Abstract
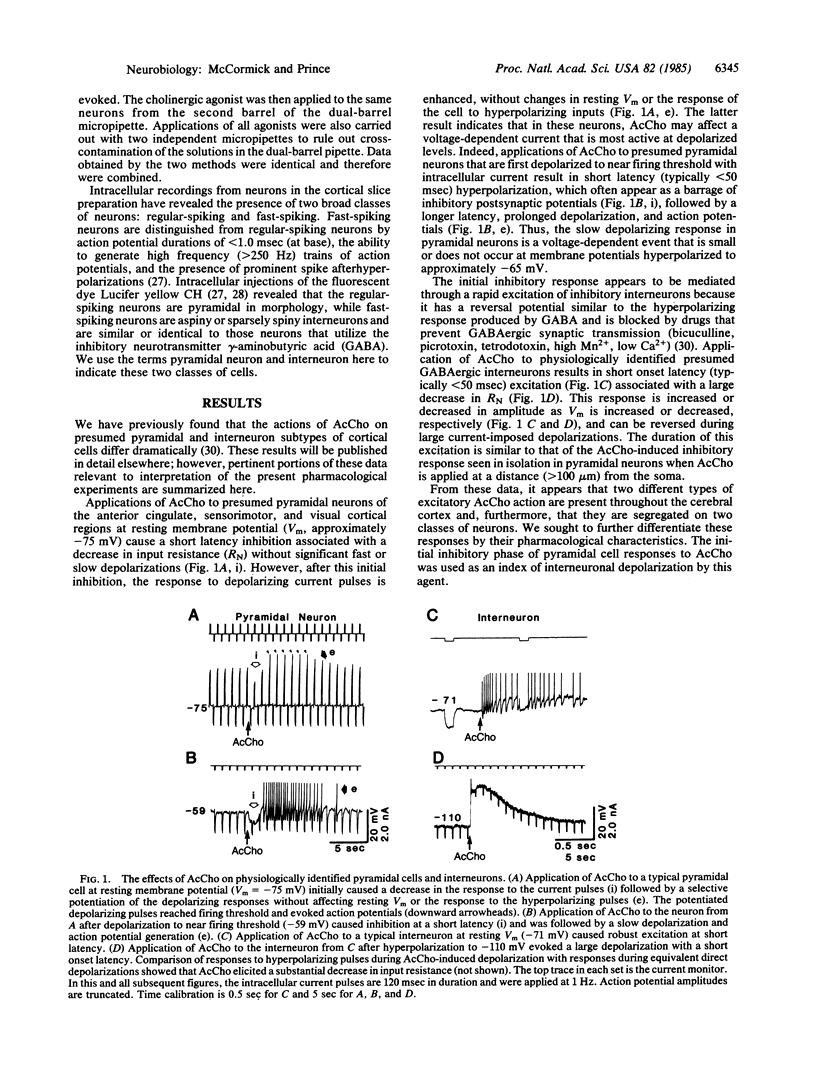
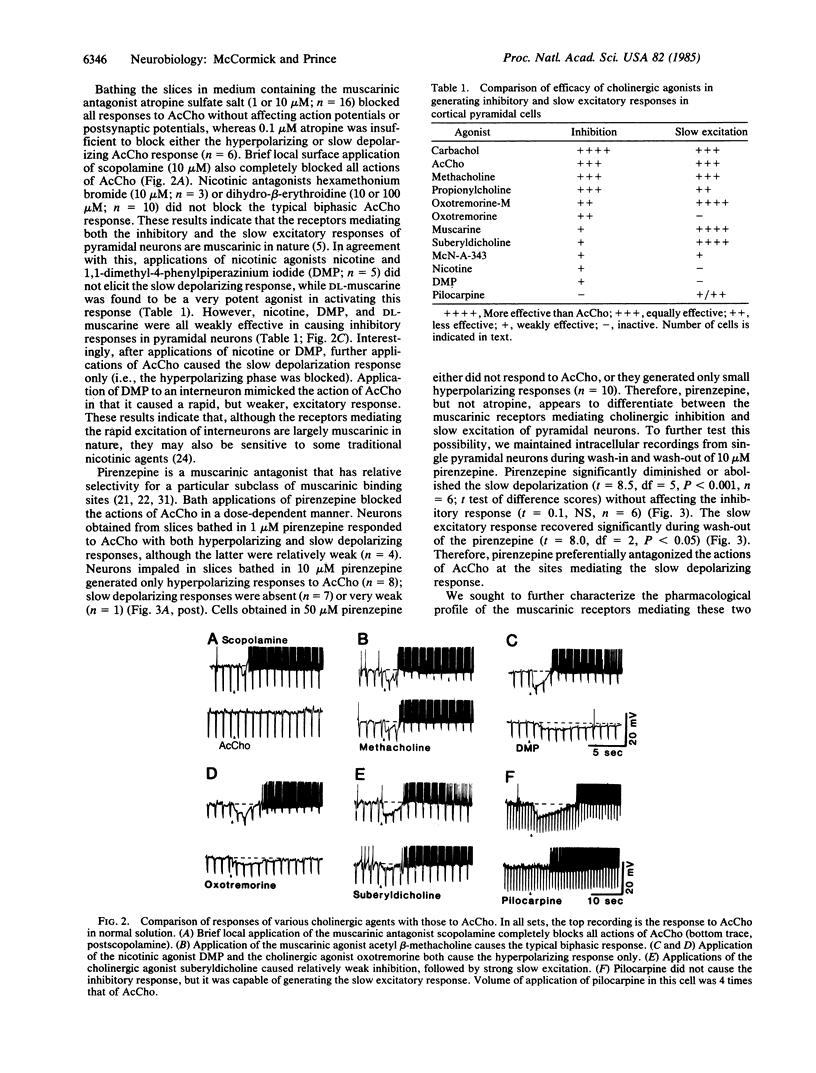
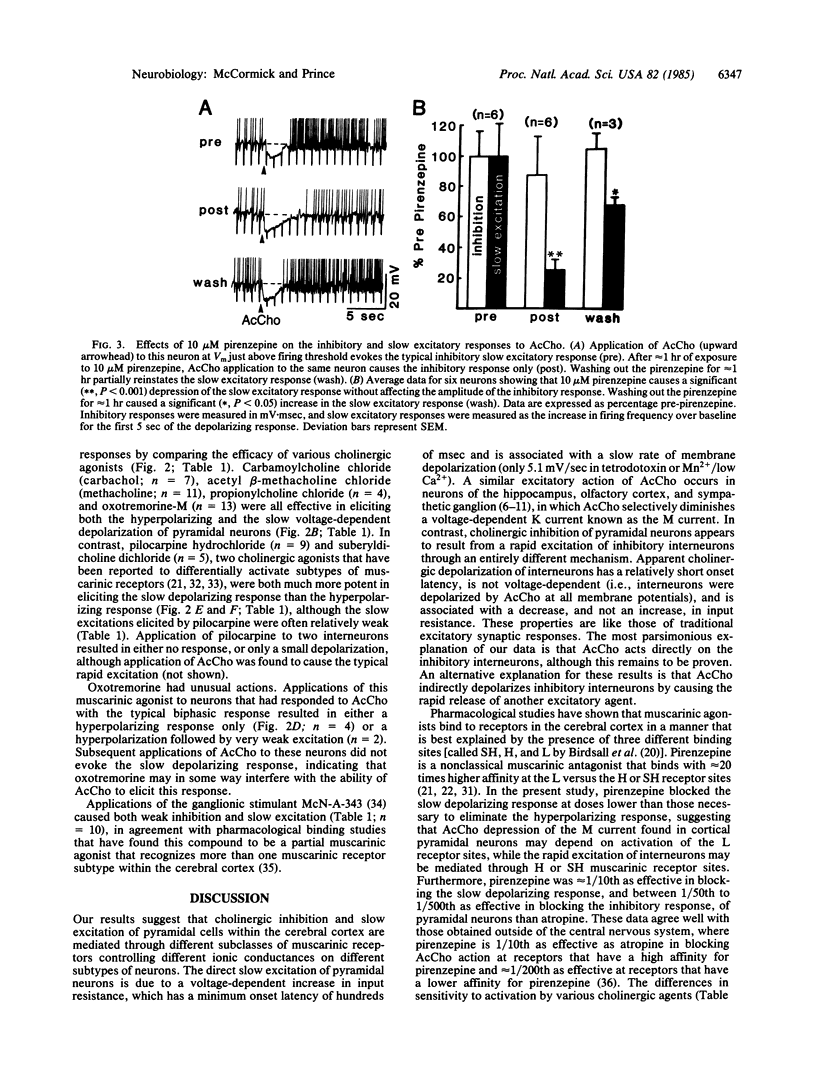
Applications of acetylcholine (AcCho) to pyramidal cells of guinea pig cingulate cortical slices maintained in vitro result in a short latency inhibition, followed by a prolonged increase in excitability. Cholinergic inhibition is mediated through the rapid excitation of interneurons that utilize the inhibitory neurotransmitter gamma-aminobutyric acid (GABA). This rapid excitation of interneurons is associated with a membrane depolarization and a decrease in neuronal input resistance. In contrast, AcCho-induced excitation of pyramidal cells is due to a direct action that produces a voltage-dependent increase in input resistance. In the experiments reported here, we investigated the possibility that these two responses are mediated by different subclasses of cholinergic receptors. The inhibitory and slow excitatory responses of pyramidal neurons were blocked by muscarinic but not by nicotinic antagonists. Pirenzepine was more effective in blocking the AcCho-induced slow depolarization than in blocking the hyperpolarization of pyramidal neurons. The two responses also varied in their sensitivity to various cholinergic agonists, making it possible to selectively activate either. These data suggest that AcCho may produce two physiologically and pharmacologically distinct muscarinic responses on neocortical neurons: slowly developing voltage-dependent depolarizations associated with an increase in input resistance in pyramidal cells and short-latency depolarizations associated with a decrease in input resistance in presumed GABAergic interneurons.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashe J. H., Yarosh C. A. Differential and selective antagonism of the slow-inhibitory postsynaptic potential and slow-excitatory postsynaptic potential by gallamine and pirenzepine in the superior cervical ganglion of the rabbit. Neuropharmacology. 1984 Nov;23(11):1321–1329. doi: 10.1016/0028-3908(84)90053-4. [DOI] [PubMed] [Google Scholar]
- Benardo L. S., Prince D. A. Ionic mechanisms of cholinergic excitation in mammalian hippocampal pyramidal cells. Brain Res. 1982 Oct 14;249(2):333–344. doi: 10.1016/0006-8993(82)90067-1. [DOI] [PubMed] [Google Scholar]
- Birdsall N. J., Burgen A. S., Hulme E. C., Stockton J. M., Zigmond M. J. The effect of McN-A-343 on muscarinic receptors in the cerebral cortex and heart. Br J Pharmacol. 1983 Feb;78(2):257–259. doi: 10.1111/j.1476-5381.1983.tb09388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsall N. J., Burgen A. S., Hulme E. C. The binding of agonists to brain muscarinic receptors. Mol Pharmacol. 1978 Sep;14(5):723–736. [PubMed] [Google Scholar]
- Brown D. A., Adams P. R. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980 Feb 14;283(5748):673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Cole A. E., Nicoll R. A. Acetylcholine mediates a slow synaptic potential in hippocampal pyramidal cells. Science. 1983 Sep 23;221(4617):1299–1301. doi: 10.1126/science.6612345. [DOI] [PubMed] [Google Scholar]
- Constanti A., Galvan M. M-current in voltage-clamped olfactory cortex neurones. Neurosci Lett. 1983 Aug 19;39(1):65–70. doi: 10.1016/0304-3940(83)90166-0. [DOI] [PubMed] [Google Scholar]
- Coyle J. T., Price D. L., DeLong M. R. Alzheimer's disease: a disorder of cortical cholinergic innervation. Science. 1983 Mar 11;219(4589):1184–1190. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- Dodd J., Horn J. P. Muscarinic inhibition of sympathetic C neurones in the bullfrog. J Physiol. 1983 Jan;334:271–291. doi: 10.1113/jphysiol.1983.sp014494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher J. P., Griffith W. H., Shinnick-Gallagher P. Cholinergic transmission in cat parasympathetic ganglia. J Physiol. 1982 Nov;332:473–486. doi: 10.1113/jphysiol.1982.sp014425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutnick M. J., Prince D. A. Dye coupling and possible electrotonic coupling in the guinea pig neocortical slice. Science. 1981 Jan 2;211(4477):67–70. doi: 10.1126/science.7444449. [DOI] [PubMed] [Google Scholar]
- Haas H. L. Cholinergic disinhibition in hippocampal slices of the rat. Brain Res. 1982 Feb 4;233(1):200–204. doi: 10.1016/0006-8993(82)90942-8. [DOI] [PubMed] [Google Scholar]
- Halliwell J. V., Adams P. R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982 Oct 28;250(1):71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Hammer R., Berrie C. P., Birdsall N. J., Burgen A. S., Hulme E. C. Pirenzepine distinguishes between different subclasses of muscarinic receptors. Nature. 1980 Jan 3;283(5742):90–92. doi: 10.1038/283090a0. [DOI] [PubMed] [Google Scholar]
- Hammer R. Subclasses of muscarinic receptors and pirenzepine. Further experimental evidence. Scand J Gastroenterol Suppl. 1982;72:59–67. [PubMed] [Google Scholar]
- Hartzell H. C., Kuffler S. W., Stickgold R., Yoshikami D. Synaptic excitation and inhibition resulting from direct action of acetylcholine on two types of chemoreceptors on individual amphibian parasympathetic neurones. J Physiol. 1977 Oct;271(3):817–846. doi: 10.1113/jphysiol.1977.sp012027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser C. R., Crawford G. D., Barber R. P., Salvaterra P. M., Vaughn J. E. Organization and morphological characteristics of cholinergic neurons: an immunocytochemical study with a monoclonal antibody to choline acetyltransferase. Brain Res. 1983 Apr 25;266(1):97–119. doi: 10.1016/0006-8993(83)91312-4. [DOI] [PubMed] [Google Scholar]
- Houser C. R., Crawford G. D., Salvaterra P. M., Vaughn J. E. Immunocytochemical localization of choline acetyltransferase in rat cerebral cortex: a study of cholinergic neurons and synapses. J Comp Neurol. 1985 Apr 1;234(1):17–34. doi: 10.1002/cne.902340103. [DOI] [PubMed] [Google Scholar]
- Libet B. Generation of slow inhibitory and excitatory postsynaptic potentials. Fed Proc. 1970 Nov-Dec;29(6):1945–1956. [PubMed] [Google Scholar]
- Mesulam M. M., Mufson E. J., Wainer B. H., Levey A. I. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6). Neuroscience. 1983 Dec;10(4):1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Phillis J. W., York D. H. Pharmacological studies on a cholinergic inhibition in the cerebral cortex. Brain Res. 1968 Sep;10(3):297–306. doi: 10.1016/0006-8993(68)90201-1. [DOI] [PubMed] [Google Scholar]
- ROSZKOWSKI A. P. An unusual type of sympathetic ganglionic stimulant. J Pharmacol Exp Ther. 1961 May;132:156–170. [PubMed] [Google Scholar]
- Segal M. Multiple action of acetylcholine at a muscarinic receptor studied in the rat hippocampal slice. Brain Res. 1982 Aug 19;246(1):77–87. doi: 10.1016/0006-8993(82)90144-5. [DOI] [PubMed] [Google Scholar]
- Vogt B. A., Gorman A. L. Responses of cortical neurons to stimulation of corpus callosum in vitro. J Neurophysiol. 1982 Dec;48(6):1257–1273. doi: 10.1152/jn.1982.48.6.1257. [DOI] [PubMed] [Google Scholar]
- Wainer B. H., Bolam J. P., Freund T. F., Henderson Z., Totterdell S., Smith A. D. Cholinergic synapses in the rat brain: a correlated light and electron microscopic immunohistochemical study employing a monoclonal antibody against choline acetyltransferase. Brain Res. 1984 Aug 6;308(1):69–76. doi: 10.1016/0006-8993(84)90918-1. [DOI] [PubMed] [Google Scholar]
- Williams M., Robinson J. L. Binding of the nicotinic cholinergic antagonist, dihydro-beta-erythroidine, to rat brain tissue. J Neurosci. 1984 Dec;4(12):2906–2911. doi: 10.1523/JNEUROSCI.04-12-02906.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody C. D., Swartz B. E., Gruen E. Effects of acetylcholine and cyclic GMP on input resistance of cortical neurons in awake cats. Brain Res. 1978 Dec 15;158(2):373–395. doi: 10.1016/0006-8993(78)90682-0. [DOI] [PubMed] [Google Scholar]


