Abstract
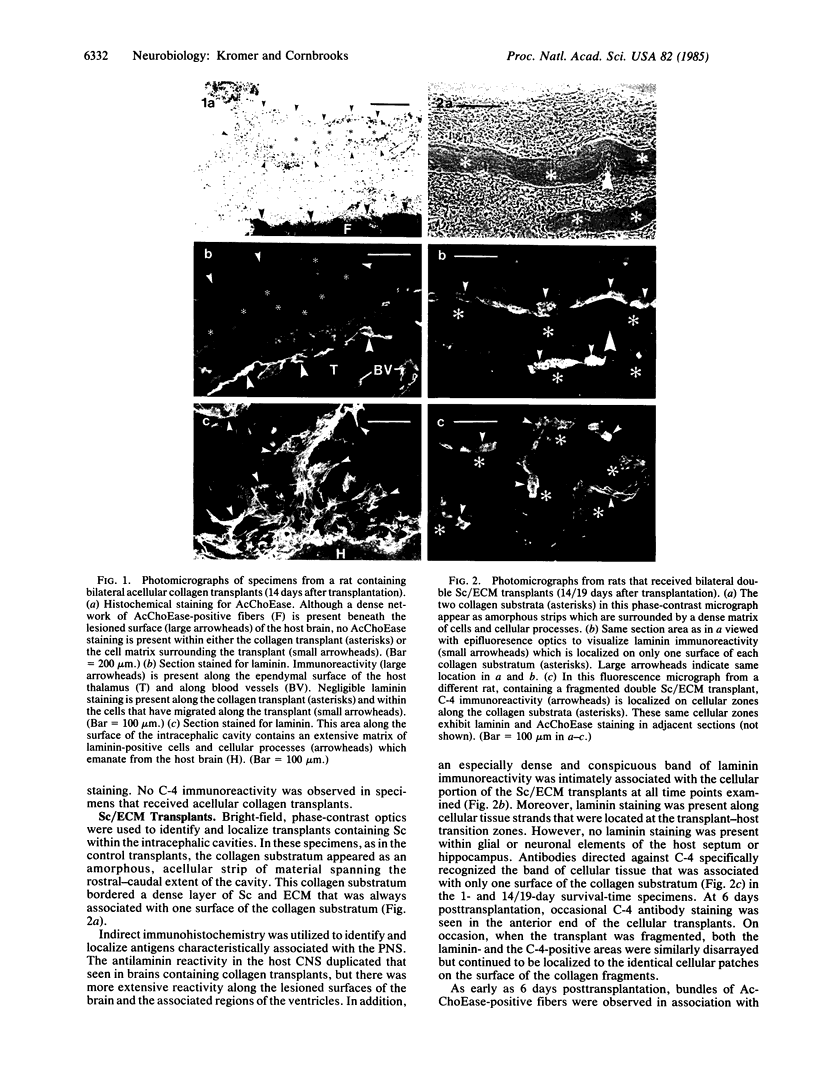
Transplantation of embryonic brain tissue or mature peripheral nerves into the adult mammalian central nervous system promotes axonal regrowth from axotomized central nervous system neurons; however, the cellular origin and molecular nature of the factors promoting axonal growth in vivo are unknown. To further characterize cellular environments that facilitate regeneration of central nervous system axons, we developed a methodology whereby cultured cell preparations can be transplanted into the brain of mature mammals. For this procedure, lesions are produced in the septal-hippocampal system of adult rats, and selected regions from collagen-supported Schwann cell/neuron cultures (consisting of Schwann cells, extracellular matrix, and degenerating neuronal processes and myelin but devoid of neuronal perikarya and fibroblasts) are positioned within the intracephalic cavity so that they bridge the lesion gap (approximately 3 mm) separating the septum and hippocampus. At various time up to 3 weeks after transplantation, specimens were prepared for acetylcholinesterase histochemistry and the immunocytochemical localization of laminin (an extracellular matrix protein) and C-4 (a Schwann cell membrane antigen). All specimens (from uninjured controls and from animals with either acellular collagen or mature Schwann cell/extracellular matrix transplants) contained laminin immunoreactivity associated with the meninges, choroid plexus, ependyma, and cerebral blood vessels. All animals with transplants showed prominent laminin staining on astrocytic processes along the intracephalic cavity, but only the Schwann cell/extracellular matrix transplants exhibited dense laminin and C-4 immunoreactivity within the cellular portion of the transplants. Regeneration of acetylcholinesterase-positive septal fibers occurred only in animals containing Schwann cell/extracellular matrix transplants. By 6 days after transplantation, acetylcholinesterase-positive fibers were observed both on laminin-positive cellular tissue strands connecting the septum and the Schwann cell/extracellular matrix transplants and on the initial portions of the transplants. By day 14, acetylcholinesterase-positive fibers traversed the entire lesion cavity in intimate association with the laminin- and C-4-positive cellular layer of the transplants and reinnervated the host hippocampus. However, cholinergic fibers were not associated with all laminin-containing processes along the lesion cavity nor did they grow along acellular collagen transplants. These results indicate the presence of factors in transplants of cultured Schwann cells and their associated extracellular matrix that promote rapid regeneration of central nervous system cholinergic axons in vivo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler R., Manthorpe M., Skaper S. D., Varon S. Polyornithine-attached neurite-promoting factors (PNPFs). Culture sources and responsive neurons. Brain Res. 1981 Feb 9;206(1):129–144. doi: 10.1016/0006-8993(81)90105-0. [DOI] [PubMed] [Google Scholar]
- Adler R., Varon S. Neuritic guidance by nonneuronal cells of ganglionic origin. Dev Biol. 1981 Aug;86(1):69–80. doi: 10.1016/0012-1606(81)90316-x. [DOI] [PubMed] [Google Scholar]
- Barde Y. A., Lindsay R. M., Monard D., Thoenen H. New factor released by cultured glioma cells supporting survival and growth of sensory neurones. Nature. 1978 Aug 24;274(5673):818–818. doi: 10.1038/274818a0. [DOI] [PubMed] [Google Scholar]
- Björklund A., Stenevi U. Regeneration of monoaminergic and cholinergic neurons in the mammalian central nervous system. Physiol Rev. 1979 Jan;59(1):62–100. doi: 10.1152/physrev.1979.59.1.62. [DOI] [PubMed] [Google Scholar]
- Bunge R. P., Wood P. Studies on the transplantation of spinal cord tissue in the rat. I. The development of a culture system for hemisections of embryonic spinal cord. Brain Res. 1973 Jul 27;57(2):261–276. doi: 10.1016/0006-8993(73)90135-2. [DOI] [PubMed] [Google Scholar]
- Collins F. Induction of neurite outgrowth by a conditioned-medium factor bound to the culture substratum. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5210–5213. doi: 10.1073/pnas.75.10.5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornbrooks C. J., Carey D. J., McDonald J. A., Timpl R., Bunge R. P. In vivo and in vitro observations on laminin production by Schwann cells. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3850–3854. doi: 10.1073/pnas.80.12.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S., Aguayo A. J. Axonal elongation into peripheral nervous system "bridges" after central nervous system injury in adult rats. Science. 1981 Nov 20;214(4523):931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- Edgar D., Timpl R., Thoenen H. The heparin-binding domain of laminin is responsible for its effects on neurite outgrowth and neuronal survival. EMBO J. 1984 Jul;3(7):1463–1468. doi: 10.1002/j.1460-2075.1984.tb01997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre-Bauman A., Puymirat J., Loudes C., Barret A., Tixier-Vidal A. Laminin promotes attachment and neurite elongation of fetal hypothalamic neurons grown in serum-free medium. Neurosci Lett. 1984 Jan 27;44(1):83–89. doi: 10.1016/0304-3940(84)90225-8. [DOI] [PubMed] [Google Scholar]
- Fallon J. R. Preferential outgrowth of central nervous system neurites on astrocytes and Schwann cells as compared with nonglial cells in vitro. J Cell Biol. 1985 Jan;100(1):198–207. doi: 10.1083/jcb.100.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUTH L. Regeneration in the mammalian peripheral nervous system. Physiol Rev. 1956 Oct;36(4):441–478. doi: 10.1152/physrev.1956.36.4.441. [DOI] [PubMed] [Google Scholar]
- Guth L. History of central nervous system regeneration research. Exp Neurol. 1975 Sep;48(3 Pt 2):3–15. doi: 10.1016/0014-4886(75)90168-5. [DOI] [PubMed] [Google Scholar]
- Ide C., Tohyama K., Yokota R., Nitatori T., Onodera S. Schwann cell basal lamina and nerve regeneration. Brain Res. 1983 Dec 12;288(1-2):61–75. doi: 10.1016/0006-8993(83)90081-1. [DOI] [PubMed] [Google Scholar]
- Kromer L. F., Björklund A., Stenevi U. Innervation of embryonic hippocampal implants by regene-rating axons of cholinergic septal neurons in the adult rat. Brain Res. 1981 Apr 6;210(1-2):153–171. doi: 10.1016/0006-8993(81)90892-1. [DOI] [PubMed] [Google Scholar]
- Kromer L. F., Björklund A., Stenevi U. Regeneration of the septohippocampal pathways in adult rats is promoted by utilizing embryonic hippocampal implants as bridges. Brain Res. 1981 Apr 6;210(1-2):173–200. doi: 10.1016/0006-8993(81)90893-3. [DOI] [PubMed] [Google Scholar]
- Lander A. D., Fujii D. K., Gospodarowicz D., Reichardt L. F. Characterization of a factor that promotes neurite outgrowth: evidence linking activity to a heparan sulfate proteoglycan. J Cell Biol. 1982 Sep;94(3):574–585. doi: 10.1083/jcb.94.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesi P., Kaakkola S., Dahl D., Vaheri A. Laminin is induced in astrocytes of adult brain by injury. EMBO J. 1984 Mar;3(3):683–686. doi: 10.1002/j.1460-2075.1984.tb01867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthorpe M., Engvall E., Ruoslahti E., Longo F. M., Davis G. E., Varon S. Laminin promotes neuritic regeneration from cultured peripheral and central neurons. J Cell Biol. 1983 Dec;97(6):1882–1890. doi: 10.1083/jcb.97.6.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthorpe M., Varon S., Adler R. Neurite-promoting factor in conditioned medium from RN22 Schwannoma cultures: bioassay, fractionation, and properties. J Neurochem. 1981 Sep;37(3):759–767. doi: 10.1111/j.1471-4159.1982.tb12552.x. [DOI] [PubMed] [Google Scholar]
- Noble M., Fok-Seang J., Cohen J. Glia are a unique substrate for the in vitro growth of central nervous system neurons. J Neurosci. 1984 Jul;4(7):1892–1903. doi: 10.1523/JNEUROSCI.04-07-01892.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popiela H., Porter T., Beach R. L., Festoff B. W. Peripheral nerve extract promotes long-term survival and neurite outgrowth in cultured spinal cord neurons. Cell Mol Neurobiol. 1984 Mar;4(1):67–77. doi: 10.1007/BF00710943. [DOI] [PubMed] [Google Scholar]
- Richardson P. M., McGuinness U. M., Aguayo A. J. Axons from CNS neurons regenerate into PNS grafts. Nature. 1980 Mar 20;284(5753):264–265. doi: 10.1038/284264a0. [DOI] [PubMed] [Google Scholar]
- Rogers S. L., Letourneau P. C., Palm S. L., McCarthy J., Furcht L. T. Neurite extension by peripheral and central nervous system neurons in response to substratum-bound fibronectin and laminin. Dev Biol. 1983 Jul;98(1):212–220. doi: 10.1016/0012-1606(83)90350-0. [DOI] [PubMed] [Google Scholar]
- Sanes J. R., Marshall L. M., McMahan U. J. Reinnervation of muscle fiber basal lamina after removal of myofibers. Differentiation of regenerating axons at original synaptic sites. J Cell Biol. 1978 Jul;78(1):176–198. doi: 10.1083/jcb.78.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonfeld A. R., Katzman R. Autoradiographic demonstration of the central origin of regenerating fibers into iris tissue implants in the rat mesencephalon. Brain Res. 1980 Sep 22;197(2):355–363. doi: 10.1016/0006-8993(80)91121-x. [DOI] [PubMed] [Google Scholar]
- Smalheiser N. R., Crain S. M., Reid L. M. Laminin as a substrate for retinal axons in vitro. Brain Res. 1984 Jan;314(1):136–140. doi: 10.1016/0165-3806(84)90184-6. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Tsuji S. On the chemical basis of thiocholine methods for demonstration of acetylcholinesterase activities. Histochemistry. 1974;42(1):99–110. doi: 10.1007/BF00498482. [DOI] [PubMed] [Google Scholar]
- Varon S., Adler R. Nerve growth factors and control of nerve growth. Curr Top Dev Biol. 1980;16:207–252. doi: 10.1016/s0070-2153(08)60157-x. [DOI] [PubMed] [Google Scholar]
- Veraa R. P., Grafstein B. Cellular mechanisms for recovery from nervous system injury: a conference report. Exp Neurol. 1981 Jan;71(1):6–75. doi: 10.1016/0014-4886(81)90071-6. [DOI] [PubMed] [Google Scholar]
- Wessells N. K., Letourneau P. C., Nuttall R. P., Ludueña-Anderson M., Geiduschek J. M. Responses to cell contacts between growth cones, neurites and ganglionic non-neuronal cells. J Neurocytol. 1980 Oct;9(5):647–664. doi: 10.1007/BF01205031. [DOI] [PubMed] [Google Scholar]
- Wood P. M. Separation of functional Schwann cells and neurons from normal peripheral nerve tissue. Brain Res. 1976 Oct 22;115(3):361–375. doi: 10.1016/0006-8993(76)90355-3. [DOI] [PubMed] [Google Scholar]
- de la Torre J. C. Catecholamine fiber regeneration across a collagen bioimplant after spinal cord transection. Brain Res Bull. 1982 Jul-Dec;9(1-6):545–552. doi: 10.1016/0361-9230(82)90162-9. [DOI] [PubMed] [Google Scholar]