Abstract
To elucidate the prevalence of HIV-1 subtypes and transmitted drug resistance in Henan, central China, HIV-1-positive blood samples from 187 antiretroviral-naive patients were collected in our study from August 2009 to November 2010. Subtype B′ (92.0%, 172 of 187) remains the predominant HIV-1 subtype in Henan province and was prevalent in all risk populations and geographic regions. Of 98 pol sequences 67 (68.4%) harbored drug resistance mutations, and only 14 (14.3%, 14 of 98) sequences have mutations associated with significantly reduced phenotypic susceptibility to antiretroviral drugs. The unexpectedly high percentage of drug resistance in Henan province is mainly due to the prevalence of minor mutations in the protease and integrase regions, especially A71T/V and L68V/I/IM/LV. In all, we detected a relatively high prevalence of drug resistance with unique mutation distributions among antiretroviral-naive patients from Henan province.
Human immunodeficiency virus type-1 (HIV-1) infection in China is a significant contributor to the global HIV-1 epidemic. In 2011, there were an estimated 780,000 people infected with HIV in China with 28,000 mortalities from AIDS and 48,000 new infections.1 To attenuate the increasing HIV infection and improve the quality of life for AIDS patients, the “four free and one care” program, launched by the Chinese government in 2003, provides free antiretroviral therapy (ART) to HIV/AIDS patients. By the end of September 2011, 332,996 patients had been treated, with 132,879 currently in treatment.1
The widespread use of ART has greatly reduced the HIV/AIDS-related mortality rate and prolonged survival for AIDS patients. Unfortunately, ART has been largely accompanied by the emergence of drug resistance mutations.2 Many treated patients develop resistance to one or more drugs, which may lead to treatment failure and patient death.3 Furthermore, the drug resistance variants may be transmitted to newly infected individuals, causing the emergence of transmitted drug resistance (TDR), which may seriously limit therapeutic options in resource-limited areas. Therefore, TDR surveillance is important to guide the optimal selection of antiviral drugs.
Henan province, located in central China, has one of the highest prevalence rates of AIDS in China. In the early 1990s, commercial plasma donation became a popular way to supplement meager incomes among rural farmers and resulted in untold numbers of HIV infection among former plasma donors (FPDs).4 Although commercial plasma donation was interrupted with the introduction and enforcement of a law banning commercial blood collection and blood products in 1996, thousands of people have been infected with HIV-1.5,6 Free ART was initiated in 2002 in Henan province and was rapidly scaled up nationwide over the next several years.7,8
In 2010, 8 years after implementing the free ART scaling-up program in Henan province, we conducted a survey to determine the prevalence of HIV drug resistance among antiretroviral-naive patients and provided recent data on the circulation of HIV-1 strains in Henan province. These findings are crucial for the further advancement of the free ART program in Henan province and can assist public health workers in implementing new ART policies in China.
Between August 2009 and November 2010, 187 HIV-1-positive blood samples were collected from six cities in Henan province, and the distribution of these subjects is shown in Fig. 1. This study was approved by the Committee for Human Research in the Center for Disease Prevention and Control (CDC) of Henan province. The clinical and epidemiological backgrounds of these patients were collected through epidemiological investigation by trained interviewers and supplied by the local CDC. The subjects included 113 males, 70 females, and 4 unrecorded sex volunteers with an age range of 5–74 years (mean: 40.97 years). All 187 subjects were serologically determined to be HIV-1 positive without HIV-2 infection detected. Baseline CD4+ T cell counts were obtained in 77 study subjects (mean: 324.91 cell/μl, range: 2–996 cell/μl).
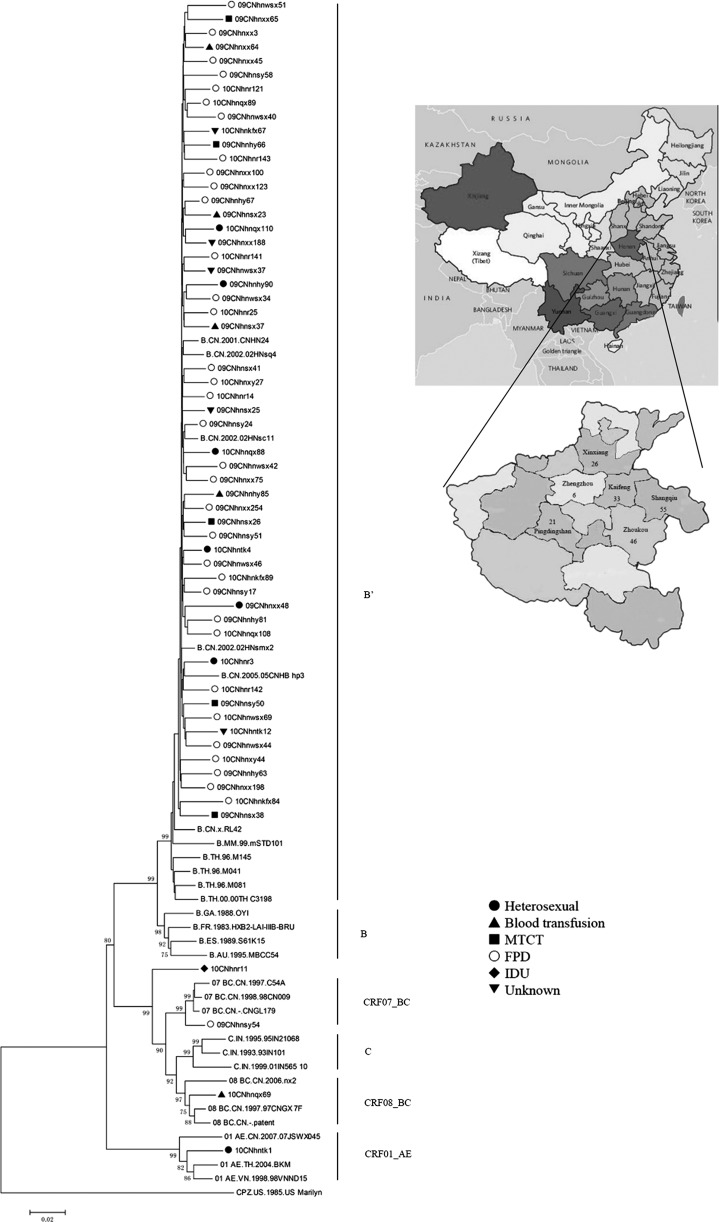
FIG. 1.
Neighbor-joining tree of HIV-1 sequences from Henan based on the 2.9-kb HIV-1 pol region (HXB2: 2244–5096 nt). The SIVcpzUS sequence is used as an outgroup. For clarity, only certain representative sequences are shown. Bootstrap values greater than 70 are shown at their corresponding nodes. Symbols represent different risk groups: ●, heterosexual; ▲, blood transfusion; ■, mother-to-child transmission (MTCT); ○, former plasma donor (FPD); ◆, injecting drug user (IDU); ▼, unknown. A map of the People's Republic of China and the geographic distribution of our specimens in Henan are depicted in the upper right corner.
Viral RNA was extracted from 200 μl of HIV-1-positive plasma specimens using the high Pure Viral RNA kit (Roche, Basel, Switzerland) according to the manufacturer's protocol. The 1.5-kb gag, 2.9-kb pol, and 1.1-kb partial env (the C2-V5 regions) sequences were determined by direct sequencing using the products amplified from plasma RNA using reverse transcriptase polymerase chain reaction (RT-PCR). In all, 129 gag genes (70.0%), 98 pol genes (52.4%), and 74 partial env C2-V5 genes (39.6%) were successfully obtained. All nucleotide sequences were screened using the BLAST program (National Center for Biotechnology Information, Bethesda, MD) to search for similarities compared with previously reported sequences in the databases and eliminate potential laboratory errors. All sequences were aligned with the Clustal X 2.0 Program and optimized by hand using the BioEdit program 7.0.9. Phylogenetic analyses were performed in gag, pol, and env C2-V5 regions by the neighbor-joining method implemented by the MEGA5.0 program based on the Kimura's two-parameter model.
The topologic reliability of the tree was evaluated by bootstrap analyses with 1,000 replicates. Bootscan analysis was used to assess the recombinant breakpoints. To understand the prevalence of HIV-1 TDR in these subjects, the 98 pol sequences were submitted to the HIVdb program, version 6.2.0, in the HIV Drug Resistance Database from Stanford University (http://hivdb.stanford.edu/). A chi-squared test was performed to detect significant differences of the frequency of specific amino acid positions in different subtypes or different risk groups. All statistical analyses were performed with Microsoft Excel software and statistical significance was defined as p<0.05.
The phylogenetic analyses based on the gag, pol, and env C2-V5 regions showed that 172 of the 187 samples were sorted into subtype B′ (92.0%) and that 15 (8.0%) samples were sorted into non-B subtypes. CRF01_AE (5/187, 2.7%), CRF07_BC (4/187, 2.1%), CRF08_BC (2/187, 1.1%), and subtype B (1/187, 0.5%) were also identified. In addition to these known subtypes and circulating recombinant forms (CRFs), three (1.6%) unique recombinant forms (URFs) were identified in our study. 10CNhnr11 [female injecting drug user (IDU)], placed outside known HIV-1 genotypes, was identified as a unique subtype B/C recombinant. 10CNhnr124 (male heterosexual) and 10CNhnr129 (male heterosexual) (data not shown) were also identified as unique B/C and C/CRF01_AE recombinants based on the recombination analysis. In contrast to the results from a previous report, our results indicate that the HIV-1 subtype B′, which is found in all risk populations and geographic regions, remains the predominant subtype in Henan province.9 In such a region where subtype B′ is predominant, the introduction of URFs could lead to the circulation of more complex recombinants with potentially different biological properties.
Figure 1 shows the neighbor-joining tree based on the 2.9-kb pol region. Subtype B′ from Henan province forms a distinct monophyletic subcluster, and intermingles with reference strains from Hubei province, which is located in central China with a large numbers of FPDs. The most basal position of the B′ clade of Henan province was occupied by subtype B′ strains from Thailand, Myanmar, and Yunnan province, indicating that subtype B′ from central China is likely a descendant lineage of subtype B′ from Yunnan and Thailand, consistent with previous findings by Li et al.10 Subtype B′ sequences from different transmission routes intermingled in the B′ cluster without a clear pattern of risk group clustering, representing tight transmission relationships among B′ strains found in Henan from different risk groups.
The current survey of HIV TDR in 98 antiretroviral-naive patients in Henan province indicated that TDR was occurring at a high rate (68.4%, 67 of 98), with 34.7% (34 of 98) for protease inhibitors (PIs), 5.1% (5 of 98) for nucleoside reverse transcriptase inhibitors (NRTIs), 11.2% (11 of 98) for nonnucleoside reverse transcriptase inhibitors (NNRTIs), and 42.9% (42 of 98) for integrase inhibitors (INIs). The detailed distribution of mutations is listed in Table 1.
Table 1.
Prevalence of Drug Resistance Mutations
| Virus subtype | ||||
|---|---|---|---|---|
| Mutations | B′ | Non-B′ | Total | Incidence (%) |
| PIs | ||||
| Major resistance mutations | ||||
| V82A | 1 | 1 | 1.0 | |
| Minor resistance mutations | ||||
| I47M | 2 | 2 | 2.0 | |
| A71T/V | 31 | 2 | 33 | 33.7 |
| NRTIs | ||||
| M41L | 1 | 1 | 1.0 | |
| K65R | 1 | 1 | 1.0 | |
| D67N | 2 | 2 | 2.0 | |
| K70R | 1 | 1 | 1.0 | |
| M184I | 2 | 2 | 2.0 | |
| L210W | 1 | 1 | 1.0 | |
| T215F/Y | 2 | 2 | 2.0 | |
| K219E | 1 | 1 | 1.0 | |
| NNRTIs | ||||
| K103N/S | 5 | 5 | 5.1 | |
| V106A | 1 | 1 | 1.0 | |
| V179D | 1 | 2 | 3 | 3.1 |
| Y188F/L | 2 | 2 | 2.0 | |
| G190A | 1 | 1 | 1.0 | |
| K238T | 1 | 1 | 1.0 | |
| N348I | 1 | 1 | 1.0 | |
| INIs | ||||
| Minor resistance mutations | ||||
| L68V/I/IM/LV | 40 | 40 | 40.8 | |
| T97A | 1 | 1 | 1.0 | |
| G163R | 1 | 1 | 1.0 | |
Drug resistance mutations potentially conferring resistance to PIs, protease inhibitors; NRTIs, nucleoside reverse transcriptase inhibitors; NNRTIs, nonnucleoside reverse transcriptase inhibitors; INIs, integrase inhibitors.
Among the detected reverse transcriptase inhibitors (RTIs)-related mutations, the prevalence of drug resistance for NRTIs and NNRTIs was 5.1% (5 of 98) and 11.2% (11 of 98), respectively, with eight NRTI-related mutations in five patients and seven NNRTI-related mutations in 11 patients. All of the mutations presented at low frequencies, and the K103N/S mutation was the most frequent (5.1%, 5 of 98). All the RTI-related mutations were detected among patients infected by subtype B′, except for one N348I and two V179D, which were detected in non- B′ subtype-infected patients. We found that almost every RTI drug-resistant strain carried a unique mutation pattern. Because of the wide use of RTIs in Henan province, it is possible that the presence of RTI-related mutations may be the result of drug selection.
Notably, primary HIV drug resistance mutations to PIs were found in 34 samples (34.7%), with one single major mutation at V82A, which confers low resistance to atazanavir and nelfinavir. A71T/V, the most predominant minor mutation in protease regions, was observed in 33.7% (31 of 92) of B′ strains and 33.3% (2 of 6) of the non- B′ strains. However, the two A71T mutations in the non-B′ strains were located at B segments of B/C recombinants based on SimPlot analysis. The B segment of 10CNhnr11, which contains A71T, was identified as subtype B′ based on phylogenetic analysis. Another B segment of URF 10CNhnr124, which contains A71T, could not have its B′ subtype status determined, because it was a short recombinant segment. The substitution of A71T/V often appears in subtype B.11 I47M, another minor mutation, was detected in two patients who were infected with subtype B′.
Our results also showed a high prevalence (42.9%, 42 of 98) of sequences from our subjects harboring INI-related drug resistance mutations, and no major INI-related mutations were found. A minor INI-related mutation, L68V/I/IM/LV, was present in 40 of these sequences. Two other minor INI-related mutations, T97A and G163R, were present in one patient each. All INIs-related mutations were detected among B′-infected patients. A high prevalence of HIV-1 TDR mutations, particularly minor drug resistance mutations to PIs and INIs, was observed among subtype B′-infected patients, indicating a special pattern for the prevalence of HIV drug resistance in Henan province.
Furthermore, the prevalence of the minor mutation A71T/V was higher in B′/FPDs (21 of 59, 35.6%) than in B′/non-FPDs (6 of 24, 25.0%), though this difference was not significant (p>0.05). However, a significant difference in the frequency of the L68V/I/IM/LV mutation was found between B′/FPD (32 of 59, 54.2%) and B′/non-FPD (6 of 24, 25.0%) (p<0.05). Subtype B′ is a single founder strain responsible for a series of HIV-1 outbreaks among FPDs in central China, which may explain the high prevalence of A71T/V and L68V/I/IM/LV in both subtype B′ and FPDs.10,12 Because PIs and INIs were seldom used in Henan province, these PIs and INI-related TDR mutations may be derived from resistant variants in foreign countries instead of drug selection, and their prevalence may accompany the epidemic of HIV-1 among FPDs in Henan province. This hypothesis may explain the different prevalence of TDR between our results and a previous report among antiretroviral-naive patients in Henan province that focused on newly HIV-1 diagnosed patients recruited from 2007 to 2008.13
Minor drug resistance mutations are not always associated with a decrease in drug susceptibility but can compensate for the fitness of resistant mutants. Some studies have reported that the combination of A71T, M89I, and L90M resulted in higher resistance to PIs,14 and that L68V/I made HIV strains more resistant to INIs, elvitegravir and raltegravir, when combined with E92Q or Q148R, another primary integrase mutation.15 If PIs or INIs are used in Henan province in the future, particularly among FPDs, the detailed effect of these two minor mutations on the development of drug resistance should be monitored before commencing further ART.
As a large-scale survey of HIV-1 drug resistance based on pol regions among antiretroviral-naive HIV-infected patients in Henan province, our results show that the overall prevalence of TDR is 68.4%. We propose that the unexpectedly high percentage (61.2%, 60 of 98) of minor mutations in the PR and IN regions resulted in the high prevalence of TDR mutations in our subjects. This prevalence of minor mutations mainly resulted from the high proportion of FPDs recruited in our research. Even if the minor mutations are excluded, there are 14 (14.3%, 14 of 98) sequences associated with significantly reduced phenotypic susceptibility to antiretroviral drugs, still higher than the 3.8% HIV drug resistance rate among 676 treatment-naive HIV patients in a nationwide investigation16 and the 7.6% among 145 antiretroviral-naive patients from 2006 to 2007 in Beijing.17 And the percentage is similar to the 12% HIV drug resistance rate reported for 55 HIV-1 newly diagnosed and treatment-naive patients recruited from 2010 to 2011.18
In conclusion, the present study demonstrates that subtype B′ is the predominant HIV subtype and detected a relatively high prevalence of TDR mutations among antiretroviral-naive individuals in Henan province. These findings are crucial for the design of further free ART program guidelines and indicate that strategies to reduce the dissemination of HIV-1 drug resistance variants are urgently needed.
Sequence data
The GenBank accession numbers of the nucleotide sequences reported in this article are KC987958 ∼ KC988259.
Acknowledgments
This work was supported by a grant from the Key National Science and Technology Program in the twelfth Five-Year Plan Period of China (2012ZX10001-002) and the Key Laboratory on Emerging Infectious Diseases and Biosafety in Wuhan.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Estimates for the HIV/AIDS Epidemic in China; P.R. China, 2011 [Google Scholar]
- 2.Liu J, Chen X, Xie Q, et al.: Drug resistance and HCV coinfection in former blood donors infected with HIV type 1 in China. AIDS Res Hum Retroviruses 2011;27(8):925–930 [DOI] [PubMed] [Google Scholar]
- 3.Wittkop L, Gunthard HF, de Wolf F, et al.: Effect of transmitted drug resistance on virological and immunological response to initial combination antiretroviral therapy for HIV (EuroCoord-CHAIN joint project): A European multicohort study. Lancet Infect Dis 2011;11(5):363–371 [DOI] [PubMed] [Google Scholar]
- 4.Wu Z, Liu Z, and Detels R: HIV-1 infection in commercial plasma donors in China. Lancet 1995;346(8966):61–62 [DOI] [PubMed] [Google Scholar]
- 5.Blood Products Regulation Act; P.R. China, December30, 1996 [Google Scholar]
- 6.Ji G, Detels R, Wu Z, and Yin Y: Correlates of HIV infection among former blood/plasma donors in rural China. AIDS 2006;20(4):585–591 [DOI] [PubMed] [Google Scholar]
- 7.Zhang F, Haberer JE, Wang Y, et al.: The Chinese free antiretroviral treatment program: Challenges and responses. AIDS 2007;21(Suppl 8):S143–148 [DOI] [PubMed] [Google Scholar]
- 8.Zhang FJ, Pan J, Yu L, Wen Y, and Zhao Y: Current progress of China's free ART program. Cell Res 2005;15(11–12):877–882 [DOI] [PubMed] [Google Scholar]
- 9.Zhao F, Wang Z, and Li WJ: Human immunodeficiency virus type 1 subtypes prevalence in central China. Yonsei Med J 2009;50(5):644–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Z, He X, Wang Z, et al.: Tracing the origin and history of HIV-1 subtype B’ epidemic by near full-length genome analyses. AIDS 2012;26(7):877–884 [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Yue J, Wu S, and Yan Y: Polymorphisms and drug resistance analysis of HIV-1 CRF01_AE strains circulating in Fujian Province, China. Arch Virol 2007;152(10):1799–1805 [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Uenishi R, Hase S, et al.: Explosive HIV-1 subtype B’ epidemics in Asia driven by geographic and risk group founder events. Virology 2010;402(2):223–227 [DOI] [PubMed] [Google Scholar]
- 13.Yuan Y, Cao XL, Liu HW, et al.: [Study on the transmission of drug resistant human immunodeficiency virus-1 in Henan province]. Zhonghua Yu Fang Yi Xue Za Zhi 2009;43(11):956–959 [PubMed] [Google Scholar]
- 14.Gonzalez LM, Santos AF, Abecasis AB, et al.: Impact of HIV-1 protease mutations A71V/T and T74S on M89I/V-mediated protease inhibitor resistance in subtype G isolates. J Antimicrob Chemother 2008;61(6):1201–1204 [DOI] [PubMed] [Google Scholar]
- 15.Goodman D, Hluhanich R, Waters J, et al.: Integrase inhibitor resistance involves complex interactions among primary and secondary resistance mutations: A novel mutation L6V/I associated with E92Q and increases resistance. Antivir Ther 2008;13(3):A15 [Google Scholar]
- 16.Liao L, Xing H, Shang H, et al.: The prevalence of transmitted antiretroviral drug resistance in treatment-naive HIV-infected individuals in China. J Acquir Immune Defic Syndr 2010;5( Suppl 1):S10–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye JR, Lu HY, Wang WS, et al.: The prevalence of drug resistance mutations among treatment-naive HIV-infected individuals in Beijing, China. AIDS Res Hum Retroviruses 2012;28(4):418–423 [DOI] [PubMed] [Google Scholar]
- 18.Xue XJ, Xing H, Cui WG, et al.: [Prevalence of HIV-1 drug resistance from those newly confirmed cases in Henan province, 2010–2011]. Zhonghua Liu Xing Bing Xue Za Zhi 2012;33(9):989–990 [PubMed] [Google Scholar]