Abstract
Background
Deep brain stimulation (DBS) of the centromedian-parafascicular (CM-Pf) thalamic nuclei has been considered an option for treating Tourette syndrome (TS). Using a large animal DBS model, this study was designed to explore the network effects of CM-Pf DBS.
Methods
The combination of DBS and functional MRI (fMRI) is a powerful means of tracing brain circuitry and testing the modulatory effects of electrical stimulation on a neuronal network in vivo. Using a with-in subjects design, we tested the proportional effects of CM and Pf DBS by manipulating current spread and varying stimulation contacts in healthy pigs (n=5).
Results
Our results suggests that CM-Pf DBS has an inhibitory modulating effect in areas that have been suggested as contributing to impaired sensory-motor and emotional processing. The results also help to define the differential neural circuitry effects of the CM and Pf with evidence of prominent sensorimotor/associative effects for CM DBS and prominent limbic/associative effects for Pf DBS.
Conclusions
Our results support the notion that stimulation of deep brain structures, such as the CM-Pf, modulates multiple networks with cortical effects. The networks affected by CM-Pf stimulation in this study reinforce the conceptualization of TS as a condition with psychiatric and motor symptoms and of CM-Pf DBS as a potentially effective tool for treating both types of symptoms.
Keywords: Deep brain stimulation (DBS), Tourette syndrome, centromedian, parafascicular, functional magnetic resonance imaging (fMRI), swine model, neural circuitry
INTRODUCTION
Increasingly, deep brain stimulation (DBS) is being considered a viable option for patients with medically refractory Tourette syndrome (TS) (1-9). TS is a complex neuropsychiatric disorder characterized by abrupt, stereotypic movements and vocalizations known as motor and vocal tics. The onset is usually in childhood with aggravation of symptoms in early adolescence (10). It is distinguished from other movement disorders by patient reports of pre-tic urges or compulsion to execute a stereotypic action (11) and by its frequent association with obsessive compulsive disorder (OCD), attention deficit hyperactivity disorder, anxiety, impulse control deficit, and other psychiatric conditions (12-14). This combination of motor and behavioral symptoms suggests that the pathophysiology of TS may be mediated by dysregulation of the corticostriatal-thalamocortical circuitry involving associative and limbic areas of the basal ganglia (BG) (15)[g484][g3]The therapeutic action of DBS and optimum targets for TS have not been established, but recent reports favor the globus pallidus internus and centromedian-parafascicular (CM-Pf) nuclei complex of the thalamus (10,16).
Studies using a variety of experimental paradigms suggest that hyperactive dopaminergic innervation and impaired function of BG circuitry play a major role in TS neuropathology (17-25). Functional magnetic resonance imaging (fMRI) studies of TS have implicated paralimbic and sensory-association areas in tic generation (25) and have suggested that distinct regions of cortico-BG networks contribute to TS (24,26). Diffusion tensor imaging in TS reveals abnormalities not only in the motor pathway, but also in associative and limbic pathway fibers (27). It has been shown, for example, that the BG of patients with TS has markedly reduced inhibitory interneuron density (28,29) and that these inhibitory interneurons are regulated by CM-Pf projections (30-32). Reports of tic reduction following CM-Pf DBS from our group (33) and improvement in behavioral symptoms (2,34-38) support the role of the CM-Pf in TS.
The CM-Pf nuclei complex is located in the posterior part of the intralaminar thalamus and is part of the nonspecific thalamocortical projection system and the internal circuit of the BG complex (39). It has been reported to have specific connections with the caudate (40), putamen, and globus pallidus (41), the nucleus accumbens (42), amygdala (43), hippocampus (HP) (44), anterior cingulate cortex, and insular cortex (IC) (45). Topographical analysis of the CM-Pf in the non-human primate shows differential connections with CM innervating the sensorimotor striatal area (32,46,47) and Pf innervating the associative-limbic striatal areas (32,48,49).
Functionally, the CM-Pf is thought to be involved in cognitive, sensory and motor processes, including sensorimotor coordination (30,50-52), arousal (53), pain processing (54,55), and sexual processing (56). CM-Pf lesions appear to cause complex attention deficits, possibly due to the role of these structures in directing attention to motivationally relevant stimuli (57).
Although MRI studies have helped identify the anatomical and structural connectivity between CM and Pf and subcortical-cortical networks (58), their individual functional effects have yet to be identified. The importance of precise targeting in DBS and the nature of the motor and behavioral symptom complex in TS warrant examination of the potential BG and cortical network effects of CM and Pf DBS.
To test the hypothesis that CM DBS and Pf DBS have differential effects on cortical and subcortical networks, we applied our previously described in vivo method of examining DBS-induced fMRI blood oxygenation-level dependent (BOLD) changes in a large animal (pig) model of DBS (59,60). We tested the proportional effects of CM and Pf DBS by manipulating current spread and varying stimulation contacts in a within-subjects design in healthy pigs implanted with DBS. Our results showed functional network differences between the two targets with CM DBS inducing prominent sensorimotor-associative BOLD effects, and Pf DBS inducing prominent associative-limbic effects. The implication of these results for CM-Pf DBS in TS patients and for the symptomotology of TS are considered within the cautionary constraints inherent in relating findings from normal animals to human pathologic conditions.
METHODS
Subjects
All study procedures were performed in accordance with the National Institutes of Health Guidelines for Animal Research and approved by Mayo Clinic Institutional Animal Care and Use Committee. The subject group consisted of 5 normal healthy domestic male pigs (30±3kg). Sedation was maintained with 1.5-3% isoflurane during surgery and with a mixture of 1.5-1.75% isoflurane and 0.5% NO2 during the fMRI experiment. Vital signs were continuously monitored throughout the procedures.
DBS Electrode Implantation
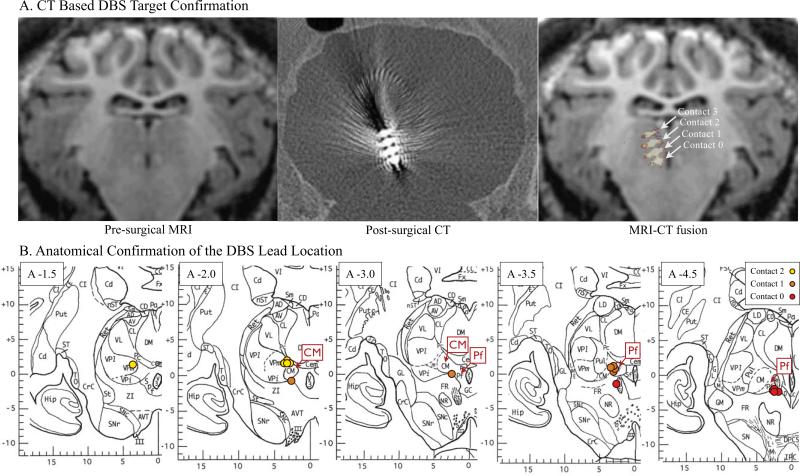
An MR image-guided Leksell stereotactic targeting system (Elekta Inc.,Stockholm, Sweden), specifically modified for large animals, was used for DBS electrode targeting and implantation (59). Imaging was conducted by a 3 Tesla MR scanner with a custom radiofrequency coil (59). Subjects were implanted with a quadripolar (contacts labeled 0,1,2, and 3) DBS electrode (Model 3389, Medtronic,Inc.). The electrode contacts were positioned so that Pf was between contacts 0 & 1 and CM was between contacts 1 & 2 based on the pig atlas (61,62) and anatomical landmarks (e.g., fasciculus retroflexus) in the MR image (Figure 1 and see Supplement for coordinate details). The location of the electrode was confirmed by a post-surgical CT scan (Image resolution 0.3×0.3×0.3mm), which was co-registered to the pre-MRI MPRAGE scan (FSL,FM-RIB Analysis group) (59,63-65).
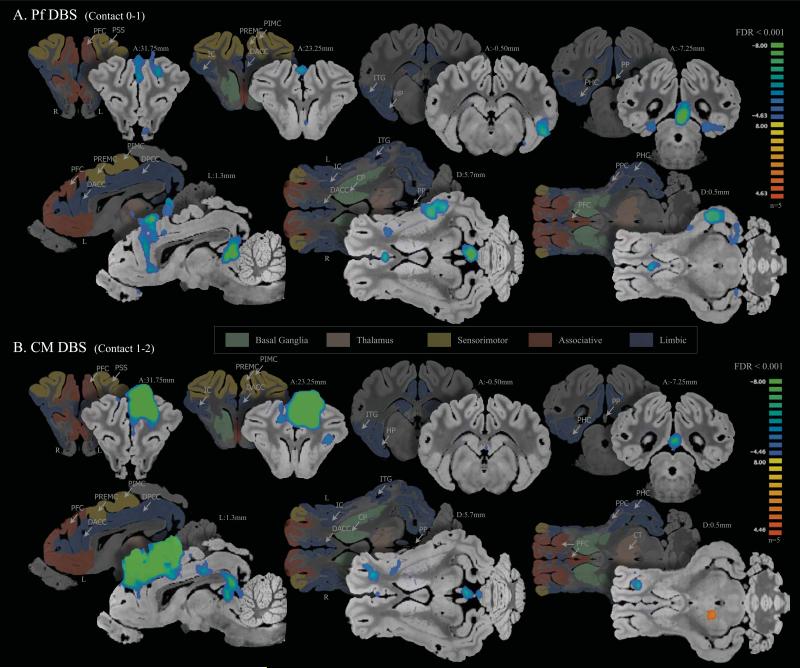
Figure 1.
CT scan of DBS electrode target localization in the pig brain and anatomical confirmation on the pig brain atlas. A) Fusion images of preoperative MRI and postoperative CT showing the electrode contacts 0,1,2 and 3; B) For each subject, the actual DBS lead contacts were marked on the coronal plane of the pig brain atlas confirmed by the MRI and CT fusion (61), reprinted with permission. Abbreviations:[g3] CM, centromedian thalamic nucleus; CT, Computed tomography; DBS, deep brain stimulation; Pf, parafascicular thalamic nucleus.
fMRI and DBS
The fMRI experiment for each subject consisted of eight conditions in which the stimulation frequency and amplitude were varied for each set of contacts. Pf (contacts 0 and 1) and CM (contacts 1 and 2) were independently stimulated at a high frequency (130Hz) at 3V and 5V using a biphasic pulse width of 250μs. Each set of contacts (0-1 and 1-2) was then stimulated at a lower frequency (60Hz) at 3V and 5V in a sequential (non-randomized) order. There was a 10min rest interval between conditions.[g3](see Supplement for MRI sequence details).
Data Processing and Analysis
Followed by standard sequence of post-processing steps, the normalized datasets were analyzed by linear regression analysis using general linear model and multi-subject analysis. To correct for multiple comparisons and exclude false positive and negative voxels, only the significance level less than the False Discovery Rate (FDR<0.001) were considered. (see Supplement for detailed post-processing steps).
The total voxel size of significant change was measured (mm3), and discrete clusters were determined by anatomically defined brain structures in the 3D pig brain atlas. The measure of interest was the maximum or minimum event-related BOLD response (e.g, the minimum BOLD signal/5 volume average of baseline BOLD signal). This BOLD signal intensity change [%], representing minimum or maximum response intensities within each cluster, was labeled “BOLD % change” (mean±SEM).
RESULTS
As can be seen in Figure 2, there was a decrease in signal intensity (negative BOLD response) as measured by BOLD % change during DBS stimulation and an increase in BOLD % change after stimulation was terminated. The negative BOLD response also increased as a function of increased stimulation amplitude. The entire BOLD signal time series can be found in Supplement Figure S1.
Figure 2.
BOLD signal intensity change [%] response by DBS stimulation. The representative BOLD signal intensity change (y axis) for a single subject, an average of the five stimulation blocks in each volume of interest, is displayed here. Pf DBS (A) and CM DBS (B) each evoked a negative BOLD response in the sensorimotor, limbic, and associative brain areas. The negative BOLD signal intensity change increased as stimulation amplitude was increased from 3V to 5V (blue: 3V, red: 5V). The negative BOLD signal intensity change maximized at the termination of the stimulation. Abbreviations: BOLD, blood oxygenation-level dependent; CM, centromedian thalamic nucleus; DBS, deep brain stimulation; ITG, inferior temporal gyrus; PFC, prefrontal cortex; PIMC, primary motor cortex; Pf, parafascicular thalamic nucleus
Both CM DBS and Pf DBS evoked a significant decrease in the BOLD % change in the following brain regions: 1) the BG circuit, including caudate and putamen (CP); 2) the sensorimotor circuit, including primary motor cortex (PIMC), premotor cortex (PREMC) and primary somatosensory cortex (PSS); 3) the associative circuit, including prefrontal cortex (PFC); and 4) the limbic circuit, including dorsoanterior cingulate cortex (DACC), dorsoposterior cingulate cortex (DPCC), hippocampus (HP), parahippocampus (PHC), insular cortex (IC), amygdala, inferior temporal gyrus (ITG), and prepyriform cortex (PPC). Additional areas affected by both targets sites were the superior colliculus (SC), pineal gland and perirhinal cortex (PP) (FDR<0.001) (See Table S1). There were condition-specific results depending on contact site (Pf contacts vs CM contacts), stimulation frequency (130Hz vs 60Hz), and applied voltage (3V vs 5V).
BOLD effects as a function of contact difference (Pf vs CM at 3V 130Hz)
With stimulation at 130Hz and 3V (Figure 3) Pf DBS evoked negative BOLD in limbic areas, including the temporal lobe network (ITG, HP, PHC, PPC) and cingulate cortex (DACC, DPCC), as well as the associative area (PFC), with minimal effects in the sensorimotor network. CM DBS evoked negative BOLD predominantly in sensorimotor cortex (PIMC, PREMC and PSS) and the associative area (PFC). CM DBS induced a small area of negative BOLD in the temporal lobe, and showed negative BOLD in the cingulate cortex (DACC, DPCC), part of the limbic network. CM DBS, but not Pf DBS evoked a BOLD response in the caudate and putamen (CP) (Table S1).
Figure 3.
130Hz CM vs Pf DBS (130Hz 3V 0.25ms) (FDR<0.001). A) Pf DBS evoked negative BOLD in limbic areas, including the temporal lobe network (ITG, HP, PHC, PPC) and cingulate cortex (DACC, DPCC) as well as the associative area (PFC), with minimal effects in the sensorimotor network; B) CM DBS evoked negative BOLD predominantly in the sensorimotor areas (PIMC, PREMC and PSS) and the associative area (PFC). Abbreviations:[g3]CM, centromedian thalamic nucleus; CP, caudate and putamen; CT, central thalamic nucleus; DACC, dorsal anterior cingulate cortex; DPCC, dorsal posterior cingulate cortex; HP, hippocampus; IC, insular cortex; ITG, inferior temporal gyrus; PFC, prefrontal cortex; PHC, parahippocampal gyrus; PIMC, primary motor cortex; Pf, parafascicular thalamic nucleus; PP, pineal gland and perirhinal cortex; PPC, prepyriform cortex; PREMC, premotor cortex; PSS, primary sensory cortex.
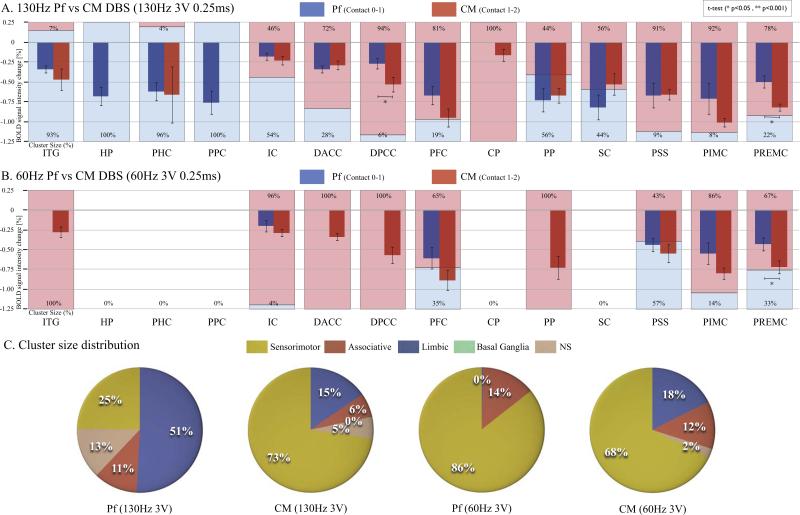
Figure 4A compares the negative peak BOLD % change and the cluster size of each brain area affected by Pf DBS and CM DBS at 3V 130Hz. There was a significantly greater BOLD % change for CM compared to Pf DBS in DPCC, and PREMC (p<0.05,t-test). When comparing cluster size in % ratio, Pf DBS induced negative BOLD predominantly in limbic areas (ITG, HP, PHC, PPC) (>70% cluster size difference in Pf than in CM), and CM DBS induced negative BOLD predominantly in sensorimotor areas (PSS, PIMC, PREMC) and the associative area (PFC) (>70% cluster size difference in CM than in Pf).
Figure 4.
Comparison of BOLD signal intensity change (bar graph) and cluster size % (shaded area) for Pf DBS and CM DBS (blue: PF; red: CM). A) At 3V, 130Hz Pf DBS induced functional inhibition predominantly in limbic areas (ITG, HP, PHC, PPC) (>70% cluster size difference in Pf than in CM), and CM DBS-induced functional inhibition predominantly in sensorimotor areas (PSS, PIMC, PREMC), limbic areas (DACC, DPCC), and the associative area (PFC) (>70% cluster size difference in CM than in Pf). There was a significantly greater BOLD signal intensity change for CM compared to Pf DBS in DPCC, and PREMC (p<0.05, t-test). B). At 3V, 60Hz DBS, cluster size differences were less pronounced in sensorimotor areas (PSS, PIMC, PREMC) than at 3V, 130Hz. Only PIMC showed a >70% cluster size difference in CM than in Pf DBS, implying that the sensorimotor effects of Pf DBS were stronger at 60Hz than at 130Hz. The only significant difference between Pf and CM DBS as measured by BOLD signal intensity change was in the PREMC (p<0.05, t-test); C). Cluster size distribution comparing CM to Pf DBS at 130Hz and 60 Hz. At 3V 130 Hz the cluster size distribution was predominantly limbic (51%) for Pf DBS and sensorimotor for CM DBS (73%). At 3V 60Hz, the cluster size distribution for both Pf and CM DBS was mainly sensorimotor (86% and 68%, respectively). Abbreviations: BOLD, blood oxygenation-level dependent; CM, centromedian thalamic nucleus; CP, caudate and putamen; DACC, dorsal anterior cingulate cortex; DPCC, dorsal posterior cingulate cortex; HP, hippocampus; IC, insular cortex; ITG, inferior temporal gyrus; NS, non-specific areas; PFC, prefrontal cortex; PHC, parahippocampal gyrus; PIMC, primary motor cortex; Pf, parafascicular thalamic nucleus; PP, pineal gland and perirhinal cortex; PPC, prepyriform cortex; PREMC, premotor cortex; PSS, primary sensory cortex; SC, superior colliculus
Figure 4C shows a distribution diagram of each cluster size % ratio in relation to known brain circuits, specifically the BG (CP), sensorimotor (PSS, PIMC, PREMC), associative (PFC), and limbic circuits (ITG, HP, PHC, PPC, IC, DACC, DPCC), and other non-specific (NS) areas (e.g., PP,SC). The distribution of areas affected by Pf DBS was predominantly limbic (51%), followed by sensorimotor (25%), associative (11%), and NS areas (13%). The distribution of areas affected by CM DBS was predominantly sensorimotor (73%), followed by limbic (15%), associative (6%) and NS (5%).
BOLD effects as a function of stimulation frequency (130Hz vs 60Hz)
With stimulation at 60Hz and 3V both Pf and CM DBS generated a negative BOLD response in sensorimotor areas (PSS, PIMC, PREMC) and the associative area (PFC) (see Figure S2). As seen in Figure 4B the only significant difference between Pf and CM DBS as measured by BOLD % change was in the PREMC (p<0.05,t-test). In addition, at 60 Hz there was a less pronounced cluster size difference in sensorimotor areas (PSS, PIMC, PREMC) between Pf and CM than there was at 130 Hz. Only PIMC showed >70% cluster size difference when comparing CM to Pf DBS, implying that the sensorimotor effects of Pf DBS were stronger at 60Hz than at 130Hz. The limbic effects for Pf DBS that were present at 130Hz disappeared at 60Hz, while at 60Hz CM DBS continued to evoke negative BOLD in the cingulate cortex, ITG, and IC. As seen in Figure 4C, the large distribution of limbic areas (51%) with Pf DBS at 130Hz decreased to 0% at 60Hz, while with CM DBS the cluster distribution stayed within the 5% range of variance across frequencies in sensorimotor, associative, limbic, and other NS areas.
BOLD effects as a function of stimulation amplitude (3V vs 5V)
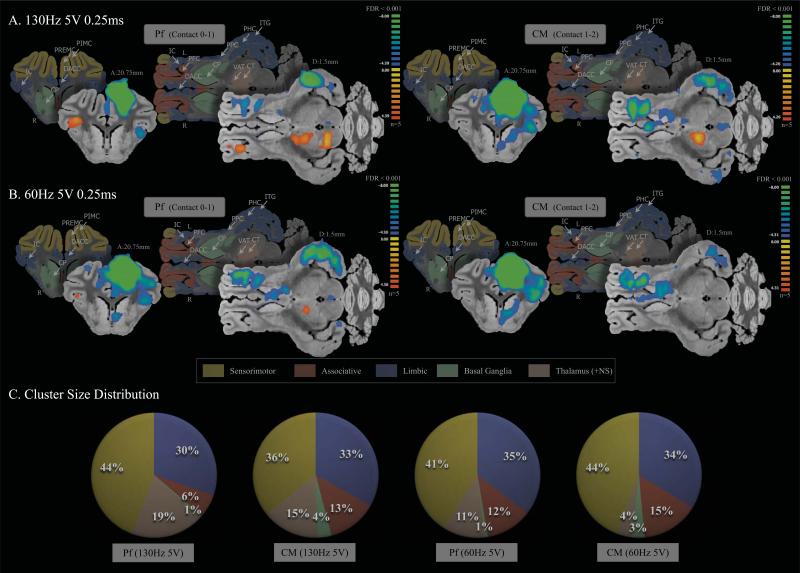
The effects of 5V of Pf and CM DBS across frequencies are depicted in Figure 5A and B. There was a similar negative BOLD response in the sensorimotor, associative, and limbic areas at 130Hz and 60Hz for both Pf and CM DBS. The cluster distribution in Figure 5C shows that regardless of stimulation frequency (130Hz vs 60Hz) or DBS contact (Pf vs CM), the distribution remained stable (<5% variance). Especially, the cluster size distribution remained stable for CM DBS (<5% variance) regardless of frequency (130Hz vs 60Hz) or amplitude (3V vs 5V) (See Figure 4C, and Figure 5C). Pf DBS was sensitive to both frequency and amplitude with major regions of BOLD signal response in limbic areas (51% of cluster size distribution) at 130Hz 3V DBS, and major regions of BOLD signal response in sensorimotor areas (86% of cluster size distribution) at 60Hz 3V DBS.
Figure 5.
fMRI BOLD effects with high amplitude (5V) stimulation (FDR<0.001). Both high frequency (A) and low frequency (B) stimulation evoked a similar negative BOLD response in the sensorimotor, associative, and limbic areas for Pf and CM DBS (C). The cluster distribution shows that regardless of stimulation frequency or DBS contact, the distribution remained stable (<5% variance). The average cluster size distribution percentage in 5V was: sensorimotor 41.25% ± 3.78; limbic 33% ± 2.16; associative 11.5% ± 3.87; BG 2.25% ± 1.5; and NS 12.25% ± 6.4. Abbreviations: NS, non-specific areas; CM, centromedian thalamic nucleus; CP, caudate and putamen; CT, central thalamic nucleus; DACC, dorsal anterior cingulate cortex; DPCC, dorsal posterior cingulate cortex; HP, hippocampus; IC, insular cortex; ITG, inferior temporal gyrus; PFC, prefrontal cortex; PHC, parahippocampal gyrus; PIMC, primary motor cortex; Pf, parafascicular thalamic nucleus; PP, pineal gland and perirhinal cortex; PPC, prepyriform cortex; PREMC, premotor cortex; PSS, primary sensory cortex; VAT, ventroanterior thalamic nucleus.
The stimulation amplitude effect on BOLD % change and cluster size % ratio are summarized in Supplement Figure S3. Across conditions, the associative area (PFC) and sensorimotor areas (PSS, PIMC, PREMC) showed a significant increase of negative BOLD % change (p<0.05,t-test) by increased amplitude (3V to 5V). Pf DBS evoked a significant BOLD % change in limbic areas (ITG, IC, DPCC at 130Hz and IC at 60Hz) as the stimulation amplitude increased, especially for both 130Hz and 60Hz the associative area (PFC) was most sensitive to stimulation amplitude showing significance of p<0.001 in BOLD % change. For CM DBS at 130Hz, the motor areas (PIMC and PREMC) BOLD % changes were most sensitive to stimulation amplitude increase (p<0.001,t-test). In most of the cases the cluster size increased as the stimulation amplitude increased, except for DACC and SC with 130Hz Pf DBS; PSS, PIMC, PREMC with 130Hz CM DBS; and PIMC with 60Hz CM DBS; which showed less then 10% cluster size difference. Of note, a negative BOLD response was present in the BG (CP) at all 5V conditions, and a positive BOLD effect was present in the contralateral hemisphere in the ventroanterior thalamic nucleus, central thalamic nucleus, cerebellar peduncle, ITG, IC, PFC, PSS and PREMC at all 5V conditions (Table S1).
DISCUSSION
The goal of this study was to test the hypothesis that CM DBS and Pf DBS have differential global functional network effects. Given the heterogeneous nature of the symptoms associated with TS and the currently known circuitry of CM and Pf, it was hoped that the results would add insight into the individual and combined contributions of CM and Pf as DBS targets.
The results of this study showed that both CM and Pf DBS significantly decreased BOLD response (66,67) in sensorimotor, associative, and limbic circuits. Differential effects appear dependent upon stimulation frequency and amplitude. Pf DBS appears more sensitive to frequency and amplitude than CM DBS. Under lower amplitude-high frequency stimulation CM DBS appears to have greater effects on sensorimotor and associative than on limbic circuits, and Pf DBS appears to have greater effects on limbic and associative than on sensoritmotor circuits. Regardless of frequency, higher amplitude stimulation of CM and of Pf resulted in a similar pattern of negative BOLD for both targets, affecting the BG, sensorimotor, associative, and limbic circuits.
These results are consistent with current understanding of the anatomical projections of CM and Pf. The CM-Pf is known to have dense topographically organized thalamostriatal projections and is thought to alter striatal activity through modulation of the thalamostriatal system. Early studies of nonhuman primates reveal that CM and Pf projections are distinct, complementary, and directed mainly to the striatum (68-70). Recently, Ding et al. (71) suggested that the corticostriatal and thalamostriatal projection systems encode information in temporally distinct ways, with the thalamostriatal system conveying precisely timed episodic signals, which may modulate corticostriatal activity. That is, CM-Pf inputs to striatal interneurons may have an important modulatory influence on medium spiny neurons, providing them with a feed-forward inhibition. The CM-Pf complex is also reported to respond to behaviorally significant multimodal stimuli, and this response is correlated with striatal activity (57,72).
CM and Pf provide distinct inputs to different parts of striatum. Using an anterograde tracer, it has been reported that the CM collects in bands and synapses on neurons of the post-commissural putamen, while Pf sends dense input to the caudate and rostral putamen (32). It has been suggested that the CM receives input from the motor cortex, reticular formation, cerebellum, vestibular nuclei, superior colliculus, pretectum, and the locus ceruleus (55) and sends projections to the motor loop which extends from the medial CM to the post-commissural putamen then to the ventrolateral GPi and back to the CM (73), and a much smaller projection to the primary motor cortex (74). That is, the CM is thought to be associated with ipsilateral central and precentral motor areas, although cortical projection neurons are less numerous and may be distinct from those projecting to the sensorimotor striatum. Nanda et al. (75) reported that CM stimulation results in striatal response patterns with excitatory and inhibitory components and that most excitatory responses in their study were transient and the response terminated before the end of the stimulation. Moreover, CM stimulation related to reductions in tonically active neurons and that are likely mediated via GABAergic transmission in the striatum. This structural network is further supported by studies showing that CM stimulation induces a decrease in striatal acetylcholine release (76,77).
Pf, on the other hand, projects to the anterior cingulate cortex, the premotor and prefrontal cortices and receives inputs from associational frontal, temporal, parietal and occipital cortices (78-80). Recent animal studies have shown that chemical activation of the Pf elicits a GABAergic inhibition of striatal acetylcholine release (81). In addition, studies of epilepsy have reported that high frequency Pf stimulation interrupted ongoing hippocampal paroxysmal discharges with possible support of GABAergic involvement (82-84).
Recognizing the inherent constraints of extrapolating findings from healthy animals to human pathologic conditions, the circuitry effects in this study may be of relevance to the heterogeneous symptoms of TS and the potential utility of CM-Pf as a clinical DBS target. Structural neuroimaging studies of patients with TS suggest that functionally segregated sensorimotor, associative and limbic cortico-BG networks contribute to the heterogenous clinical expressions of TS (26), including simple (85,86) and complex tics (26) and psychiatric co-morbidities (87,88).
fMRI in patients with TS, shows excessive activity in motor pathways and reduced activation in the control portions of cortico-striato-thalamo-cortical circuits (89), amygdala hypersensitivity in response to emotional facial expressions (90), functional disorganization of cortico-BG and a correlation between severity of OCD and functional abnormalities in associative and limbic networks, specifically in the orbito-frontal and dorsolateral prefrontal cortices (24). Tic severity has also been correlated with abnormalities in the premotor, sensorimotor, parietal and cingulate cortices, and the medial thalamus, and tic complexity with abnormalities in the sensorimotor area and associative networks, specifically in the insula and putamen (24). Bohlhalter et al. (25) found that during tic production, there was significant activity in sensorimotor areas, including the superior parietal lobule bilaterally and the cerebellum. In contrast, during the feeling of “urge” immediately prior to tic onset, there was activation in paralimbic areas, including the anterior cingulate and insular cortex, the supplementary motor area, and the parietal operculum.
CM-PF DBS was first conducted (7) based on a 1970 paper by Hassler and Dieckmann (91), in which prior to conducting bilateral thalamotomy to treat TS, they reported that stimulating different areas of the thalamus (intralaminar nucleus and lamella medialis) showed indications of emotional regulation through electrical stimulation (8-50Hz). Based on their previous experience with thalamotomy for OCD, they suggested that the rostral intraliminar nucleus might control the impulse component of TS, affecting the thalamo-cortical system between the medial nucleus and the prefrontal cortex (92).
Using [18F]fallypride PET in patients with TS, Kuhn et al. (23) found that bithalamic DBS was associated with reduction in dopaminergic transmission. Prior to stimulation, the patients were found to have increased dopaminergic transmission compared to healthy controls. During Pf stimulation, the dorsomedial nucleus and lamella medialis of the thalamus induced decreased dopaminergic transmission in the thalamus and inferior temporal cortex, but had no effect on the caudate and putamen.
Our results suggest that CM-Pf stimulation spreads via orthodromic and/or antidromic connections. When the electrical spread was large enough (via high amplitude DBS), CM and Pf DBS had similar circuitry effects; when the spread was reduced (via lower amplitude DBS), more specific network manipulations were possible. Given that the stimulation parameters for clinical applications of CM-Pf DBS in TS range from ~2V to 6V at ~90 to 130Hz (33,93), our finding of network differences between CM and Pf DBS suggests that DBS frequency and amplitude may be important considerations. Pf DBS appears to be more closely associated with the associative-limbic circuitry of the BG and also more sensitive to frequency and amplitude than CM DBS. Thus, manipulations of Pf may be of particular interest when treating behavioral symptoms associated with TS and possibly other conditions considered to be paralimbic in origin.
The CM-Pf circuit also may mediate response to unexpected brain events. Minamimoto (72) suggested that CM-Pf can induce an externally driven re-biasing process in the striatum that assists in selecting and executing actions appropriate for unexpected situations. This possibility implies that CM-Pf DBS might be a powerful tool in controlling multiple abnormal hyperactive BG functions and connectivity in neuropsychiatric conditions such as TS (2,3). On the other hand, adverse effects of CM-Pf DBS in TS patients, which includes impaired concentration, subjective gaze impairment, and alteration of sexual behavior (10,36), may also be related to the network effects found in our results.
Part of the central thalamic diffuse arousal system, CM-Pf has shown some benefit as a DBS target for patients in a chronic minimally conscious state (94-96). Our results suggest that CM-Pf DBS has a strong effect on the pineal gland, which produces several important circadian hormones including melatonin. Of note, CM-Pf stimulation may affect the sleep-wake cycle and sexual development, as well as regulation of endocrine functions (7,95).
Recently, McCairn and Worbe et al. (97-99) describe steps toward the development of a primate model of TS in which injections of GABA-antagonist into the striatum resulted in TS-like symptoms. They found that tic-like movements were associated with sensorimotor network, hyperactivity to the associative territories, and stereotypic behaviors linked to orbitofrontal cortex and limbic part of BG. Future disease animal model studies are needed to elucidate the links between the circuitry effects of DBS and behavioral outcomes.
Our use of a high precision Leksell stereotactic targeting system modified for large animals enhances accuracy in positioning the multi-contact DBS electrodes for CM and Pf respectively (59,100). However, despite confirmation of targeting accuracy by CT, the pig brain atlas notation of the electrode position is based on AC-PC alignment, proportionally resized by AC-PC length. Thus, accuracy can only be reached within a certain probability on the basis of the atlas.
Additional limitations includes that the animals were anesthetized and under muscle relaxant that was based on previous animal fMRI studies showing robust visual and electrical stimulation-dependent BOLD responses, electrophysiological, and neurotransmitter responses (101-105). Also of note, the Medtronic DBS electrodes used in this study have been reported to have an effective electric field up to 2~3mm (106). Thus, it is possible that adjacent thalamic nuclei, such as mediodorsal thalamic nucleus, may have been affected. Also in our sequential experimental design, we cannot rule out the possibility that the previous stimulation parameter may have affected the one following in the next condition. Future studies using selective neuronal activation methods (107-109) applied to substructures and different TS DBS targets, such as GPi and NAc (59,60) which have shown to have different effect in TS (10) could provide enhanced identification of the neuromodulary network effects of neural stimulation.
Safety risk, including increased temperature near the electrode tips from MRI scanner RF field focusing (110-112) prohibits conducting fMRI in humans with implanted DBS systems. However, in our recent study of in-vivo temperature measurements during MRI in a DBS-implanted pig, we confirmed that EPI sequence induced temperature change within the safety range for the animal brain (113).
fMRI is an indirect measure of neuronal activity via changes in hemodynamic parameters, and further research is needed to characterize the actual physiologic changes in the affected areas and to identify and interpret their role in behavioral change using concurrent alternate methodologies (114). The dynamic inter-relationships and effects of neuronal mechanisms, involving different system modules, during DBS are complex, but this complexity does not negate the possibility that the net output of the circuitry effect of DBS is reflected in the cortex (59,60,115), findings that are not limited to MR coil sensitivity distribution (data not included).
In summary, our results suggests that CM-Pf DBS has an inhibitory modulating effect in areas that have been suggested as contributing to impaired sensory-motor and emotional processing. Our findings support the notion that stimulation of deep brain structures, such as the CM-Pf, modulates multiple networks with cortical effects. The networks affected by CM-Pf stimulation in this study are consistent with current knowledge of their anatomic projections. While these results cannot be generalized to the clinical utility of DBS for TS, nor serve as an explanatory construct for TS symptom heterogeneity, they do provide evidence of differential motor, associative and limbic DBS-induced changes and stimulation parameter manipulation with CM and Pf DBS.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the Oxley Foundation, The Grainger Foundation, and by the National Institutes of Health (K08 NS 52232 and R01 NS 70872). We thank the Center for Advanced Imaging Research, Opus Building, Mayo Clinic for their support. MAF has served as an unpaid consultant with Allergan, Merck, Myriad, Sanofi-Aventis, Sunovion, and Teva Pharmaceuticals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All other authors reports no biomedical financial interests or potential conflicts of interest.
REFERENCE
- 1.Houeto JL, Karachi C, Mallet L, Pillon B, Yelnik J, Mesnage V, et al. Tourette's syndrome and deep brain stimulation. J Neurol Neurosurg Psychiatry. 2005;76:992–995. doi: 10.1136/jnnp.2004.043273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maciunas RJ, Maddux BN, Riley DE, Whitney CM, Schoenberg MR, Ogrocki PJ, et al. Prospective randomized double-blind trial of bilateral thalamic deep brain stimulation in adults with Tourette syndrome. J Neurosurg. 2007;107:1004–1014. doi: 10.3171/JNS-07/11/1004. [DOI] [PubMed] [Google Scholar]
- 3.Welter ML, Mallet L, Houeto JL, Karachi C, Czernecki V, Cornu P, et al. Internal pallidal and thalamic stimulation in patients with Tourette syndrome. Arch Neurol. 2008;65:952–957. doi: 10.1001/archneur.65.7.952. [DOI] [PubMed] [Google Scholar]
- 4.Porta M, Servello D, Zanaboni C, Anasetti F, Menghetti C, Sassi M, et al. Deep brain stimulation for treatment of refractory Tourette syndrome: long-term follow-up. Acta neurochirurgica. 2012 doi: 10.1007/s00701-012-1497-8. [DOI] [PubMed] [Google Scholar]
- 5.Ackermans L, Temel Y, Visser-Vandewalle V. Deep brain stimulation in Tourette's Syndrome. Neurotherapeutics. 2008;5:339–344. doi: 10.1016/j.nurt.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Visser-Vandewalle V, Temel Y, Boon P, Vreeling F, Colle H, Hoogland G, et al. Chronic bilateral thalamic stimulation: a new therapeutic approach in intractable Tourette syndrome. Report of three cases. J Neurosurg. 2003;99:1094–1100. doi: 10.3171/jns.2003.99.6.1094. [DOI] [PubMed] [Google Scholar]
- 7.Vandewalle V, van der Linden C, Groenewegen HJ, Caemaert J. Stereotactic treatment of Gilles de la Tourette syndrome by high frequency stimulation of thalamus. Lancet. 1999;353:724. doi: 10.1016/s0140-6736(98)05964-9. [DOI] [PubMed] [Google Scholar]
- 8.Okun MS, Foote KD, Wu SS, Ward HE, Bowers D, Rodriguez RL, et al. A Trial of Scheduled Deep Brain Stimulation for Tourette Syndrome: Moving Away From Continuous Deep Brain Stimulation Paradigms. Archives of neurology. 2012:1–10. doi: 10.1001/jamaneurol.2013.580. [DOI] [PubMed] [Google Scholar]
- 9.Savica R, Stead M, Mack KJ, Lee KH, Klassen BT. Deep brain stimulation in tourette syndrome: a description of 3 patients with excellent outcome. Mayo Clinic proceedings. 2012;87:59–62. doi: 10.1016/j.mayocp.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neuner I, Podoll K, Janouschek H, Michel TM, Sheldrick AJ, Schneider F. From psychosurgery to neuromodulation: deep brain stimulation for intractable Tourette syndrome. The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry. 2009;10:366–376. doi: 10.1080/15622970802513317. [DOI] [PubMed] [Google Scholar]
- 11.Hallett M. Neurophysiology of tics. Advances in neurology. 2001;85:237–244. [PubMed] [Google Scholar]
- 12.Cavanna AE, Eddy C, Rickards HE. Cognitive functioning in Tourette syndrome. Discov Med. 2009;8:191–195. [PubMed] [Google Scholar]
- 13.Freeman RD, Fast DK, Burd L, Kerbeshian J, Robertson MM, Sandor P. An international perspective on Tourette syndrome: selected findings from 3,500 individuals in 22 countries. Dev Med Child Neurol. 2000;42:436–447. doi: 10.1017/s0012162200000839. [DOI] [PubMed] [Google Scholar]
- 14.Jankovic J. Tourette's syndrome. The New England journal of medicine. 2001;345:1184–1192. doi: 10.1056/NEJMra010032. [DOI] [PubMed] [Google Scholar]
- 15.Krack P, Hariz MI, Baunez C, Guridi J, Obeso JA. Deep brain stimulation: from neurology to psychiatry? Trends Neurosci. 2010;33:474–484. doi: 10.1016/j.tins.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Lyons MK. Deep brain stimulation: current and future clinical applications. Mayo Clinic proceedings. 2011;86:662–672. doi: 10.4065/mcp.2011.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mink JW. The Basal Ganglia and involuntary movements: impaired inhibition of competing motor patterns. Archives of neurology. 2003;60:1365–1368. doi: 10.1001/archneur.60.10.1365. [DOI] [PubMed] [Google Scholar]
- 18.Du JC, Chiu TF, Lee KM, Wu HL, Yang YC, Hsu SY, et al. Tourette syndrome in children: an updated review. Pediatrics and neonatology. 2010;51:255–264. doi: 10.1016/S1875-9572(10)60050-2. [DOI] [PubMed] [Google Scholar]
- 19.Wong DF, Brasic JR, Singer HS, Schretlen DJ, Kuwabara H, Zhou Y, et al. Mechanisms of dopaminergic and serotonergic neurotransmission in Tourette syndrome: clues from an in vivo neurochemistry study with PET. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:1239–1251. doi: 10.1038/sj.npp.1301528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ernst M, Zametkin AJ, Jons PH, Matochik JA, Pascualvaca D, Cohen RM. High presynaptic dopaminergic activity in children with Tourette's disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:86–94. doi: 10.1097/00004583-199901000-00024. [DOI] [PubMed] [Google Scholar]
- 21.Singer HS. The treatment of tics. Current neurology and neuroscience reports. 2001;1:195–202. doi: 10.1007/s11910-001-0016-8. [DOI] [PubMed] [Google Scholar]
- 22.Gilbert D. Treatment of children and adolescents with tics and Tourette syndrome. Journal of child neurology. 2006;21:690–700. doi: 10.1177/08830738060210080401. [DOI] [PubMed] [Google Scholar]
- 23.Kuhn J, Janouschek H, Raptis M, Rex S, Lenartz D, Neuner I, et al. In vivo evidence of deep brain stimulation-induced dopaminergic modulation in Tourette's syndrome. Biological psychiatry. 2012;71:e11–13. doi: 10.1016/j.biopsych.2011.09.035. [DOI] [PubMed] [Google Scholar]
- 24.Worbe Y, Malherbe C, Hartmann A, Pelegrini-Issac M, Messe A, Vidailhet M, et al. Functional immaturity of cortico-basal ganglia networks in Gilles de la Tourette syndrome. Brain. 2012 doi: 10.1093/brain/aws056. [DOI] [PubMed] [Google Scholar]
- 25.Bohlhalter S, Goldfine A, Matteson S, Garraux G, Hanakawa T, Kansaku K, et al. Neural correlates of tic generation in Tourette syndrome: an event-related functional MRI study. Brain. 2006;129:2029–2037. doi: 10.1093/brain/awl050. [DOI] [PubMed] [Google Scholar]
- 26.Worbe Y, Gerardin E, Hartmann A, Valabregue R, Chupin M, Tremblay L, et al. Distinct structural changes underpin clinical phenotypes in patients with Gilles de la Tourette syndrome. Brain : a journal of neurology. 2010;133:3649–3660. doi: 10.1093/brain/awq293. [DOI] [PubMed] [Google Scholar]
- 27.Neuner I, Kupriyanova Y, Stocker T, Huang R, Posnansky O, Schneider F, et al. White-matter abnormalities in Tourette syndrome extend beyond motor pathways. Neuroimage. 2010;51:1184–1193. doi: 10.1016/j.neuroimage.2010.02.049. [DOI] [PubMed] [Google Scholar]
- 28.Kalanithi PS, Zheng W, Kataoka Y, DiFiglia M, Grantz H, Saper CB, et al. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proc Natl Acad Sci U S A. 2005;102:13307–13312. doi: 10.1073/pnas.0502624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kataoka Y, Kalanithi PS, Grantz H, Schwartz ML, Saper C, Leckman JF, et al. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. J Comp Neurol. 2010;518:277–291. doi: 10.1002/cne.22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith Y, Raju D, Nanda B, Pare JF, Galvan A, Wichmann T. The thalamostriatal systems: anatomical and functional organization in normal and parkinsonian states. Brain Res Bull. 2009;78:60–68. doi: 10.1016/j.brainresbull.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Groenewegen HJ, Berendse HW. The specificity of the ‘nonspecific’ midline and intralaminar thalamic nuclei. Trends in neurosciences. 1994;17:52–57. doi: 10.1016/0166-2236(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 32.Sadikot AF, Rymar VV. The primate centromedian-parafascicular complex: anatomical organization with a note on neuromodulation. Brain Res Bull. 2009;78:122–130. doi: 10.1016/j.brainresbull.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 33.Savica R, Stead M, Mack KJ, Lee KH, Klassen BT. Deep brain stimulation in tourette syndrome: a description of 3 patients with excellent outcome. Mayo Clin Proc. 2012;87:59–62. doi: 10.1016/j.mayocp.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Servello D, Porta M, Sassi M, Brambilla A, Robertson MM. Deep brain stimulation in 18 patients with severe Gilles de la Tourette syndrome refractory to treatment: the surgery and stimulation. J Neurol Neurosurg Psychiatry. 2008;79:136–142. doi: 10.1136/jnnp.2006.104067. [DOI] [PubMed] [Google Scholar]
- 35.Porta M, Brambilla A, Cavanna AE, Servello D, Sassi M, Rickards H, et al. Thalamic deep brain stimulation for treatment-refractory Tourette syndrome: two-year outcome. Neurology. 2009;73:1375–1380. doi: 10.1212/WNL.0b013e3181bd809b. [DOI] [PubMed] [Google Scholar]
- 36.Ackermans L, Duits A, van der Linden C, Tijssen M, Schruers K, Temel Y, et al. Double-blind clinical trial of thalamic stimulation in patients with Tourette syndrome. Brain. 2011;134:832–844. doi: 10.1093/brain/awq380. [DOI] [PubMed] [Google Scholar]
- 37.Ackermans L, Duits A, Temel Y, Winogrodzka A, Peeters F, Beuls EA, et al. Long-term outcome of thalamic deep brain stimulation in two patients with Tourette syndrome. J Neurol Neurosurg Psychiatry. 2010;81:1068–1072. doi: 10.1136/jnnp.2009.176859. [DOI] [PubMed] [Google Scholar]
- 38.Visser-Vandewalle V. DBS in tourette syndrome: rationale, current status and future prospects. Acta Neurochir Suppl. 2007;97:215–222. doi: 10.1007/978-3-211-33081-4_24. [DOI] [PubMed] [Google Scholar]
- 39.Minamimoto T, Kimura M. Participation of the thalamic CM-Pf complex in attentional orienting. J Neurophysiol. 2002;87:3090–3101. doi: 10.1152/jn.2002.87.6.3090. [DOI] [PubMed] [Google Scholar]
- 40.Macchi G, Bentivoglio M, Molinari M, Minciacchi D. The thalamo caudate versus thalamo-cortical projections as studied in the cat with fluorescent retrograde double labeling. Exp Brain Res. 1984;54:225–239. doi: 10.1007/BF00236222. [DOI] [PubMed] [Google Scholar]
- 41.Berendse HW, Groenewegen HJ. Restricted cortical termination fields of the midline and intralaminar thalamic nuclei in the rat. Neuroscience. 1991;42:73–102. doi: 10.1016/0306-4522(91)90151-d. [DOI] [PubMed] [Google Scholar]
- 42.Jayaraman A. Organization of thalamic projections in the nucleus accumbens and the caudate nucleus in cats and its relation with hippocampal and other subcortical afferents. J Comp Neurol. 1985;231:396–420. doi: 10.1002/cne.902310309. [DOI] [PubMed] [Google Scholar]
- 43.Ottersen OP. Afferent connections to the amygdaloid complex of the rat with some observations in the cat. III. Afferents from the lower brain stem. J Comp Neurol. 1981;202:335–356. doi: 10.1002/cne.902020304. [DOI] [PubMed] [Google Scholar]
- 44.Cavdar S, Onat FY, Cakmak YO, Yananli HR, Gulcebi M, Aker R. The pathways connecting the hippocampal formation, the thalamic reuniens nucleus and the thalamic reticular nucleus in the rat. J Anat. 2008;212:249–256. doi: 10.1111/j.1469-7580.2008.00858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Brain Res Rev. 2002;39:107–140. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- 46.Sidibe M, Bevan MD, Bolam JP, Smith Y. Efferent connections of the internal globus pallidus in the squirrel monkey: I. Topography and synaptic organization of the pallidothalamic projection. J Comp Neurol. 1997;382:323–347. [PubMed] [Google Scholar]
- 47.Sidibe M, Pare JF, Smith Y. Nigral and pallidal inputs to functionally segregated thalamostriatal neurons in the centromedian/parafascicular intralaminar nuclear complex in monkey. J Comp Neurol. 2002;447:286–299. doi: 10.1002/cne.10247. [DOI] [PubMed] [Google Scholar]
- 48.Sadikot AF, Parent A, Francois C. Efferent connections of the centromedian and parafascicular thalamic nuclei in the squirrel monkey: a PHA-L study of subcortical projections. J Comp Neurol. 1992;315:137–159. doi: 10.1002/cne.903150203. [DOI] [PubMed] [Google Scholar]
- 49.Parent M, Parent A. Single-axon tracing and three-dimensional reconstruction of centre median-parafascicular thalamic neurons in primates. The Journal of comparative neurology. 2005;481:127–144. doi: 10.1002/cne.20348. [DOI] [PubMed] [Google Scholar]
- 50.Pavlides C, Aoki C, Chen JS, Bailey WH, Winson J. Differential glucose utilization in the parafascicular region during slow-wave sleep, the still-alert state and locomotion. Brain Res. 1987;423:399–402. doi: 10.1016/0006-8993(87)90871-7. [DOI] [PubMed] [Google Scholar]
- 51.Saade NE, Al Amin H, Abdel Baki S, Chalouhi S, Jabbur SJ, Atweh SF. Reversible attenuation of neuropathic-like manifestations in rats by lesions or local blocks of the intralaminar or the medial thalamic nuclei. Exp Neurol. 2007;204:205–219. doi: 10.1016/j.expneurol.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 52.Liu C, Wang Y, Smallwood PM, Nathans J. An essential role for Frizzled5 in neuronal survival in the parafascicular nucleus of the thalamus. J Neurosci. 2008;28:5641–5653. doi: 10.1523/JNEUROSCI.1056-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kinomura S, Larsson J, Gulyas B, Roland PE. Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science. 1996;271:512–515. doi: 10.1126/science.271.5248.512. [DOI] [PubMed] [Google Scholar]
- 54.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weigel R, Krauss JK. Center median-parafascicular complex and pain control. Review from a neurosurgical perspective. Stereotact Funct Neurosurg. 2004;82:115–126. doi: 10.1159/000079843. [DOI] [PubMed] [Google Scholar]
- 56.Coolen LM, Veening JG, Wells AB, Shipley MT. Afferent connections of the parvocellular subparafascicular thalamic nucleus in the rat: evidence for functional subdivisions. J Comp Neurol. 2003;463:132–156. doi: 10.1002/cne.10739. [DOI] [PubMed] [Google Scholar]
- 57.Matsumoto N, Minamimoto T, Graybiel AM, Kimura M. Neurons in the thalamic CM-Pf complex supply striatal neurons with information about behaviorally significant sensory events. J Neurophysiol. 2001;85:960–976. doi: 10.1152/jn.2001.85.2.960. [DOI] [PubMed] [Google Scholar]
- 58.Eckert U, Metzger CD, Buchmann JE, Kaufmann J, Osoba A, Li M, et al. Preferential networks of the mediodorsal nucleus and centromedian parafascicular complex of the thalamus-A DTI tractography study. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Min HK, Hwang SC, Marsh MP, Kim I, Knight E, Striemer B, et al. Deep brain stimulation induces BOLD activation in motor and non-motor networks: An fMRI comparison study of STN and EN/GPi DBS in large animals. NeuroImage. 2012;63:1408–1420. doi: 10.1016/j.neuroimage.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Knight EJ, Min HK, Hwang SC, Marsh MP, Paek S, Kim I, et al. Nucleus accumbens deep brain stimulation results in insula and prefrontal activation: a large animal FMRI study. PLoS One. 2013;8:e56640. doi: 10.1371/journal.pone.0056640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Felix B, Leger ME, Albe-Fessard D, Marcilloux JC, Rampin O, Laplace JP. Stereotaxic atlas of the pig brain. Brain Res Bull. 1999;49:1–137. doi: 10.1016/s0361-9230(99)00012-x. [DOI] [PubMed] [Google Scholar]
- 62.Saikali S, Meurice P, Sauleau P, Eliat PA, Bellaud P, Randuineau G, et al. A three-dimensional digital segmented and deformable brain atlas of the domestic pig. J Neurosci Methods. 2010;192:102–109. doi: 10.1016/j.jneumeth.2010.07.041. [DOI] [PubMed] [Google Scholar]
- 63.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen- Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 64.Starr PA, Christine CW, Theodosopoulos PV, Lindsey N, Byrd D, Mosley A, et al. Implantation of deep brain stimulators into the subthalamic nucleus: technical approach and magnetic resonance imaging-verified lead locations. Journal of neurosurgery. 2002;97:370–387. doi: 10.3171/jns.2002.97.2.0370. [DOI] [PubMed] [Google Scholar]
- 65.Cho ZH, Min HK, Oh SH, Han JY, Park CW, Chi JG, et al. Direct visualization of deep brain stimulation targets in Parkinson disease with the use of 7-tesla magnetic resonance imaging. Journal of neurosurgery. 2010;113:639–647. doi: 10.3171/2010.3.JNS091385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mandeville JB, Choi JK, Jarraya B, Rosen BR, Jenkins BG, Vanduffel W. fMRI of cocaine self-administration in macaques reveals functional inhibition of basal ganglia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:1187–1198. doi: 10.1038/npp.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shmuel A, Augath M, Oeltermann A, Logothetis NK. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nature neuroscience. 2006;9:569–577. doi: 10.1038/nn1675. [DOI] [PubMed] [Google Scholar]
- 68.Francois C, Percheron G, Parent A, Sadikot AF, Fenelon G, Yelnik J. Topography of the projection from the central complex of the thalamus to the sensorimotor striatal territory in monkeys. J Comp Neurol. 1991;305:17–34. doi: 10.1002/cne.903050104. [DOI] [PubMed] [Google Scholar]
- 69.Lanciego JL, Rodriguez-Oroz MC, Blesa FJ, Alvarez-Erviti L, Guridi J, Barroso-Chinea P, et al. Lesion of the centromedian thalamic nucleus in MPTP-treated monkeys. Mov Disord. 2008;23:708–715. doi: 10.1002/mds.21906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sadikot AF, Parent A, Smith Y, Bolam JP. Efferent connections of the centromedian and parafascicular thalamic nuclei in the squirrel monkey: a light and electron microscopic study of the thalamostriatal projection in relation to striatal heterogeneity. J Comp Neurol. 1992;320:228–242. doi: 10.1002/cne.903200207. [DOI] [PubMed] [Google Scholar]
- 71.Ding J, Peterson JD, Surmeier DJ. Corticostriatal and thalamostriatal synapses have distinctive properties. J Neurosci. 2008;28:6483–6492. doi: 10.1523/JNEUROSCI.0435-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Minamimoto T, Hori Y, Kimura M. Complementary process to response bias in the centromedian nucleus of the thalamus. Science. 2005;308:1798–1801. doi: 10.1126/science.1109154. [DOI] [PubMed] [Google Scholar]
- 73.Smith Y, Raju DV, Pare JF, Sidibe M. The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends in neurosciences. 2004;27:520–527. doi: 10.1016/j.tins.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 74.Galvan A, Smith Y. The primate thalamostriatal systems: Anatomical organization, functional roles and possible involvement in Parkinson's disease. Basal Ganglia. 2011;1:179–189. doi: 10.1016/j.baga.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nanda B, Galvan A, Smith Y, Wichmann T. Effects of stimulation of the centromedian nucleus of the thalamus on the activity of striatal cells in awake rhesus monkeys. Eur J Neurosci. 2009;29:588–598. doi: 10.1111/j.1460-9568.2008.06598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Anderson JJ, Kuo S, Chase TN, Engber TM. GABAA and GABAB receptors differentially regulate striatal acetylcholine release in vivo. Neurosci Lett. 1993;160:126–130. doi: 10.1016/0304-3940(93)90395-2. [DOI] [PubMed] [Google Scholar]
- 77.DeBoer P, Westerink BH. GABAergic modulation of striatal cholinergic interneurons: an in vivo microdialysis study. J Neurochem. 1994;62:70–75. doi: 10.1046/j.1471-4159.1994.62010070.x. [DOI] [PubMed] [Google Scholar]
- 78.Goldman PS, Nauta WJ. An intricately patterned prefronto-caudate projection in the rhesus monkey. J Comp Neurol. 1977;72:369–386. doi: 10.1002/cne.901710305. [DOI] [PubMed] [Google Scholar]
- 79.Van Hoesen GW, Yeterian EH, Lavizzo-Mourey R. Widespread corticostriate projections from temporal cortex of the rhesus monkey. J Comp Neurol. 1981;199:205–219. doi: 10.1002/cne.901990205. [DOI] [PubMed] [Google Scholar]
- 80.Yeterian EH, Pandya DN. Corticostriatal connections of the superior temporal region in rhesus monkeys. J Comp Neurol. 1998;399:384–402. [PubMed] [Google Scholar]
- 81.Zackheim J, Abercrombie ED. Thalamic regulation of striatal acetylcholine efflux is both direct and indirect and qualitatively altered in the dopamine-depleted striatum. Neuroscience. 2005;131:423–436. doi: 10.1016/j.neuroscience.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 82.Velasco F, Velasco AL, Velasco M, Jimenez F, Carrillo-Ruiz JD, Castro G. Deep brain stimulation for treatment of the epilepsies: the centromedian thalamic target. Acta Neurochir Suppl. 2007;97:337–342. doi: 10.1007/978-3-211-33081-4_38. [DOI] [PubMed] [Google Scholar]
- 83.Velasco AL, Velasco F, Jimenez F, Velasco M, Castro G, Carrillo-Ruiz JD, et al. Neuromodulation of the centromedian thalamic nuclei in the treatment of generalized seizures and the improvement of the quality of life in patients with Lennox-Gastaut syndrome. Epilepsia. 2006;47:1203–1212. doi: 10.1111/j.1528-1167.2006.00593.x. [DOI] [PubMed] [Google Scholar]
- 84.Langlois M, Polack PO, Bernard H, David O, Charpier S, Depaulis A, et al. Involvement of the thalamic parafascicular nucleus in mesial temporal lobe epilepsy. J Neurosci. 2010;30:16523–16535. doi: 10.1523/JNEUROSCI.1109-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sowell ER, Kan E, Yoshii J, Thompson PM, Bansal R, Xu D, et al. Thinning of sensorimotor cortices in children with Tourette syndrome. Nature neuroscience. 2008;11:637–639. doi: 10.1038/nn.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thomalla G, Siebner HR, Jonas M, Baumer T, Biermann-Ruben K, Hummel F, et al. Structural changes in the somatosensory system correlate with tic severity in Gilles de la Tourette syndrome. Brain : a journal of neurology. 2009;132:765–777. doi: 10.1093/brain/awn339. [DOI] [PubMed] [Google Scholar]
- 87.Bloch MH, Leckman JF, Zhu H, Peterson BS. Caudate volumes in childhood predict symptom severity in adults with Tourette syndrome. Neurology. 2005;65:1253–1258. doi: 10.1212/01.wnl.0000180957.98702.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ludolph AG, Pinkhardt EH, Tebartz van Elst L, Libal G, Ludolph AC, Fegert JM, et al. Are amygdalar volume alterations in children with Tourette syndrome due to ADHD comorbidity? Developmental medicine and child neurology. 2008;50:524–529. doi: 10.1111/j.1469-8749.2008.03014.x. [DOI] [PubMed] [Google Scholar]
- 89.Wang Z, Maia TV, Marsh R, Colibazzi T, Gerber A, Peterson BS. The neural circuits that generate tics in Tourette's syndrome. Am J Psychiatry. 2011;168:1326–1337. doi: 10.1176/appi.ajp.2011.09111692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Neuner I, Kellermann T, Stocker T, Kircher T, Habel U, Shah JN, et al. Amygdala hypersensitivity in response to emotional faces in Tourette's patients. The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry. 2010;11:858–872. doi: 10.3109/15622975.2010.480984. [DOI] [PubMed] [Google Scholar]
- 91.Hassler R, Dieckmann G. [Stereotaxic treatment of tics and inarticulate cries or coprolalia considered as motor obsessional phenomena in Gilles de la Tourette's disease]. Rev Neurol (Paris) 1970;123:89–100. [PubMed] [Google Scholar]
- 92.Rickards H, Wood C, Cavanna AE. Hassler and Dieckmann's seminal paper on stereotactic thalamotomy for Gilles de la Tourette syndrome: translation and critical reappraisal. Mov Disord. 2008;23:1966–1972. doi: 10.1002/mds.22238. [DOI] [PubMed] [Google Scholar]
- 93.Hariz MI, Robertson MM. Gilles de la Tourette syndrome and deep brain stimulation. The European journal of neuroscience. 2010;32:1128–1134. doi: 10.1111/j.1460-9568.2010.07415.x. [DOI] [PubMed] [Google Scholar]
- 94.Schiff ND. Central thalamic contributions to arousal regulation and neurological disorders of consciousness. Ann N Y Acad Sci. 2008;1129:105–118. doi: 10.1196/annals.1417.029. [DOI] [PubMed] [Google Scholar]
- 95.Yamamoto T, Katayama Y, Kobayashi K, Oshima H, Fukaya C, Tsubokawa T. Deep brain stimulation for the treatment of vegetative state. Eur J Neurosci. 2010;32:1145–1151. doi: 10.1111/j.1460-9568.2010.07412.x. [DOI] [PubMed] [Google Scholar]
- 96.Yamamoto T, Kobayashi K, Kasai M, Oshima H, Fukaya C, Katayama Y. DBS therapy for the vegetative state and minimally conscious state. Acta neurochirurgica Supplement. 2005;93:101–104. doi: 10.1007/3-211-27577-0_17. [DOI] [PubMed] [Google Scholar]
- 97.McCairn KW, Bronfeld M, Belelovsky K, Bar-Gad I. The neurophysiological correlates of motor tics following focal striatal disinhibition. Brain : a journal of neurology. 2009;132:2125–2138. doi: 10.1093/brain/awp142. [DOI] [PubMed] [Google Scholar]
- 98.Worbe Y, Sgambato-Faure V, Epinat J, Chaigneau M, Tande D, Francois C, et al. Towards a primate model of Gilles de la Tourette syndrome: anatomo-behavioural correlation of disorders induced by striatal dysfunction. Cortex; a journal devoted to the study of the nervous system and behavior. 2013;49:1126–1140. doi: 10.1016/j.cortex.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 99.Worbe Y, Baup N, Grabli D, Chaigneau M, Mounayar S, McCairn K, et al. Behavioral and movement disorders induced by local inhibitory dysfunction in primate striatum. Cerebral cortex. 2009;19:1844–1856. doi: 10.1093/cercor/bhn214. [DOI] [PubMed] [Google Scholar]
- 100.Bjarkam CR, Cancian G, Glud AN, Ettrup KS, Jorgensen RL, Sorensen JC. MRI-guided stereotaxic targeting in pigs based on a stereotaxic localizer box fitted with an isocentric frame and use of SurgiPlan computer-planning software. Journal of neuroscience methods. 2009;183:119–126. doi: 10.1016/j.jneumeth.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 101.Angenstein F, Kammerer E, Scheich H. The BOLD response in the rat hippocampus depends rather on local processing of signals than on the input or output activity. A combined functional MRI and electrophysiological study. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:2428–2439. doi: 10.1523/JNEUROSCI.5015-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Angenstein F, Krautwald K, Scheich H. The current functional state of local neuronal circuits controls the magnitude of a BOLD response to incoming stimuli. Neuroimage. 2010;50:1364–1375. doi: 10.1016/j.neuroimage.2010.01.070. [DOI] [PubMed] [Google Scholar]
- 103.Jin T, Kim SG. Cortical layer-dependent dynamic blood oxygenation, cerebral blood flow and cerebral blood volume responses during visual stimulation. Neuroimage. 2008;43:1–9. doi: 10.1016/j.neuroimage.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Masamoto K, Kim T, Fukuda M, Wang P, Kim SG. Relationship between neural, vascular, and BOLD signals in isoflurane-anesthetized rat somatosensory cortex. Cerebral cortex. 2007;17:942–950. doi: 10.1093/cercor/bhl005. [DOI] [PubMed] [Google Scholar]
- 105.Krautwald K, Min HK, Lee KH, Angenstein F. Synchronized electrical stimulation of the rat medial forebrain bundle and perforant pathway generates an additive BOLD response in the nucleus accumbens and prefrontal cortex. Neuroimage. 2013;77:14–25. doi: 10.1016/j.neuroimage.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 106.Lozano AM, Dostrovsky J, Chen R, Ashby P. Deep brain stimulation for Parkinson's disease: disrupting the disruption. Lancet neurology. 2002;1:225–231. doi: 10.1016/s1474-4422(02)00101-1. [DOI] [PubMed] [Google Scholar]
- 107.Miocinovic S, Zhang J, Xu W, Russo GS, Vitek JL, McIntyre CC. Stereotactic neurosurgical planning, recording, and visualization for deep brain stimulation in non-human primates. Journal of neuroscience methods. 2007;162:32–41. doi: 10.1016/j.jneumeth.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee JH. Informing brain connectivity with optogenetic functional magnetic resonance imaging. Neuroimage. 2012;62:2244–2249. doi: 10.1016/j.neuroimage.2012.01.116. [DOI] [PubMed] [Google Scholar]
- 110.Carmichael DW, Pinto S, Limousin-Dowsey P, Thobois S, Allen PJ, Lemieux L, et al. Functional MRI with active, fully implanted, deep brain stimulation systems: safety and experimental confounds. NeuroImage. 2007;37:508–517. doi: 10.1016/j.neuroimage.2007.04.058. [DOI] [PubMed] [Google Scholar]
- 111.Pictet J, Meuli R, Wicky S, van der Klink JJ. Radiofrequency heating effects around resonant lengths of wire in MRI. Phys Med Biol. 2002;47:2973–2985. doi: 10.1088/0031-9155/47/16/312. [DOI] [PubMed] [Google Scholar]
- 112.Rezai AR, Baker KB, Tkach JA, Phillips M, Hrdlicka G, Sharan AD, et al. Is magnetic resonance imaging safe for patients with neurostimulation systems used for deep brain stimulation? Neurosurgery. 2005;57:1056–1062. doi: 10.1227/01.neu.0000186935.87971.2a. discussion 1056-1062. [DOI] [PubMed] [Google Scholar]
- 113.Gorny KR, Presti MF, Goerss SJ, Hwang SC, Jang DP, Kim I, et al. Measurements of RF heating during 3.0-T MRI of a pig implanted with deep brain stimulator. Magn Reson Imaging. 2013;31:783–788. doi: 10.1016/j.mri.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- 115.Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324:354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.