the caval saturations are unequal, and their relationship may be influenced by factors such as patient age and size, cardiac output, and sedation strategy.17, 18 It is not clear whether the same relationships would be observed after SCPC, although several groups have attempted to model this in a theoretical sense.19, 20 Diller and colleagues hypothesized that the SVC and IVC saturations would be equal in the subset of SCPC patients whose upper body conducted >50% of the total body metabolism. Figure 4 plots SVC flow fraction against upper body metabolic fraction for our cohort. SVC flow increased linearly with metabolic fraction across the entire range, but flow almost always exceeded metabolic demand. Physiologically this demands that the SVC saturation be higher than the IVC saturation, which we suspect contributes to the observed error in the Fick-derived QS.
Figure 4.
Scatter plot comparing percentage of systemic blood flow to upper body vs. percentage of oxygen metabolism in upper body. Reduced major axis best-fit line and line of identity are overlaid. Qs indicates systemic blood flow; r, Pearson’s linear correlation.
Inaccuracies in assumed oxygen consumption may also contribute to error in Fick-derived flow parameters. In these patients, the assumed VO2 underestimated the calculated value by an average of 12%, which should translate into an underestimate of both QP and QS by Fick. Because Fick-derived QS overestimated the CMR value, the error in VO2 cannot be directly implicated. In fact, it is likely that the discrepancy between Fick and CMR-derived QS would be even greater if the precise VO2 were known for each patient. The underestimate of VO2 almost certainly accounts for some of the error in Fick QP. However, because the magnitude of the VO2 error is modest compared with a 33% error in Fick QP, it appears that it is not the primary source of error in the oximetry calculation.
Having described significant (and opposite) errors in both pulmonary and systemic blood flows as calculated by oximetry, it follows that the Fick Qp:Qs ratio was also found to be inaccurate. We observed that all oximetry-derived values of Qp:Qs fell within a fixed range between 0.4 and 0.8, with no correlation to the actual ratio measured by CMR. As an example, one patient whose Fick Qp:Qs ratio would have been deemed “low” at 0.6 had an actual Qp:Qs by CMR of 1.6, owing to extensive systemic to pulmonary arterial collateral flow. One can imagine that the management of a patient with low Qp and high PVR would differ substantially from that of a patient with high (but perhaps low effective) Qp, low PVR, and a volume-loaded systemic ventricle.
It seems, therefore, that the best physiologic characterization of these patients can be obtained by combining flow measurements obtained by CMR with catheterization-derived pressure data. Ultimately, a completely non-invasive strategy might be desirable, but the need to calculate PVR precludes this in many cases. Other groups have investigated using MRI indices alone to predict PVR,21 or employed MRI-guided catheterization as a means to reduce radiation exposure.22 These techniques warrant further study in the single ventricle population.
Limitations
This study is limited by its retrospective design. Most importantly, our cohort may not represent the SCPC population as a whole because all study patients had a clinical indication for XMR. This could produce a relevant selection bias if patients referred for CMR have more systemic to pulmonary arterial collateral flow than the general SCPC population. It is difficult to ascertain whether this is the case, however, because to date there are no prospective studies examining collateral flow in the general SCPC population.
The data on SVC/IVC flow and metabolic ratios must be interpreted understanding that all of these patients received general anesthesia for the procedure. It is therefore possible that our findings do not extend to catheterization procedures performed without general anesthesia. Some patients studied early in the series received supplemental oxygen during the CMR portion of the procedure. Although this has the potential to produce slightly different physiology compared to the catheterization, recent data from our group suggest that oxygen does not significantly alter systemic to pulmonary arterial collateral flow.23
Finally, the limitations of PC-MRI for flow assessment must be recognized. The target spatial resolution was achieved in 98% of acquisitions in this study, and internal consistency assessments showed excellent reproducibility of the velocity mapping data. In similar SCPC patients, the coefficients of intra- and inter-observer reliability for pulmonary vein flow measurements were high at 0.98 and 0.86, respectively. Nevertheless, errors may occur, and anatomic features such as turbulent flow around anastomotic sites may exacerbate these errors in certain patients. There is no clear reason, however, to suspect a systematic bias in PC-MRI measurements when compared to Fick.
Conclusions
In patients with SCPC presenting for XMR, oximetry consistently underestimates Qp and overestimates PVR, in part due to the presence of systemic to pulmonary arterial collateral flow. In contrast, Fick calculations of Qs overestimate CMR-measured Qs when conventional assumptions are applied. This may occur because the upper body consistently receives a disproportionate amount of blood flow relative to its metabolic demand, making the SVC saturation a systematic overestimate of the mixed venous saturation. Finally, the Qp:Qs ratio generated by oximetry bears no relationship to the patient’s actual flow balance as measured by CMR.
We conclude that catheterization-derived flow measurements are generally unreliable in SCPC patients. Addition of CMR can significantly enhance the understanding of an individual patient’s physiology. When CMR is contraindicated or unavailable, oximetry data should be interpreted with caution, understanding the nature and causes of its inaccuracy. Further study of combined cardiac catheterization and CMR techniques is warranted in the single ventricle population.
Clinical Perspective.
This research compares traditional oximetry-based methods for calculating pulmonary and systemic blood flows with phase-contrast MRI measurements, in the population of single ventricle patients palliated with superior cavopulmonary connection. We found that oximetry significantly underestimates pulmonary blood flow, especially in patients with a greater burden of systemic to pulmonary arterial collaterals. This, in turn, leads to inaccurate calculation of pulmonary vascular resistance. Oximetry also produces a systematic overestimate of systemic blood flow and a Qp:Qs ratio that does not correlate with that measured by MRI. Clinically, the implication is that oximetry, while still widely employed, may yield information that inaccurately describes the true physiology of this patient population. Supplementing traditional hemodynamic catheterization with flow measurements by MRI will likely provide a better assessment of the superior cavopulmonary circuit, both for diagnosing suspected physiologic derangements and assessing patient candidacy for Fontan completion.
Acknowledgments
Sources of Funding
Dr. Kevin Whitehead was supported in part by NIH K23 Grant HL089647 from the National Heart, Lung, and Blood Institute.
Footnotes
Disclosures
None.
References
- 1.Valverde I, Nordmeyer S, Uribe S, Greil G, Berger F, Kuehne T, Beerbaum P. Systemic-to-pulmonary collateral flow in patients with palliated univentricular heart physiology: Measurement using cardiovascular magnetic resonance 4d velocity acquisition. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2012;14:25. doi: 10.1186/1532-429X-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitehead KK, Gillespie MJ, Harris MA, Fogel MA, Rome JJ. Noninvasive quantification of systemic-to-pulmonary collateral flow: A major source of inefficiency in patients with superior cavopulmonary connections. Circ Cardiovasc Imaging. 2009;2:405–411. doi: 10.1161/CIRCIMAGING.108.832113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grosse-Wortmann L, Al-Otay A, Yoo SJ. Aortopulmonary collaterals after bidirectional cavopulmonary connection or fontan completion: Quantification with mri. Circ Cardiovasc Imaging. 2009;2:219–225. doi: 10.1161/CIRCIMAGING.108.834192. [DOI] [PubMed] [Google Scholar]
- 4.Prakash A, Rathod RH, Powell AJ, McElhinney DB, Banka P, Geva T. Relation of systemic-to-pulmonary artery collateral flow in single ventricle physiology to palliative stage and clinical status. Am J Cardiol. 2012;109:1038–1045. doi: 10.1016/j.amjcard.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glatz AC, Rome JJ, Small AJ, Gillespie MJ, Dori Y, Harris MA, Keller MS, Fogel MA, Whitehead KK. Systemic-to-pulmonary collateral flow, as measured by cardiac magnetic resonance imaging, is associated with acute post-fontan clinical outcomes. Circ Cardiovasc Imaging. 2012;5:218–225. doi: 10.1161/CIRCIMAGING.111.966986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dori Y, Glatz AC, Hanna BD, Gillespie MJ, Harris MA, Keller MS, Fogel MA, Rome JJ, Whitehead KK. Acute effects of embolizing systemic-to-pulmonary arterial collaterals on blood flow in patients with superior cavopulmonary connections: A pilot study. Circ Cardiovasc Interv. 2013;6:101–106. doi: 10.1161/CIRCINTERVENTIONS.112.972265. [DOI] [PubMed] [Google Scholar]
- 7.Odenwald T, Quail MA, Giardini A, Khambadkone S, Hughes M, Tann O, Hsia TY, Muthurangu V, Taylor AM. Systemic to pulmonary collateral blood flow influences early outcomes following the total cavopulmonary connection. Heart. 2012;98:934–940. doi: 10.1136/heartjnl-2011-301599. [DOI] [PubMed] [Google Scholar]
- 8.Grosse-Wortmann L, Drolet C, Dragulescu A, Kotani Y, Chaturvedi R, Lee KJ, Mertens L, Taylor K, La Rotta G, van Arsdell G, Redington A, Yoo SJ. Aortopulmonary collateral flow volume affects early postoperative outcome after fontan completion: A multimodality study. J Thorac Cardiovasc Surg. 2012;144:1329–1336. doi: 10.1016/j.jtcvs.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 9.Goo HW, Al-Otay A, Grosse-Wortmann L, Wu S, Macgowan CK, Yoo SJ. Phase-contrast magnetic resonance quantification of normal pulmonary venous return. Journal of magnetic resonance imaging : JMRI. 2009;29:588–594. doi: 10.1002/jmri.21691. [DOI] [PubMed] [Google Scholar]
- 10.Hundley WG, Li HF, Hillis LD, Meshack BM, Lange RA, Willard JE, Landau C, Peshock RM. Quantitation of cardiac output with velocity-encoded, phase-difference magnetic resonance imaging. Am J Cardiol. 1995;75:1250–1255. doi: 10.1016/s0002-9149(99)80772-3. [DOI] [PubMed] [Google Scholar]
- 11.Hundley WG, Li HF, Lange RA, Pfeifer DP, Meshack BM, Willard JE, Landau C, Willett D, Hillis LD, Peshock RM. Assessment of left-to-right intracardiac shunting by velocity-encoded, phase-difference magnetic resonance imaging. A comparison with oximetric and indicator dilution techniques. Circulation. 1995;91:2955–2960. doi: 10.1161/01.cir.91.12.2955. [DOI] [PubMed] [Google Scholar]
- 12.Powell AJ, Maier SE, Chung T, Geva T. Phase-velocity cine magnetic resonance imaging measurement of pulsatile blood flow in children and young adults: In vitro and in vivo validation. Pediatr Cardiol. 2000;21:104–110. doi: 10.1007/s002469910014. [DOI] [PubMed] [Google Scholar]
- 13.Bellsham-Revell HR, Tibby SM, Bell AJ, Witter T, Simpson J, Beerbaum P, Anderson D, Austin CB, Greil GF, Razavi R. Serial magnetic resonance imaging in hypoplastic left heart syndrome gives valuable insight into ventricular and vascular adaptation. J Am Coll Cardiol. 2013;61:561–570. doi: 10.1016/j.jacc.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muthurangu V, Taylor A, Andriantsimiavona R, Hegde S, Miquel ME, Tulloh R, Baker E, Hill DL, Razavi RS. Novel method of quantifying pulmonary vascular resistance by use of simultaneous invasive pressure monitoring and phase-contrast magnetic resonance flow. Circulation. 2004;110:826–834. doi: 10.1161/01.CIR.0000138741.72946.84. [DOI] [PubMed] [Google Scholar]
- 15.Tang C, Blatter DD, Parker DL. Accuracy of phase-contrast flow measurements in the presence of partial-volume effects. Journal of magnetic resonance imaging : JMRI. 1993;3:377–385. doi: 10.1002/jmri.1880030213. [DOI] [PubMed] [Google Scholar]
- 16.Kappagoda CT, Greenwood P, Macartney FJ, Linden RJ. Oxygen consumption in children with congenital diseases of the heart. Clin Sci. 1973;45:107–114. doi: 10.1042/cs0450107. [DOI] [PubMed] [Google Scholar]
- 17.LaFarge CG, Miettinen OS. The estimation of oxygen consumption. Cardiovasc Res. 1970;4:23–30. doi: 10.1093/cvr/4.1.23. [DOI] [PubMed] [Google Scholar]
- 18.Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–268. [PubMed] [Google Scholar]
- 19.Diller GP, Uebing A, Willson K, Davies LC, Dimopoulos K, Thorne SA, Gatzoulis MA, Francis DP. Analytical identification of ideal pulmonary-systemic flow balance in patients with bidirectional cavopulmonary shunt and univentricular circulation: Oxygen delivery or tissue oxygenation? Circulation. 2006;114:1243–1250. doi: 10.1161/CIRCULATIONAHA.106.616870. [DOI] [PubMed] [Google Scholar]
- 20.Santamore WP, Barnea O, Riordan CJ, Ross MP, Austin EH. Theoretical optimization of pulmonary-to-systemic flow ratio after a bidirectional cavopulmonary anastomosis. The American journal of physiology. 1998;274:H694–700. doi: 10.1152/ajpheart.1998.274.2.H694. [DOI] [PubMed] [Google Scholar]
- 21.Bell A, Beerbaum P, Greil G, Hegde S, Toschke AM, Schaeffter T, Razavi R. Noninvasive assessment of pulmonary artery flow and resistance by cardiac magnetic resonance in congenital heart diseases with unrestricted left-to-right shunt. JACC Cardiovasc Imaging. 2009;2:1285–1291. doi: 10.1016/j.jcmg.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Razavi R, Hill DL, Keevil SF, Miquel ME, Muthurangu V, Hegde S, Rhode K, Barnett M, van Vaals J, Hawkes DJ, Baker E. Cardiac catheterisation guided by mri in children and adults with congenital heart disease. Lancet. 2003;362:1877–1882. doi: 10.1016/S0140-6736(03)14956-2. [DOI] [PubMed] [Google Scholar]
- 23.Whitehead KK, Dori Y, Harris M, Glatz A, Keller MS, Rome J, Fogel M. Effect of hyperoxia and hypercarbia on systemic to pulmonary collateral flow in patients with cavopulmonary connections. Journal of the American College of Cardiology. 2012;59:E750–E750. [Google Scholar]