Abstract
Coronary heart disease (CHD) is characterized by abnormal intercellular communication and circulating microRNAs (miRNAs) are likely involved in this process. Here, we show that CHD was associated with changes in the transport of circulating miRNA, particularly decreased miRNA enrichment in microparticles (MPs). Additionally, MPs from CHD patients were less efficient at transferring miRNA to cultured HUVECs, which correlated with their diminished capacity to bind developmental endothelial locus-1 (Del-1). In summary, CHD was associated with distinct changes in circulating miRNA transport and these changes may contribute to the abnormal intercellular communication that underlies CHD initiation and progression.
Keywords: Coronary Heart Disease, Atherosclerosis, Intercellular signaling, microRNA, Microparticles, Developmental Endothelial Locus-1
Introduction
Atherosclerosis is a chronic inflammatory disease characterized by abnormal cellular interactions between leukocytes, platelets, endothelial, and smooth muscle cells. These interactions lead to the production of inflammatory cytokines, such as tumor necrosis factor α (TNF α)[1], which, in turn, potentiate inflammatory signals between and within cells. Intercellular signaling, therefore, is a critical aspect of atherosclerotic plaque formation, impacting not only the initiation of coronary heart disease (CHD), but also its progression[2].
MicroRNAs (miRNAs) are short, single-stranded, non-coding RNAs that modulate intracellular gene expression through posttranscriptional regulation of targeted mRNAs[3,4,5]. In vivo and in vitro studies have demonstrated that extracellular miRNAs, in a manner suggestive of paracrine or endocrine signaling, can be shuttled between cells to orchestrate a diverse array of cellular responses[6,7,8]. These findings, together with observations by our group[9] and others that circulating miRNA levels are altered in different pathological states[10,11,12,13], point to the importance of circulating miRNA transport in normal physiology and disease
Extracellular miRNAs have been identified in serum and plasma and are associated with distinct transport modalities, including encapsulation within membrane-bound vesicles[14,15,16] (microparticles, exosomes) or bound to circulating proteins and lipoproteins[8,17]. Specific miRNAs have been shown to be preferentially enriched in particular transport modalities and this enrichment can be altered by disease[8,16,18,19]. In the present study, we investigated the impact of coronary heart disease (CHD) on the transport characteristics of a small subset of 8 miRNAs whose abundances are known to be altered in CHD (miR-17, miR-19a, miR-21, miR-92a, miR-126, miR-146a, miR-222, and miR-223) [20]. Our findings indicate that CHD was primarily associated with decreased miRNA loading of MPs as well as their transmission to recipient cells, which, taken together, may contribute to the abnormal intercellular signaling that accompanies CHD initiation and progression.
Materials and Methods
Sample Population
Frozen human serum samples from healthy subjects (n = 27), patients with significant CHD (n = 21) and patients with only CHD risk factors (n = 20) were obtained from the Emory Cardiovascular Biobank. Blood from patients with significant CHD and individuals with only CHD risk factors was collected through an arterial sheath placed during elective cardiac catheterization. Blood from healthy subjects was collected by venipuncture at the time of enrollment in the Biobank data base. Healthy donors met the following criteria: 1) 18 – 64 yrs old; 2) no history of major systemic medical conditions/diseases; 3) no major modifiable risk factors for CHD; 4) no prescription medications or hospitalizations within one year of serum collection. Patients with significant CHD were individuals with > 50% stenosis in ≥ 1 epicardial coronary artery documented by coronary angiogram. Analysis of angiograms was based on visual estimates by two independent operators, who utilized the modified form of the AHA/ACC classification of the coronary tree[21,22]. Patients with only CHD risk factors exhibited similar clinical characteristics as CHD donors, except none had an epicardial coronary artery with > 50% stenosis. The clinical characteristics of the three study groups are shown in Table 1. The study was approved by the Institutional Review Board at Emory University, Atlanta, GA, USA. All subjects provided written informed consent at the time of enrollment.
Table I.
Characteristics of Study Subjects
| Healthy Subjects (n =27) | Significant CHD (n = 21) | CHD Risk Factors Only (n = 20) | |
|---|---|---|---|
|
| |||
| Gender Male | 10 (37%) | 12 (57%) | 8(40%) |
|
| |||
| Race White | 17 (63.0%) | 16 (76.2%) | 15 (75%) |
|
| |||
| Age (years) | 46.68 ± 13.86### | 64.14 ± 11.75 | 58.60 ± 9.31 |
|
| |||
| Significant CHD (> 50%) | 0### | 100% | 0### |
|
| |||
| Hypertension | 0### | 16 (76.2%) | 15 (75.0%) |
|
| |||
| Active Smoker | 1 (3.7%) | 1 (4.8%) | 1 (5.0%) |
|
| |||
| Adipositas (BMI > 25)* | 5 (18.5%)### | 16 (94.1%) | 20 (100%) |
|
| |||
| Diabetes mellitus | 0# | 7 (33.3%) | 4 (20%) |
|
| |||
| History of (AMI, PCI, Stroke)*** | 0### | 13 (61.9%) | 1 (5%)### |
|
| |||
| Concurrent Medication: | |||
| Aspirin / Plavix | 0### | 20 (95.2%) | 17 (85%) |
| ACE-Inhibitor / ARB | 0### | 19 (90.5%) | 16 (80%) |
| Statin therapy | 0### | 19 (90.5%) | 15 (75%) |
BMI = Body Mass Index
PCI = Percutaneous Coronary Intervention
p < 0.05
p < 0.01
p < 0.001 significance vs. CHD (ANOVA, Bonferroni Posthoc)
Serum Ultracentrifugation
Serum samples were thawed at 37°C. After thawing, serum was diluted 1:3 with phosphate buffered saline (PBS). Using a previously described ultracentrifugation protocol[16] (Supplemental Fig. S1), diluted serum was separated into four distinct fractions: a microparticle fraction, an exosome fraction, an aggregated protein fraction that contained miRNAs complexed with proteins such as Argonaut 2 (Ago2)[17], and a lipoprotein fraction, containing miRNA-lipoprotein complexes. Fractions were characterized by flow cytometry for annexin V, CD63, and CD81 staining in MPs and exosomes; lipid/lipoprotein/apolipoprotein contamination was determined for each fraction (Supplemental Fig. S1).
Quantitative qRT-PCR Analysis for miRNA Enrichment
MiRNA was isolated using the commercially available miRNeasy isolation kit according to manufacturer’s protocol (Qiagen Inc, Valencia, CA). MiRNA reverse transcription was performed using the TaqMan microRNA Reverse transcription Kit (Life Technologies, Foster City, CA). TaqMan microRNA assays (Life Technologies) were performed using the 7500 Fast Real-Time PCR System at the 9600 emulation run mode. Quantitative PCR was carried out using a StepOne real-time thermocycler (Applied Biosystems, San Francisco, CA). MiRNA detection was performed using specific primer pairs and TaqMan probes, which were predesigned by Applied Biosystems. Fluorescence data were quantified using the mak3 module of the qpcR software library in the R environment[23,24,25]. Quantitative results were normalized to the average values of miR-346 as an internal control. This miRNA was found suitable for normalization because it met the threshold level of gene variability [26] and presented minimal changes between different disease samples and serum fractions (Supplemental Fig. S2). MiRNA abundance in a particular serum fraction was calculated by dividing the normalized quantitative levels of miRNA in a particular serum fraction by the sum of the normalized quantitative levels of the same miRNA obtained for all four serum fractions [% of Total = (miRNA serum fraction × / ΣmiRNA all serum fractions) × 100].
qRT-PCR Analysis for miRNA Relative Expression
Reverse transcription was performed on miRNA isolated from serum using the TaqMan microRNA Reverse transcription Kit. Ct values of the analyzed miRNAs were converted into relative expression levels (relative expression = 2(−Ct)). Normalized relative expression values for miRNAs were calculated by dividing the relative expression of each miRNA by the relative expression obtained for miR-346.
Transfer of Circulating miRNA to Cultured Human Endothelial Cells
Human umbilical vein endothelial cells (HUVECs), passages 2 – 5, were grown on 75 cm2 cell culture flasks (Corning Inc, Corning, NY). Cells were split onto 6-well cell culture plates (Corning) 1 day prior to miRNA transfer experiments. On the day of experiments, cell confluence was confirmed by visual estimation and media in each well was replaced with 1 mL of fresh medium in addition to 100 μL of MPs (re-suspended in PBS) or PBS (as control). HUVECs were incubated at 37°C for 2 h. After incubation, media was removed and HUVECs were washed rigorously with PBS then harvested. MiRNA was isolated from HUVECs using the mirVana miRNA isolation kit (Life Technologies) according to manufacturer’s protocol and relative miRNA expression was quantified using qRT-PCR analysis. Intracellular miR-223 and miR-92a abundance in treated HUVECs was represented as a fold change over the miRNA abundance measured in HUVECs that were incubated with PBS only. Transfer index was defined as intracellular miRNA abundance divided by the qRT-PCR-determined relative expression of the same miRNA as measured in a 100 μL volume of MPs.
ELISA for Circulating Del-1 in Human Sera
A commercially available sandwich ELISA kit for Developmental endothelial locus-1 (Del-1) (Cusabio via Antibodies-Online, Atlanta, GA) was used, according to manufacturer’s protocol, to assess circulating Del-1 levels in unfractionated human sera.
Flow Cytometry Analysis of Microparticles
Using 10 μm Flow-Count fluorescent beads (Beckman Coulter), MPs were counted by flow cytometry, according to the previously described protocol[27]. Del-1 expression in MPs isolated from human sera was assessed as previously described[28]. In brief, MPs were incubated with a primary antibody to Del-1 (rabbit polyclonal EDIL3, Abcam, Cambridge, MA), followed by a FITC-tagged secondary antibody (goat polyclonal anti-rabbit, Abcam). FITC mean fluorescence intensity (MFI) was analyzed by flow cytometry and results were reported as MFI per MP count. In order to quantify available phosphatidylserine (PS) on serum MPs, isolated MPs were labeled with FITC-tagged annexin V (BD Biosciences, San Jose, CA). FITC MFI was subsequently analyzed by flow cytometry, and the results were reported as MFI per MP count.
Quantification of Microparticle Interaction with Recombinant Del-1
MPs isolated from human sera were incubated with varying concentrations of recombinant Del-1 (R&D Systems) for 30 minutes at 37°C. Subsequently, MPs were incubated with FITC-labeled annexin V, according to manufacturer’s protocol (BD Biosciences). FITC MFI was quantified via flow cytometry. Results were reported as MFI per MP count and plotted as a function of initial recombinant Del-1 concentration (μg Del-1 / MP) to produce a Del-1 binding curve. To quantify the relative efficiency of the Del-1/MP interaction, the slope of the linear region of the Del-1 binding curve was calculated using GraphPad Prism statistical software tools.
Statistical Analysis
Statistical analyses were performed using the GraphPad Prism software package (GraphPad Software Inc, LaJolla, CA). Values are expressed as means ± SEM. Statistical tests, including the unpaired Student t test, analysis of variance (ANOVA) and the Bonferroni posthoc test were used to compare data. P ≤ 0.05 was considered statistically significant.
Results
CHD-Associated Abnormalities in miRNA Distribution Are Exhibited Primarily in Microparticles
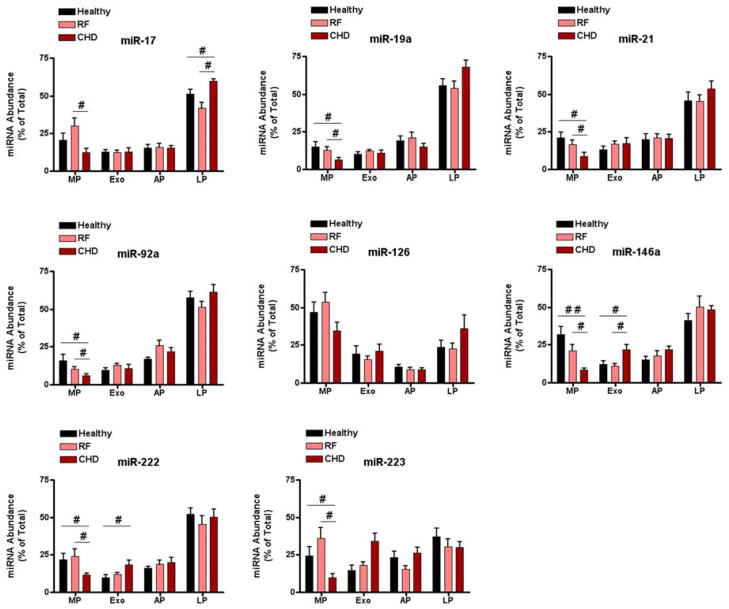
Coronary heart disease (CHD) is associated with changes in circulating levels of miRNA[20], so we examined whether the distribution of miRNAs across different transport modalities was also affected by disease. CHD-associated differences in miRNA distribution were detected for 7 out of 8 miRNAs assessed; these differences were observed primarily in the microparticle (MP) fraction of human sera (Figure 1). The enrichment of miR-17, -19a, -21, -92a, -146a, -222, and -223 in MPs was significantly lower in serum obtained from CHD patients compared to serum obtained from healthy subjects or patients with CHD risk factors only. For the three groups assessed, miR-126 was the only miRNA that showed no significant differences in distribution across all four circulating miRNA transport modalities. Interestingly, several miRNAs that had similar levels in unfractionated sera of healthy subjects compared to patients with significant CHD (Supplemental Fig. S3) showed significant differences in their distribution across distinct fractions (Figure 1). Taken together, these results show that significant CHD was associated with changes in the transport profile of miRNAs, and these changes were observed predominantly in the MP fraction.
Figure 1. Circulating miRNA distributions across distinct serum fractions.
Serum samples were obtained from healthy subjects (n = 8, black bars), individuals with risk factors for CHD (n = 8, pink bars) and individuals with significant CHD (n = 8, red bars) were separated into distinct fractions: MP – microparticles; Exo – exosomes; AP – aggregated proteins; LP – lipoprotein. MiRNA was isolated from the fractions and quantified by qRT-PCR analysis; miR-346 was used for normalization. MiRNA abundance in each fraction was calculated as described in materials and methods and is represented as % of total miRNA. #p < 0.05, ##p < 0.01 (ANOVA, Bonferroni Posthoc). CHD risk factor group and significant CHD group were not significantly different in terms of age, gender, CHD risk factors and medications.
Although there were differences in miRNA distribution between healthy subjects and patients with significant CHD, no such differences were seen between healthy subjects and patients with CHD risk factors only (Figure 1). Moreover, differences in miRNA distribution were detected between patients with CHD risk factors only and those with significant CHD. Taken together, these findings suggest that miRNA distribution was associated with disease progression, as opposed to differences in age, gender, or medication. For this reason, subsequent analysis included healthy subjects and patients with significant CHD. This allowed the use of extreme cases, in terms of CHD phenotype, to assess the impact of disease on circulating miRNA transport.
CHD is Associated with Decreased miRNA Loading of Circulating MPs
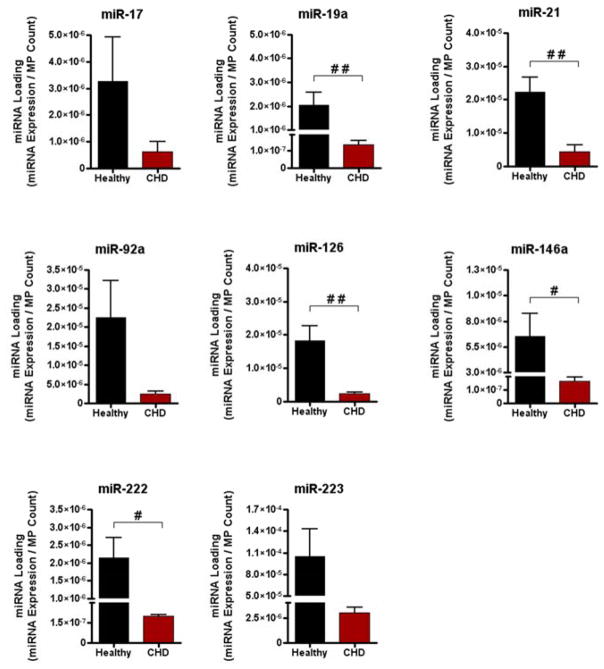
Circulating MPs are thought to play a role in intercellular signaling because they contain a variety of biological components, including proteins and RNA molecules[29], and can effectively transfer these components from one cell type to the next[30,31,32]. Here, CHD-associated decreases in miRNA enrichment were seen primarily in the MP fraction of human serum; this phenomenon occurred largely in the absence of significant increases in miRNA enrichment in the other fractions. One possible explanation for this observation is decreased miRNA loading of circulating MPs in patients with CHD. We found that for a majority of the miRNAs studied, miRNA content per MP was significantly decreased in MPs isolated from the sera of patients with significant CHD, (Figure 2). These data suggest that the CHD-associated decrease in miRNA enrichment of the MP fraction was due to decreased miRNA loading of circulating MPs.
Figure 2. MPs isolated from CHD patients are loaded with less miRNA compared to MPs isolated from healthy subjects.
MiRNA loading in MPs was calculated by dividing relative miRNA expression by average MP count, as described in Materials and Methods (healthy, n = 8; CHD, n = 8). #p < 0.05, ##p < 0.01 (Student t test).
MiRNA transfer from Microparticles to Cultured HUVECs
To test whether miRNA-containing MPs isolated from human sera can transmit miRNA to recipient cells, human umbilical vein endothelial cells (HUVECs) were incubated with serum-isolated MPs for 2 h (Figure 3A). We utilized qRT-PCR analysis to assess the transmission of miRNA from isolated MPs to cultured HUVECs. The 2 h time point was chosen to minimize the possibility of MP-induced changes in miRNA transcription that are independent of MP uptake, which can occur within a 4 – 8 h time period[33]. Intracellular levels of miR-92a and miR-223 were assessed in treated HUVECs. MiR-92a and miR-223 were chosen because of their high expression levels in serum (Supplemental Fig. S4). Intracellular levels of miR-92a and miR-223 were higher in HUVECs treated with MPs compared to HUVECs treated with PBS (Figure 3A), thus supporting transfer of MP content to cultured HUVECs.
Figure 3. MPs from healthy subjects transfer miRNA to cultured endothelial cells more efficiently than MPs from CHD patients.

A) MP-mediated miRNA transfer to cultured HUVECs. HUVECs were incubated at 37°C for 2 h with MPs obtained from the sera of healthy subjects or with PBS (as control). After incubation, intracellular levels of miR-92a and miR-223 were quantified using qRT-PCR analysis. B) Impact of CHD on MP-mediated miRNA transfer. HUVECs were incubated with MP obtained from the sera of healthy subjects or patients with significant CHD. After incubation, HUVECs were harvested and intracellular miR-92a and miR-223 were assessed. The miRNA transfer index was calculated from qRT-PCR-based measurement of intracellular miRNA abundance, as described in Materials and Methods (Healthy n = 4, CHD n = 4 for panels A, B). #p < 0.05 (Student t test), *p < 0.05 (ANOVA, Bonferroni Posthoc).
Next, we assessed whether MPs from healthy subjects, compared to MPs from patients with significant CHD, differed in their ability to transmit miRNA to cultured HUVECs. MPs from healthy subjects or CHD patients, respectively, were incubated with cultured HUVECs for 2 h. In parallel, relative miRNA expression levels were measured in MPs isolated from healthy subjects and CHD patients, respectively. After incubation, uptake of miR-92a and miR-223 was assessed. MPs isolated from CHD patients had a significantly lower capacity for transfer miR-92a and miR-223 to cultured HUVECs compared to MPs isolated from healthy subjects (Figure 3B).
MPs from CHD Patients Have Less Del-1
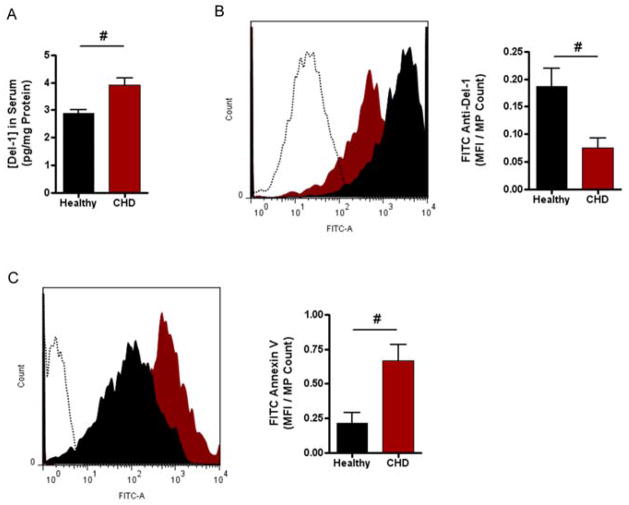
The decreased ability of MPs isolated from patients with significant CHD to transfer miRNA to cultured HUVECs suggests that MP uptake was impaired in the disease state. Developmental endothelial locus-1 (Del-1) is a 52-kDa bridging glycoprotein (also known as EDIL3) that is secreted by endothelial cells[34]. Del-1 has been shown to mediate endothelial uptake of MPs, in vivo, via a phosphatidylserine-mediated mechanism[28]. To investigate whether CHD is associated with changes in Del-1 expression, levels of Del-1 in unfractionated sera were assessed. We found a significantly higher amount of Del-1 in the sera of CHD patients (3.9 ± 0.2 pg/mg total protein) than we did in the sera of healthy subjects (2.9 ± 0.1 pg/mg total protein; P < 0.05) (Figure 4A). However, when we used flow cytometry to measure Del-1 bound to the surface of MPs isolated from human sera, we found that MPs from CHD patients had significantly less Del-1 bound than MPs from healthy subjects (Figure 4B).
Figure 4. MPs from CHD patients have altered Del-1 expression compared to MPs from healthy subjects.
A) Del-1 in whole sera. The concentration of circulating Del-1 in whole sera from healthy subjects and CHD patients, respectively, was analyzed using a sandwich ELISA kit. B) Del-1 expression on surface of circulating MPs. MPs isolated from healthy subjects and CHD patients were incubated with a primary antibody to Del-1 followed by a FITC-tagged, anti-rabbit secondary antibody. FITC mean fluorescence intensity (MFI) was analyzed by flow cytometry (left: histogram profiles; healthy = filled black; CHD = filled red; PBS control = dotted line; right: mean grouped data +/− SEM). C) Available phosphatidylserine on surface of MPs of circulating MPs. MPs isolated from healthy subjects and CHD patients were incubated with a primary antibody to Annexin V followed by a FITC-tagged, anti-rabbit secondary antibody. FITC mean fluorescence intensity (MFI) was analyzed by flow cytometry. Right panel is mean grouped data +/− SEM. n = 6 for each group in panels A, B; n = 4 for each group in panel C. #p < 0.05 (Student t test).
Del-1 binds to phosphatidylserine on the external surface of MPs, which is an important part of the mechanism by which it mediates MP uptake by endothelial cells[35,36]. Based on the observed differences in Del-1 bound to MPs, we hypothesized that MPs from CHD patients would have more available PS on their surface. The abundance of available (i.e unbound) phosphatidylserine on the surface of MPs isolated from healthy subjects and CHD patients, respectively, was assessed by annexin V staining and flow cytometry analysis. MPs isolated from CHD patients had a significantly higher amount of available PS compared to MPs isolated from healthy subjects (Figure 4C).
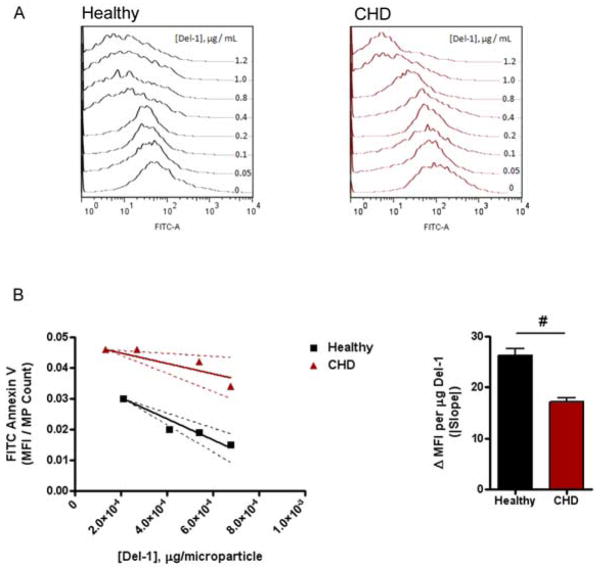
These results suggest that even in the presence of increased concentrations of circulating Del-1, MPs from sera of CHD patients did not bind to circulating Del-1 as efficiently as did MPs from healthy subjects. To further examine this possibility, we incubated MPs from CHD patients and healthy subjects, respectively, with increasing concentrations of recombinant Del-1. After Del-1 incubation, MPs were stained with FITC-labeled annexin V to assess the amount of unbound PS that remained on surface of the MPs. The resulting flow cytometry histograms revealed distinct annexin binding responses for MPs isolated from CHD patients compared to those isolated from healthy subjects (Figure 5A). MPs from healthy subjects, when incubated with increasing concentrations of recombinant Del-1, showed an abrupt decrease in fluorescence at concentrations above 0.4 μg/ml Del-1, suggesting saturation of Del-1 binding above this concentration. In contrast, MPs from CHD patients exhibited a more gradual decrease in fluorescence, which only appreciably changed above a Del-1 concentration of 0.8 μg/ml.
Figure 5. MPs from CHD patients interact abnormally with recombinant Del-1 protein.
A) Binding of recombinant Del-1 to MPs from healthy subjects and patients with significant CHD. MPs from healthy subjects or CHD patients were incubated with increasing concentrations of recombinant Del-1 protein then stained with FITC-labeled annexin V; representative flow cytometry histograms are shown. B) Binding efficiency of recombinant Del-1 to MPs from healthy subjects and patients with CHD. The slope of the linear region of the Del-1 binding curve (showing the 95% CI) estimates the efficiency of Del-1 binding to PS on MPs. (n = 10 for each group in panel A; n = 12 for each group in panel B (pooled samples). #p < 0.05 (Student t test).
Binding curves describing the relationship between average fluorescence per microparticle and Del-1 concentration were generated from the flow cytometry data. The slope of the linear region of the curve was taken as an estimate of the efficiency of recombinant Del-1 binding to PS on MPs (Figure 5B). The binding efficiency of recombinant Del-1 was significantly less for MPs isolated from CHD patients (slope = −17.1 ± 3.8) compared to MPs isolated from healthy subjects (slope = −34.44 ± 3.1; P< 0.05), indicating that MPs from CHD patients interact less avidly with Del-1 compared to MPs from healthy subjects. This finding offers a potential mechanism for the difference in MP-mediated miRNA transfer that was observed between MPs from healthy subjects and CHD patients: Del-1 binding to MPs is impaired in CHD sera, thereby leading to decreased uptake by recipient cells.
Discussion
Selective packaging of miRNAs into distinct transport modalities is likely an important factor in the biological function of secreted miRNAs[8,37]. Here, we show that CHD, a disease that is characterized by abnormal cell-to-cell communication, altered circulating miRNA transport. In particular, this study is the first to demonstrate CHD-associated changes in miRNA distribution across different circulating miRNA transport modalities. Moreover, we show that CHD was associated with diminished miRNA loading into MPs and miRNA transfer from MPs to recipient cells.
The mechanisms responsible for the uptake of extracellular miRNAs from the circulation are dependent on specific molecular interactions. For example, the uptake of miRNA bound to HDL is mediated through the scavenger receptor class B type I (SR-BI)[8]. Additionally, the glycoproteins Del-1 and lactedherin mediate the clearance of circulating MPs by endothelial cells and splenic macrophages, respectively, via bridging interactions with αvβ3 integrin on the cell and phosphatidylserine on the MP[28]. Given that MPs facilitate the transfer of circulating miRNA between cells[38,39], changes in the membrane composition of MPs likely influence miRNA-mediated intercellular communication.
In this study, we present data suggesting that CHD altered the membrane interactions of circulating MPs, leading to impaired MP-mediated miRNA transfer. Despite increased circulating levels of Del-1 in patients with CHD, there was less Del-1 bound to MPs from these patients. Recombinant Del-1 demonstrated a lower affinity for MPs from CHD patients compared to those from healthy subjects, indicating that CHD-associated alterations in MP function (i.e. interaction with Del-1) were at least partly responsible for differences in miRNA transfer to endothelial cells. It is also possible that CHD is associated with a defect in Del-1, although this was not assessed here. Further studies are required to fully characterize the impact of CHD on the morphological properties and surface protein composition of circulating MPs.
Although the results presented in this study offer a novel mechanism by which coronary heart disease alters miRNA-mediated intercellular communication, it is important to note the study’s limitations. Firstly, the subset of miRNAs that were assessed reflects only a select group of miRNAs and is by no means exhaustive. A high-throughput screening of more miRNAs is likely to reveal additional information about the effects of CHD on extracellular miRNA transport. Secondly, a repository of frozen human serum was utilized to develop protocols that focused on assessing extracellular miRNA transport. Although the freezing process did not induce significant losses in miRNA (Supplemental Fig. S5), serum storage at low temperatures has been associated with a decrease in annexin V+ labeled MPs. While this phenomenon is expected to equally affect all of the groups examined in the current study, the effect of freezing could potentially bias the MP population being analyzed by preferentially depleting annexin V+ labeled MPs[40].
Given the mechanism by which MPs are formed, the cellular source of circulating MPs is likely to be another factor that impacts MP surface composition[38,39] and therefore the ability of MPs to transfer miRNA to recipient cells. We conducted a preliminary assessment of the cellular origin of circulating MPs in the sera of healthy subjects (Supplemental Fig. S6) that indicated most MPs originate from platelets and red cells, with less than 20% originated from ECs and monocytes. However, a more in-depth analysis of the cellular origin of circulating MPs will be necessary to fully understand the impact of CHD on miRNA-mediated intercellular communication. Future studies will need to characterize not only the cellular source of the circulating microparticles and exosomes, but also how export of miRNAs from these cellular sources may be affected by disease progression.
The current study focused on serum, but there are likely differences in the MP population present in serum compared to that in plasma. Our analysis of plasma samples from healthy subjects and CHD patients showed that CHD-associated decreases in miRNA loading of MPs were also observed in plasma samples (Supplemental Fig. S7). Moreover, other studies have shown that miRNA levels are comparable between serum and plasma[10]. Nonetheless, a more comprehensive analysis of the effect of sample preparation and storage will be necessary to extrapolate the current findings to a diverse array of biological fluids.
In summary, the transfer of miRNA from one cell to another via the circulation is dependent on how circulating miRNAs are transported. CHD was associated with distinct changes in extracellular miRNA transport which can impact miRNA-mediated intercellular communication (Figure 6). The current study focused on the role of MPs, but, the impact of CHD on other miRNA transport modalities should not be ignored. Currently, there is a limited understanding of the molecular mechanisms that regulate miRNA sorting and export, and as a result, there may be unknown levels of connectivity, or inhibition, between the pathways that are responsible for extracellular miRNA transport. Future work will need to elucidate disease-associated molecular mechanisms responsible for the sorting and release of miRNAs into the circulation. The identification of the mechanisms responsible for disease-associated alterations in miRNA transport will likely enhance the use of extracellular miRNAs as biomarkers and novel therapeutics for disease.
Figure 6. Schema of the proposed impact of coronary heart disease on miRNA-mediated intercellular communication.
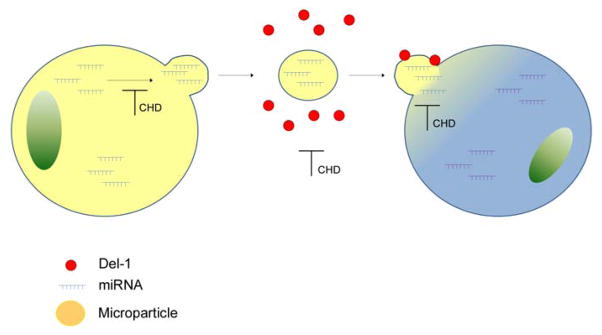
CHD impacts miRNA-mediated intercellular communication via three mechanisms: i) by decreasing miRNA loading of MPs, ii) by altering Del-1 binding to MPs, and iii) by decreasing MP uptake. Altered miRNA profile of MPs and decreased miRNA content in MPs suggests CHD-associated abnormalities within the donor cell. Impaired MP uptake is likely to impact the behavior and function of the target cell.
Supplementary Material
Highlights.
The circulating miRNA transport profile is altered in coronary heart disease (CHD) patients.
CHD is associated with decreased miRNA loading of microparticles (MPs).
CHD is associated with decreased transfer of miRNA from MPs to recipient cells.
MPs isolated from CHD patients have diminished capacity to bind Del-1.
Acknowledgments
Sources of funding:
This work was by NHLBI Program of Excellence in Nanotechnology (HHSN268201000043C to CDS), VA Merit Award (I01 BX000704 to CDS) and a NHLBI R01 Award (HL 109559 to CDS).
Abbreviations
- CHD
Coronary Heart Disease
- MP/MPs
Microparticles
- Del-1
Developmental Endothelial Locus-1
- Exo
Exosomes
- AP
Aggregated Protein
- LP
Lipoprotein
- RF
Risk Factor
Footnotes
Disclosures
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 2.Burnier L, Fontana P, Angelillo-Scherrer A, Kwak BR. Intercellular communication in atherosclerosis. Physiology (Bethesda) 2009;24:36–44. doi: 10.1152/physiol.00036.2008. [DOI] [PubMed] [Google Scholar]
- 3.Dennis C. The brave new world of RNA. Nature. 2002;418:122–124. doi: 10.1038/418122a. [DOI] [PubMed] [Google Scholar]
- 4.Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466:835–840. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 6.Aliotta JM, Pereira M, Johnson KW, de Paz N, Dooner MS, et al. Microvesicle entry into marrow cells mediates tissue-specific changes in mRNA by direct delivery of mRNA and induction of transcription. Exp Hematol. 2010;38:233–245. doi: 10.1016/j.exphem.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen TS, Lai RC, Lee MM, Choo AB, Lee CN, et al. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2010;38:215–224. doi: 10.1093/nar/gkp857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13:423–433. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weber M, Baker MB, Patel RS, Quyyumi AA, Bao G, et al. MicroRNA Expression Profile in CAD Patients and the Impact of ACEI/ARB. Cardiol Res Pract. 2011;2011:532915. doi: 10.4061/2011/532915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ai J, Zhang R, Li Y, Pu J, Lu Y, et al. Circulating microRNA-1 as a potential novel biomarker for acute myocardial infarction. Biochem Biophys Res Commun. 2010;391:73–77. doi: 10.1016/j.bbrc.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Corsten MF, Dennert R, Jochems S, Kuznetsova T, Devaux Y, et al. Circulating MicroRNA-208b and MicroRNA-499 reflect myocardial damage in cardiovascular disease. Circ Cardiovasc Genet. 2010;3:499–506. doi: 10.1161/CIRCGENETICS.110.957415. [DOI] [PubMed] [Google Scholar]
- 13.Tijsen AJ, Creemers EE, Moerland PD, de Windt LJ, van der Wal AC, et al. MiR423–5p as a circulating biomarker for heart failure. Circ Res. 2010;106:1035–1039. doi: 10.1161/CIRCRESAHA.110.218297. [DOI] [PubMed] [Google Scholar]
- 14.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 15.Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, et al. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285:17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang K, Zhang S, Weber J, Baxter D, Galas DJ. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res. 2010;38:7248–7259. doi: 10.1093/nar/gkq601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108:5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diehl P, Fricke A, Sander L, Stamm J, Bassler N, et al. Microparticles: major transport vehicles for distinct microRNAs in circulation. Cardiovasc Res. 2012;93:633–644. doi: 10.1093/cvr/cvs007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zipf GK. Selected Studies of the Prinicple of Relative Frequency in Language. Cambridge: Harvard University Press; 1932. p. 51. [Google Scholar]
- 20.Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, et al. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010;107:677–684. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- 21.Ryan TJ, Faxon DP, Gunnar RM, Kennedy JW, King SB, 3rd, et al. Guidelines for percutaneous transluminal coronary angioplasty. A report of the American College of Cardiology/American Heart Association Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures (Subcommittee on Percutaneous Transluminal Coronary Angioplasty) Circulation. 1988;78:486–502. doi: 10.1161/01.cir.78.2.486. [DOI] [PubMed] [Google Scholar]
- 22.Sianos G, Morel MA, Kappetein AP, Morice MC, Colombo A, et al. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1:219–227. [PubMed] [Google Scholar]
- 23.Boggy GJ, Woolf PJ. A mechanistic model of PCR for accurate quantification of quantitative PCR data. PLoS ONE. 2010;5:e12355. doi: 10.1371/journal.pone.0012355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruijter JM, Pfaffl MW, Zhao S, Spiess AN, Boggy G, et al. Evaluation of qPCR curve analysis methods for reliable biomarker discovery: bias, resolution, precision, and implications. Methods. 2013;59:32–46. doi: 10.1016/j.ymeth.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Ritz C, Spiess AN. qpcR: an R package for sigmoidal model selection in quantitative real-time polymerase chain reaction analysis. Bioinformatics. 2008;24:1549–1551. doi: 10.1093/bioinformatics/btn227. [DOI] [PubMed] [Google Scholar]
- 26.Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007;8:R19. doi: 10.1186/gb-2007-8-2-r19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jansen F, Yang X, Hoyer FF, Paul K, Heiermann N, et al. Endothelial microparticle uptake in target cells is annexin I/phosphatidylserine receptor dependent and prevents apoptosis. Arterioscler Thromb Vasc Biol. 2012;32:1925–1935. doi: 10.1161/ATVBAHA.112.253229. [DOI] [PubMed] [Google Scholar]
- 28.Dasgupta SK, Le A, Chavakis T, Rumbaut RE, Thiagarajan P. Developmental endothelial locus-1 (Del-1) mediates clearance of platelet microparticles by the endothelium. Circulation. 2012;125:1664–1672. doi: 10.1161/CIRCULATIONAHA.111.068833. [DOI] [PubMed] [Google Scholar]
- 29.Hugel B, Martinez MC, Kunzelmann C, Freyssinet JM. Membrane microparticles: two sides of the coin. Physiology (Bethesda) 2005;20:22–27. doi: 10.1152/physiol.00029.2004. [DOI] [PubMed] [Google Scholar]
- 30.Wang JG, Williams JC, Davis BK, Jacobson K, Doerschuk CM, et al. Monocytic microparticles activate endothelial cells in an IL-1beta-dependent manner. Blood. 2011;118:2366–2374. doi: 10.1182/blood-2011-01-330878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mesri M, Altieri DC. Endothelial cell activation by leukocyte microparticles. J Immunol. 1998;161:4382–4387. [PubMed] [Google Scholar]
- 32.Chen X, Liang H, Zhang J, Zen K, Zhang CY. Secreted microRNAs: a new form of intercellular communication. Trends Cell Biol. 2012;22:125–132. doi: 10.1016/j.tcb.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res. 2010;107:810–817. doi: 10.1161/CIRCRESAHA.110.226357. [DOI] [PubMed] [Google Scholar]
- 34.Hidai C, Zupancic T, Penta K, Mikhail A, Kawana M, et al. Cloning and characterization of developmental endothelial locus-1: an embryonic endothelial cell protein that binds the alphavbeta3 integrin receptor. Genes Dev. 1998;12:21–33. doi: 10.1101/gad.12.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willekens FL, Werre JM, Kruijt JK, Roerdinkholder-Stoelwinder B, Groenen-Dopp YA, et al. Liver Kupffer cells rapidly remove red blood cell-derived vesicles from the circulation by scavenger receptors. Blood. 2005;105:2141–2145. doi: 10.1182/blood-2004-04-1578. [DOI] [PubMed] [Google Scholar]
- 36.Hanayama R, Tanaka M, Miwa K, Nagata S. Expression of developmental endothelial locus-1 in a subset of macrophages for engulfment of apoptotic cells. J Immunol. 2004;172:3876–3882. doi: 10.4049/jimmunol.172.6.3876. [DOI] [PubMed] [Google Scholar]
- 37.Zhang C. MicroRNAs: role in cardiovascular biology and disease. Clin Sci (Lond) 2008;114:699–706. doi: 10.1042/CS20070211. [DOI] [PubMed] [Google Scholar]
- 38.Boulanger CM, Dignat-George F. Microparticles: an introduction. Arterioscler Thromb Vasc Biol. 2011;31:2–3. doi: 10.1161/ATVBAHA.110.220095. [DOI] [PubMed] [Google Scholar]
- 39.Dignat-George F, Boulanger CM. The many faces of endothelial microparticles. Arterioscler Thromb Vasc Biol. 2011;31:27–33. doi: 10.1161/ATVBAHA.110.218123. [DOI] [PubMed] [Google Scholar]
- 40.Gasper-Smith N, Crossman DM, Whitesides JF, Mensali N, Ottinger JS, et al. Induction of Plasma (TRAIL), TNFR-2, Fas Ligand, and Plasma Microparticles after Human Immunodeficiency Virus Type 1 (HIV-1) Transmission: Implications for HIV-1 Vaccine Design. Journal of Virology. 2008;82:7700–7710. doi: 10.1128/JVI.00605-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.