Abstract
The World Health Organization (WHO) recommends periodic surveillance of transmitted drug resistance (TDR) in communities in which antiretroviral therapy (ART) has been scaled-up for greater than 3 years. We conducted a survey of TDR mutations among newly detected HIV-infected antiretroviral (ARV)-naive pregnant women. From May 2010 to March 2012, 38 ARV-naive pregnant women were recruited in three hospitals in Jos, Plateau state, north central Nigeria. Eligible subjects were recruited using a modified version of the binomial sequential sampling technique recommended by WHO. HIV-1 genotyping was performed and HIV-1 drug resistance mutations were characterized according to the WHO 2009 surveillance drug resistance mutation (SDRM) list. HIV subtypes were determined by phylogenetic analysis. The women's median age was 25.5 years; the median CD4+ cell count was 317 cells/μl and the median viral load of 16 was 261 copies/ml. Of the 38 samples tested, 34 (89%) were successfully genotyped. The SDRM rate was <5% for all ART drug classes, with 1/34 (2.9%) for NRTIs/NNRTIs and none for protease inhibitors 0/31 (0%). The specific SDRMs detected were M41L for nucleoside reverse transcriptase inhibitors (NRTIs) and G190A for nonnucleoside reverse transcriptase inhibitors (NNRTIs). HIV-1 subtypes detected were CRF02_AG (38.2%), G′ (41.2%), G (14.7%), CRF06-CPX (2.9%), and a unique AG recombinant form (2.9%). The single ARV-native pregnant woman with SDRMs was infected with HIV-1 subtype G′. Access to ART has been available in the Jos area for over 8 years. The prevalence of TDR lower than 5% suggests proper ART administration, although continued surveillance is warranted.
Introduction
Effective use of antiretroviral drugs for therapy (ART) markedly reduces the morbidity and mortality associated with HIV infection.1,2 In addition, the appropriate use of a combination of antiretroviral drugs (ARVs) in both breastfeeding and nonbreastfeeding populations has resulted in reductions of mother-to-child transmission rates from 15% to 45% to less than 2%.3–5 However, the rapid scale-up of ART in resource-limited countries is not without its challenges including inadequate capacity for treatment monitoring, limited options of ARV drug choices for those failing therapy, intermittent interruptions in drug supply, treatment adherence problems, and emerging HIV genetic diversity.6–8 These are factors that could fuel the emergence of HIV drug resistance. It is feared that HIV-infected individuals may transmit drug-resistant viruses to others in the society and infants may acquire resistant virus from their infected mothers at birth and during breastfeeding.9,10 The periodic surveillance of the prevalence, pattern, and trends of HIV transmitted drug resistance and the identification of circulating subtypes are therefore recommended so that timely interventions can be implemented as needed.11 This practice will help in ensuring that the efficacy of antiretroviral drugs used for ART, prevention of mother-to-child transmission (PMTCT), treatment for prevention, and preexposure and postexposure prophylaxis are preserved.
The population of Nigeria is about 162 million people, with an estimated 3.3 million people living with HIV/AIDS.12 The number of HIV/AIDS-related deaths in the country is estimated at 217,000.2,12 In Jos, Nigeria, the site of this study, only a small number of HIV-infected patients could afford to purchase ARV drugs in the late 1990s. In 2002, the federal government rolled-out ART to 10,000 adult patients and 5,000 HIV-positive children in 25 tertiary Health Institutions in the six geopolitical regions of the country and subsequent expansion of ART services has continued to date with support from the President's Emergency Plan For AIDS Relief (PEPFAR), Global Fund, and other international funders, thus increasing the critical mass of HIV-infected patients receiving ARVs to about 400,000; yet, an estimated 1.5 million eligible patients are still in need.12 Jos University Teaching Hospital (JUTH) located in Plateau state was one of the federal hospitals designated to provide ART. Plateau state has a population of ∼3.2 million and to date it is estimated that over 20,000 HIV-positive patients have been enrolled on ART in government, faith-based, and private health institutions.12 The use of single dose nevirapine (sdNVP) intervention for PMTCT in labor for mother and infants, which is known to be associated with the rapid emergence of drug resistance, also started in Nigeria within the same period of ART scale-up.9,12,13 Compared to other countries with high burdens of HIV, studies reporting HIV genotypes and drug resistance in Nigeria are few and there is a dearth of information in this challenging area of HIV infection control.14–16 Studies that have surveyed drug resistance among antiretroviral-naive patients in Nigeria have reported low rates of <5%.14,16,17 This is comparable to similar reports from other sub-Sahara African countries.18–20 However, a recent report from Kampala, Uganda described a dramatic increase from 0% to 8.6% in transmitted drug resistance within 10 years of the introduction of ART.21 This finding further justifies the need for vigilance in areas where ART has been rolled out.
WHO has recommended a simple and cost-effective protocol for determining the prevalence of TDR in resource-limited settings.11,22 This employs the use of a binomial sequential sampling technique to obtain 34–47 samples for genotyping from ARV-naive primigravid women less than 25 years old who are newly detected as HIV infected at antenatal enrollment or during national sentinel surveillance surveys. The prevalence of transmitted drug resistance (TDR) mutations among the women is determined and classified for each drug or drug class as <5%, 5–15%, and >15%.11,22 However, recent reports have indicated that the methodology may lack sensitivity for newly infected individuals and revisions to the protocol are ongoing.23,24 Questions have also been raised about the rationale for limiting the age of ARV-naive pregnant women required for the survey to less than 25 years, especially in urban settings where people tend to marry much later in life due to educational and career pursuits compared to rural settings.24 Other studies have found that the practice of using subjects less than 25 years old underestimates TDR in communities where ART has been rolled-out for upward of 3 years. Revision of the WHO criteria to include older pregnant women who are ARV naive and the use of laboratory-based assays for selecting recently infected subjects for testing have been proposed.19,25,26 We report the results of our study aimed at determining the prevalence and pattern of HIV TDR mutations and subtypes among HIV-infected ART-naive pregnant women detected during antenatal enrollment in Jos, north central Nigeria.
Materials and Methods
The study was conducted at JUTH, a federal government tertiary health institution, Our Lady of Apostles (OLA) Hospital, a faith-based hospital, and SOLAT Women's Hospital, a private health facility. All three hospitals have been supported for prevention, care, and treatment services by the Harvard PEPFAR program and its successor in 2012, the AIDS Prevention Initiative in Nigeria (APIN) PEPFAR program. The hospitals were chosen to ensure the inclusion of women across the different social economic strata in the Jos community. From May 2010 to March 2012, 38 ART-naive pregnant women newly detected as HIV infected at antenatal visits in the three hospitals were recruited for enrollment.
The recruitment of study subjects followed a modified version of the binomial sequential sampling technique recommended by WHO along with one additional modification. The first criteria recommended by WHO included newly diagnosed HIV infection, the primigravid status of the pregnant woman, ARV-naïve, and age ≤30 years. The second criteria involved a combination of laboratory results including a positive Aware BED EIA HIV-1 incidence test-Calypte capture enzyme immunoassay (BED-CEIA) with CD4+ cell count greater than 200 cells/μl and a plasma viral load greater than 400 copies/ml. Therefore, the second criteria allowed HIV-1-infected ARV-naive pregnant women who were older than 30 year or were multigravid to also be included in the study.18,25 All the pregnant women were interviewed by clinic staff in order to verify that there was no prior exposure to ARVs. All eligible women provided written informed consent prior to study enrollment. Institutional ethical review board approvals from the Ethics Committee at JUTH and the Human Subject Committee at the Harvard School of Public Health were obtained for the study.
The Nigerian National rapid HIV test serial algorithm was implemented for HIV diagnosis using a provider initiated opt-out option approach at antenatal clinic enrollment.27 Whole blood sample was collected and plasma tested for HIV antibodies with rapid HIV test kits including Determine (Alere Medical Co., Japan) followed by Unigold (Trinity Biotech PLC, Ireland) with Statpak (Chembio Diagnostic Systems, New York) as the tiebreaker assay for resolving discordant results. Enumeration of CD4+ cells was performed using the flow cytometry technique (Partec Cyflow, Munster, Germany). The plasma portion of the sample was separated after centrifugation and stored at −80°C for viral load and HIV genotyping. Viral load determinations were performed using Roche Amplicor version 1.5 (Roche Diagnostics, Newark, NJ). The Aware BED Enzyme Immunoassay was performed according to the manufacturer's instructions (Calypte Biomedical Corporation, Portland, OR).
The ViroSeq v 2.0 HIV-1 Genotyping system (Abbott Molecular, Des Plains, IL) was used to reverse transcribe and amplify a 1.8-kb region of the pol gene that spans the entire protease (PR) gene and approximately two-third of the reverse transcriptase (RT) gene. Sequence was obtained with ABI Genetic Analyser 3130xl (Applied Biosystems, Foster City, CA). Sequences were edited and compared with that of a reference HXB2 subtype B. Using the manufacturer's software and the Stanford University drug resistance algorithm database online, the lists of mutations and polymorphisms were generated. HIV-1 transmitted drug resistance mutations were further characterized according to the WHO 2009 surveillance drug resistance mutation (SDRM) list.23 Phylogenetic analysis for determining HIV-1 subtype was performed by aligning the viral sequences along with the reference sequences obtained from Los Alamos repository using Clustal X (www.softedia.com). NJPlot (www.soft82.com) was used to visualize the phylogenetic reconstruction and neighbor-joining trees were used to classify them by subtype. The data obtained from this study were analyzed and calculated in proportions using EPI Info 7 (www.cdc.gov/epiinfo).
Results
During the period of the study, a total of 8,357 pregnant women were tested for HIV at antenatal enrollment at the three hospitals and 364 (4.4%) were found to be HIV positive. Thirty eight ARV-naive pregnant women met the study criteria and were recruited for HIV-1 genotyping. Of the 38 samples obtained, 34 (89%) were successfully amplified and genotyped. The median age, CD4+ cell count, and viral load were 25.5 years (IQR 23–28 years), 317 cells/μl (IQR 204–917 cells/μl), and 16,261 copies/ml (5,271–110,007) copies/ml), respectively (Table 1). Two HIV-1 SDRMs were detected including the nucleoside reverse transcriptase inhibitor (NRTI) mutation (M41L) and nonnucleoside reverse transcriptase inhibitor (NNRTI) mutation (G190A) in only one out of the 34 genotyped samples. The only SDRMs found were in an ARV-naive primigravid, 27-year-old woman (Table 2). The woman was interviewed and her medical records reviewed to confirm that she had no prior ARV use. The TDR mutation rates were <5% by ARV drug class: 1 of 34 (2.9%) for NRTIs/NNRTIs and 0 of 34 (0%) for protease inhibitors (PIs) (Table 2). Other minor mutations or polymorphisms that were detected but not classified on the 2009 WHO SDRM list were PI mutations L10V (1/34; 2.9%), L10I (2/34; 5.9%), V11I (1/34; 2.9%), and the NNRTI mutations A71T (1/34; 2.9%) and A98G (2/34; 5.9%).
Table 1.
Characteristics of Antiretroviral-Naive Pregnant Women
| Age (years) | CD4 count (cells/μl) | Viral load (copies/ml) | ||||
|---|---|---|---|---|---|---|
| Group | Median | IQR | Median | IQR | Median | IQR |
| ≤25 years (n=19) | 24.5 | 22–25 | 386 | 233–457 | 16,881 | 6,172–30,986 |
| 26–30 years (n=14) | 28.5 | 27.5–28.5 | 361 | 137–561 | 36,528 | 4,136–219,679 |
| BED-CEIA+Multiassaya: CD4+VL (n=5) | 33.0 | 32–37.5 | 290 | 224–313 | 5,182 | 2,041–170,928 |
| Amplified and genotyped plasma samples (n=34) | 25.5 | 20–38 | 317 | 204–917 | 16,261 | 5,271–110,007 |
Multiassay=CD4 >200 cells/μl+viral load >400 copies/ml.
BED-CEIA, BED capture enzyme immunoassay; IQR, interquartile range.
Table 2.
HIV-1 Drug Resistance Mutations by Patient Group
| WHO surveillance drug resistance mutation detected | |||||
|---|---|---|---|---|---|
| Sample group | Number of samples (%) | Number of WHO SDRM (%) | NRTI | NNRTI | PI |
| ARV naive primigravid ≤25 years | 18 (52.9) | 0 (0) | 0 | 0 | 0 |
| ARV naive primigravid 26–30 years | 11 (32.4) | 1 (2.9) | M41L | G190A | 0 |
| Recent infection detected by BED and multiassays | 5 (14.7) | 0 (0) | 0 | 0 | 0 |
| Total | 34 (100) | 1 (2.9) | 1 | 1 | 0 |
SDRM, surveillance drug resistance mutation; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; ARV, antiretroviral.
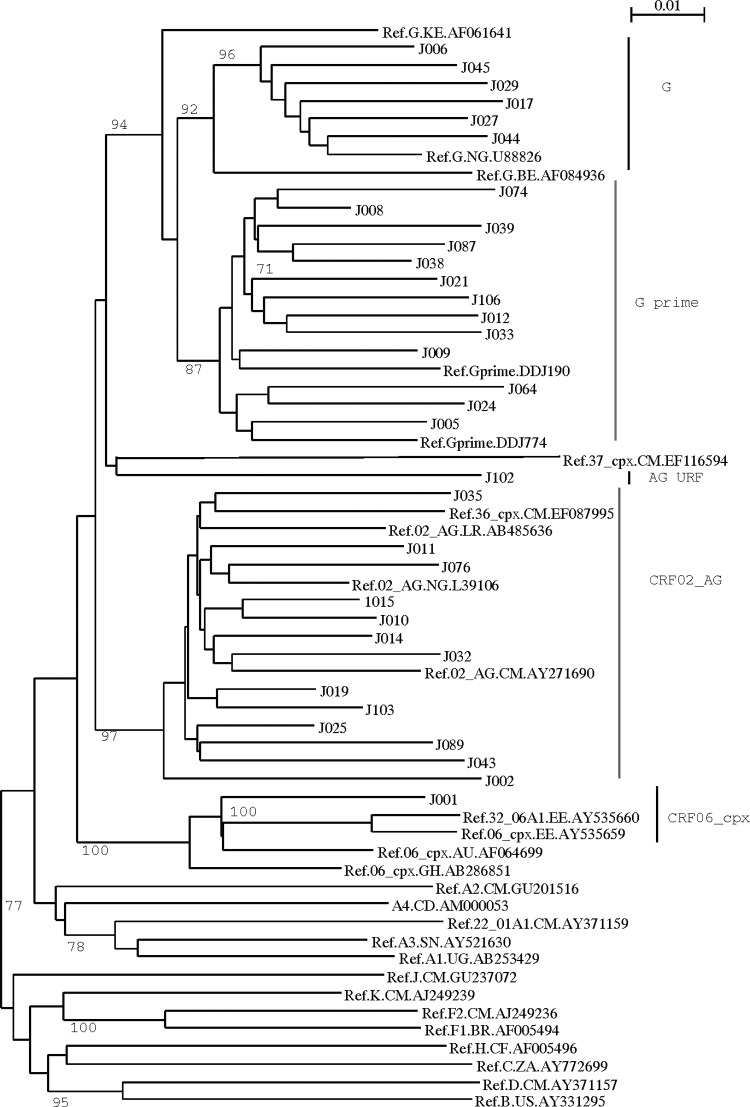
The HIV-1 subtypes classified by phylogenetic analysis are as shown in Figs. 1 and 2. The G and G′ subtypes were the most prevalence subtypes with 19 of 34 (55.9%), subtype G accounting for 14% (5/34) of the subtotal while G′, a unique variant of G prevalent in Nigeria,28 accounted for 41.2 % (14/34). Other subtypes included CRF02_AG (13/34, 38.2%), CRF06_cpx (1/34; 2.9%), and a unique AG recombinant form (1/34; 2.9%). In general, the majority of the mutations (7/9; 77.8%) found in this study (i.e., G190A, A98Gx2, M41L, V11I, and L10Ix2) were associated with subtype G (G′: 5 of 9 and G: 2 of 9). The subtype G′-infected individual harbored the two SDRMs, G190A and M41L (Table 2).
FIG. 1.
Phylogenetic tree of HIV-1 subtypes detected in antiretroviral (ARV)-naive pregnant women in Jos, north central Nigeria.
FIG. 2.
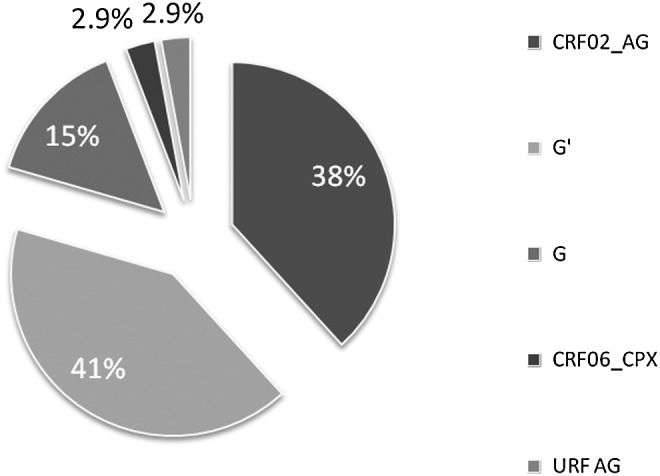
HIV-1 subtypes among antiretroviral-naive women in Jos, Nigeria. The total proportion of subtype G 19/34 (55.9%) detected included subtypes G′ and G indicated in the pie chart.
Discussion
This survey revealed a low prevalence of WHO SDRM of <5% among 34 ARV-naive, newly diagnosed HIV-infected pregnant women evaluated in Jos, north central Nigeria. There were no SDRMs detected against PIs and only one SDRM (2.9%, 1/34) was found for NRTIs (M41L) and one SRDRM for NNRTIs (G190A) in the 34 (2.9%, 1/34) ARV-naive pregnant women evaluated.
This finding suggests that generalized use of ARVs in the HIV treatment programs in Jos and its surrounding communities for over 8 years has been optimal and does not appear to have promoted the emergence of TDR to a concerning level. Although this finding is encouraging, caution is required in its interpretation and generalization of the result. HIV transmitted drug resistance results obtained by population survey methods are location and site specific and it may not be representative of the entire state or nation. This means that location and community specific surveys are preferred for a better understanding of TDR prevention and control. While our findings are similar to recent survey reports emerging from some sub-Saharan African countries such as Botswana, Malawi, Zimbabwe, Kenya, and South Africa, higher rates ranging from 8% to 12% have been reported for Yaoundé in Cameroun, Dar es Salaam in Tanzania, and Kampala in Uganda, respectively.18–21,29,30 TDR rates as high as 6%–23% have been reported in Europe and the United States among recent and acute HIV-infected patients.31,32 The highest rates of TDR in Europe and the United States were recorded during the early years of ART when mono and dual ART regimens with zidovudine were used. With the advent of HAART and improved options of ARVs accompanied by HIV drug resistance testing to guide antiretroviral regimen selection, the prevalence rates of TDR are stable and beginning to decline in North America and Europe.32–34
Much of our knowledge regarding ARV efficacy and drug resistance is based on HIV-1 subtype B, the subtype prevalence in the western world, i.e., North America, Western Europe, Japan, and Australia. However, a disproportionate burden of HIV is found in low-income and middle-income countries where non-B subtypes predominate. The HIV-1 subtypes that have been reported in Nigeria include CRF02_AG, G, G′ CRF06_cpx, and A.14–16,28,35 In an analysis of HIV sequences obtained from 338 HIV patients failing first line regimen in four Harvard/APIN PEPFAR sites across Nigeria, a relationships between subtypes and emergence of drug resistance mutations and polymorphic forms was reported.15 While many of the major drug resistance mutations observed were similar to what would be found in HIV-1 subtype B infections, there were subtype-specific differences for both NRTI and NNRTI major mutations.15 In their study, HIV-1 subtype A patients showed a 42.5-fold increased risk for the L210W mutation. Patients infected with subtype G patients had an increased risk for A98G and V106I, whereas subtype CRF02_AG patients had an increased risk for V90I and a decreased risk for A98G.15 The clinical relevance of these findings deserves further study.
In our study, the only two SDRM mutations (M41L and G190A) detected were found in a patient infected with subtype G′. Apart from the two SDRM detected, other minor mutations or polymorphisms included A98G for NNRTIs and V11I, L10I, L10V, and A71T for PIs. It is worth noting that the majority of these mutations (7/9; 77.8%) were found in patients infected with HIV-1 subtype G including G′. Only a minority of the PI non-SDRMs, L10V and A71T (2/9; 22.2%), was associated with CRF02_AG. The relevance of these findings and possible future impact of these mutations on these patients as they receive ART or PMTCT interventions are currently not known, but are worthy of further study. Current concepts support the notion that naturally occurring polymorphisms among different non-subtype B subtypes can affect HIV-1 susceptibility to antiretroviral drugs, the magnitude of resistance conferred by major mutations, and the propensity to acquire some resistance mutations.36 This can be further elucidated by studying the treatment outcomes of patients infected with viruses with wild-type minor mutations or polymorphisms.
Best practice guidelines in ART recommend HIV genotyping for HIV-infected patients before the initiation of ART.37 While this may be the norm in most high-income countries, the prohibitive cost of the HIV genotyping assay has made this impossible in resource-limited settings. As a result, WHO developed a simple and cost-effective population-based surveillance approach to assess the TDR threat to patients initiating ART.11,22,23 This population approach has been critical to early surveillance efforts, but recent reports are suggesting a review and modification of the protocol to address issues arising from its current use. In this study, the only subject found to harbor the two transmitted drug resistance mutations was a 27-year-old ARV-naive pregnant woman who would have been excluded from the study based on age if WHO criteria (i.e., <25 years) were the only selection criteria used. Higher rates of TDR among ARV-naive pregnant women older than 25 years have also been reported in Tanzania.26 In addition, it is often challenging to determine the actual age of pregnant women among the unschooled subpopulations often found in resource-limited settings. Furthermore, urban women's desire for tertiary education and career pursuit before marriage may be contributing to the older age of marriage and child-bearing. The use of newly diagnosed HIV-infected subjects does not necessarily equate to recent infections. It is also known that some TDR mutants may revert to wild type and, as such, may not be detected in recently infected patients.36 The use of laboratory-based assays to identify recently HIV-infected individuals, irrespective of age, has been suggested as an alternative surveillance strategy but it may not be cost effective.24
We noted some limitations and challenges in our study. It was difficult to obtaining the WHO required sample size of 34–47 eligible pregnant women during the study period; this population has demonstrated declining HIV prevalence and incidence in recent years.38 We therefore modified the WHO criteria using the BED-early infection assay for five women in order to obtain the 38. Despite these limitations, the results we obtained provide local and relevant evidence of the low prevalence of TDR in Jos, north central Nigeria. Concerted efforts should be made through government and other stakeholders to provide continuous funding and involvement to ensure that the current robust and effective HIV and ART programs are maintained. This will increase the probability that the low TDR prevalence described in our study can be sustained.
Sequence Data
The sequences of the data reported in this article have been submitted to the GenBank Sequence Database under the following accession numbers: KC755236–KC755269.
Acknowledgments
We are thankful to all the women who participated in this study. The support of the leadership of the three hospitals and the antenatal clinic staff at the sites where the study was conducted is gratefully acknowledged. We are thankful to Prof. John Idoko, Director General, National Agency for AIDS Control (NACA), Nigeria and former PI, APIN JUTH for his support and Chindak Lekuk for sample handling and transportation. This research was supported in part by the President's Emergency Plan for AIDS Relief (PEPFAR) through the Harvard/APIN PEPFAR funded by the U.S. Health Resources and Services Administration (U51HA02522-01-01). Data analysis and manuscript writing for this study were done through funding received by Godwin Imade from the Educational Trust Fund (ETF), University of Jos, and the Harvard AIDS Initiative, Harvard School of Public Health Fogarty grant (2D43W000004-24).
Partial presentation of these data was made at the 7th International Workshop on HIV Transmission held on July 19–20, 2012 in Washington, DC.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Hong SY, Nachega JB, Kelley K, Bertagnolio S, Marconi VC, and Jordan MR: The global status of HIV drug resistance; clinical and public health approaches for detection, treatment, and prevention. Infect Disord Drug Targets 2011;11(92):124–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UNAIDS Global Report 2012 UNAIDS Report on the global AIDS. www.unaids.org/globalreport/global_report.htm
- 3.Fowler MG, Lampe MA, Jamieson DJ, Kourtis AP, and Rogers MF: Reducing the risk of mother-to-child human immunodeficiency virus transmission: Past successes, current progress and challenges, and future directions. Am J Obstet Gynecol 2007;197(3 Suppl):S3–S9 [DOI] [PubMed] [Google Scholar]
- 4.World Health Organisation: Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: Towards a universal access. Recommendations for a public health approach. 2006version.
- 5.Ekouevi DK, Tonwe-Gold B, and Dabis F: Advances in the prevention of mother-to-child of HIV-1 infection in resource-limited settings. AIDS Res Hum Retroviruses 2005;15(9):479–480,487–493. [PubMed] [Google Scholar]
- 6.Nkengasong JN, Adjie-Toure , and Weidle PJ: HIV antiretroviral drug resistance in Africa. AIDS Rev 2004;6:4–12 [PubMed] [Google Scholar]
- 7.Broder S: The development of antiretroviral therapy and its impact on the HIV-1/AIDS pandemic. Antiviral Res 2010;85(1):1–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor BS, Sobieszczyk ME, McCutchan FE, and Hammer SM: The challenge of HIV-1 subtype diversity. N Engl J Med 2008;358:1590–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arrive E, Newell M-L, Ekouevi DK, et al. : Prevalence of resistance to nevirapine in mothers and children after single dose exposure to prevent vertical transmission of HIV-1: A meta-analysis. Int J Epidemiol 2007;36(5):1009–1021 [DOI] [PubMed] [Google Scholar]
- 10.Paredes R, Marconi VC, Lockman S, Abrams EJ, and Kuhn L: Impact of antiretroviral drugs in pregnant women and their children in Africa: HIV resistance and treatment outcomes. J Infect Dis 2013;207(S2):S93–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett DE, Myatt M, Bertagnolio , Sutherland D, and Gilks CF: Recommendations for surveillance of transmitted HIV drug resistance in countries scaling up antiretroviral treatment. Antivir Ther 2008;13:25–36 [PubMed] [Google Scholar]
- 12.National Agency for the Control of AIDS (NACA): Federal Republic of Nigeria. Global AIDS Response. Country Progress Report. Nigeria GARPR 2012. www.unaids.org/en/dataanalysis/knowyourresponse/countryprogressreports/2012countries/Nigeria
- 13.UNICEF 2010 PMTCT: Facts Sheets of Nigeria. www.unicef.org/aids/files/Nigeria_PMTCTFactsheet_2010.pdf
- 14.Ajoge HO, Gordon ML, Ibrahim S, Shiite OS, Ndung'u T, and Olonitola SO: Drug resistance pattern of HIV type 1 isolates sampled in 2007 from therapy-naïve pregnant women in north-central Nigeria. AIDS Res Hum Retroviruses 2012;28:115–118 [DOI] [PubMed] [Google Scholar]
- 15.Chaplin B, Eisen G, Idoko J, et al. : Impact of HIV subtype on drug resistance mutation in Nigerian patients failing first-line therapy. AIDS Res Hum Retroviruses 2010;20:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ojesina AI, Sankalé J-L, Odaibo G, et al. : Subtype-specific patterns in HIV-1 reverse transcriptase and protease in Oyo state, Nigeria: Implications for drug resistance and host response. AIDS Res Hum Retroviruses 2006;22(8):770–779 [DOI] [PubMed] [Google Scholar]
- 17.Ugbena R, Aberle-Grasse J, Diallo K, et al. : Virological response and HIV drug resistance 12 months after antiretroviral therapy initiation at 2 clinics in Nigeria. Clin Infect Dis 2012;54(S4):S375–S380 [DOI] [PubMed] [Google Scholar]
- 18.Hamers RL, Wallis CL, Kityo C, Siwale M, et al.: HIV-1 drug resistance in antiretroviral-naïve individuals in sub-Sahara Africa after rollout of antiretroviral therapy: A multicentre observational study. Lancet Infect Dis 2011;11:750–759 [DOI] [PubMed] [Google Scholar]
- 19.Bussmann H, de la Hoz Gomez F, Roels TH, Wester RC, Boldika SM, et al. : Prevalence of transmitted HIV drug resistance in Botswana: Lesson learned from the HIVDR-threshold survey conducted among women presenting for routine antenatal care as part of the 2007 national sentinel survey. AIDS Res Hum Retroviruses 2011;27:365–373 [DOI] [PubMed] [Google Scholar]
- 20.Tsabalala M, Manasa J, Zijenah LS, et al. : Surveillance of transmitted antiretroviral drug resistance among HIV-1 infected women attending antenatal clinics in Chitungwiza, Zimbabwe. PloS One 2011;6:e21241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ndembi N, Hamers RL, Sigaloff KCE, Lyagoba F, Magambo B, et al.: Transmitted antiretroviral drug resistance among newly infected diagnosed young individuals in Kampala. AIDS 2011;25:905–910 [DOI] [PubMed] [Google Scholar]
- 22.Myatt M. and Bennett DE. A novel sequential sampling technique for the surveillance of transmitted HIV drug resistance by cross-sectional survey for use in low resource settings. Antivir Ther 2008;13(Suppl 2):37–48 [PubMed] [Google Scholar]
- 23.Bennett DE, Camacho RJ, Otelea D, et al. : Drug resistance mutations for surveillance of transmitted HIV-1 drug resistance. 2009 update. PloS One 2009;4:e4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamoto K. and Aberle-Grasse J: Malawi HIV drug resistance task force. Surveillance of transmitted HIV drug resistance with the World Health Organization threshold survey method in Lilongwe, Malawi. Antivir Ther 2008;13(Suppl 2):83–87 [PubMed] [Google Scholar]
- 25.Laeyendecker O, Brookmeyer R, Mullis C, et al. : Specificity of four laboratory approaches for cross-sectional HIV incidence determination: Analysis of samples from adults with known non-recent HIV infection from five African countries. AIDS Res Hum Retroviruses 2012;28(10):1177–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kasang C, Kalluvya S, Majinge C, Stich A, Bodem J, Kongola G, et al.: HIV drug resistance (HIVDR) in antiretroviral therapy-naïve patients in Tanzania not eligible for WHO threshold HIVDR survey is dramatically high. PLos One 2011;6:e23091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO: 2007, Guidance on provider-initiated HIV testing and counselling in health facilities
- 28.Meloni S, Sankale JL, Odaibo G, Olaleye D, and Kanki P: A Nigeria-specific cluster of HIV-1 subtype G is sustained in full-length genome sequencing. 13th Conference on Retroviruses and Opportunistic Infections, Session 66 poster abstract session [Google Scholar]
- 29.Ceccarelli L, Salpini R, and Moudourou S: Characterization of drug resistance mutations in naïve and ART-treated patients infected with HIV-1 in Yaounde, Cameroon. J Med Virol 2012;84(5):721–727 [DOI] [PubMed] [Google Scholar]
- 30.Mosha F, Urassa W, Aboud S, Lyamuya E, Sandstrom E, Bredell H, and Williamson C: Prevalence of genotypic resistance to antiretroviral drugs in treatment-naive youths infected with diverse HIV type 1 subtypes and recombinant forms in Dar es Salaam, Tanzania. AIDS Res Hum Retroviruses 2011;27(4):377–382 [DOI] [PubMed] [Google Scholar]
- 31.Colafigli M, Torti C, Trecarichi EM, et al. : Evolution of transmitted HIV-1 drug resistance in HIV-1-infected patients in Italy from 2000 to 2010. Clin Microbiol Infect 2012;18(8):E299–E304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vercauteren J, Wensing AM, van de Vijver DA, et al. : Transmission of drug-resistant HIV-1 is stabilizing in Europe. J Infect Dis 2009;(10):1503–1508 [DOI] [PubMed] [Google Scholar]
- 33.Rahim S, Fredrick LM, da Silva BA, Bernstein B, and King MS: Geographic and temporal trends of transmitted HIV-1 drug resistance among antiretroviral-naïve subjects screening for two clinical trials in North America and Western Europe. HIV Clin Trials 2009;10(2):94–103 [DOI] [PubMed] [Google Scholar]
- 34.Truong HM, Kellogg TA, McFarland W, Louie B, Klausner JD, Philip SS, and Grant RM: Sentinel surveillance of HIV-1 transmitted drug resistance, acute infection and recent infection. PLoS One 2011;6(10):e25281.(1–7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sankale JL, Langevin S, Odaibo G, Meloni ST, Ojesinal AI, Olaleye D, and Kanki P: The complexity of circulating HIV type 1 strains in Oyo state, Nigeria. AIDS Res Hum Retroviruses 2007;23(8):1020–1025 [DOI] [PubMed] [Google Scholar]
- 36.Wainberg MA. and Brenner BG: The role of subtype diversity in development of resistance to antiviral drugs. Viruses 2010;2:2493–2508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirsch MS, Gunthard HF, Schapiro JM, Brun-Vezinet F, Clotet B, Hammer SM, Johnson VA, Kuritzkes DR, Mellors JW, Pillay D, Yeni PG, Jacobsen DM, and Richman DD: Antiretroviral drug resistance testing in adult HIV-1 infection: 2008 recommendations of an international AIDS society–USA Panel. Clin Infect Dis 2008;47:266–285 [DOI] [PubMed] [Google Scholar]
- 38.Imade GE, Sagay AS, Musa J, et al. : Declining rates of maternal HIV infection detected at delivery settings in north central Nigeria. AIDS 2012-International AIDS Conference, July22–27, Washington, DC Abstract No. TUPE209. [Google Scholar]