Abstract
Many pharmaceutical and personal care products (PPCPs) and endocrine-disrupting chemicals (EDCs) are present in reclaimed water, leading to concerns of human health risks from the consumption of food crops irrigated with reclaimed water. This study evaluated the potential for plant uptake and accumulation of four commonly occurring PPCP/EDCs, i.e., bisphenol A (BPA), diclofenac sodium (DCL), naproxen (NPX), and 4-nonylphenol (NP), by lettuce (Lactuca sativa) and collards (Brassica oleracea) in hydroponic culture, using 14C-labeled compounds. In both plant species, plant accumulation followed the order of BPA > NP > DCL > NPX and accumulation in roots was much greater than in leaves and stems. Concentrations of 14C-PPCP/EDCs in plant tissues ranged from 0.22±0.03 to 927± 213 ng/g, but nearly all 14C-residue was non-extractable. PPCP/EDCs, particularly BPA and NP, were also extensively transformed in the nutrient solution. Dietary uptake of these PPCP/EDCs by humans was predicted to be negligible.
Keywords: Reclaimed water, pharmaceuticals, endocrine disrupting chemicals, plant uptake, non-extractable residue
1. Introduction
Treated wastewater, commonly called reclaimed or recycled water, is a valuable water source in arid and semi-arid areas where fresh water sources are becoming increasingly scarce due to urbanization and climate change (Benotti and Snyder, 2009). Reclaimed water may have many beneficial applications, including agriculture irrigation and landscape irrigation. In the state of California, these irrigation uses account for 37% and 18%, respectively, of the 650,000 acre-feet (8.0 × 108 cubic meters) per year of water reuse (Anderson et al., 2010). State policy calls to increase the use of reclaimed water to more than 2.5 million acre-feet (3.1× 109 cubic meters) per year by 2030 (California State Water Resources Control Board, 2009). Accompanying increased reuse, the presence and environmental risks of unregulated organic contaminants in reclaimed water are drawing attention (Anderson et al., 2010; Daughton and Ternes, 1999).
Pharmaceutical and personal care products (PPCPs) and endocrine disrupting compounds (EDCs) are typically anthropogenic chemicals with known biological effects (Daughton and Ternes, 1999) that may interfere with normal metabolism and behaviors of organisms (Daughton and Ternes, 1999; Marwick, 1999). Many PPCP/EDCs are routinely found in reclaimed water (Anderson et al., 2010; Kinney et al., 2006), as well as in surface water impacted by wastewater treatment plant effluent (Kolpin et al., 2002) and in groundwater (Barnes et al., 2008). When reclaimed water is used for irrigation, the associated PPCP/EDCs may interact with the soil matrix (Chefetz et al., 2008; Kinney et al., 2006) and may contaminate groundwater (Avisar et al., 2009) and food crops (Eggen and Lillo, 2012; Herklotz et al., 2010; Shenker et al., 2011). Accumulation of PPCP/EDCs into food crops that are consumed fresh, such as many leafy vegetables, is relevant due to the likelihood of unintentional human exposure. If research demonstrates that accumulation of PPCP/EDCs by crops is unlikely to result in human health risks, this will provide scientific basis to promote use of reclaimed water, as well as enhance positive public perception of water reuse.
Many factors influence the uptake of organic compounds into plants, such as by affecting diffusion through cell membranes. Briggs et al. (1982) suggested that chemical hydrophobicity is an important factor affecting uptake by diffusion and that chemicals with a log Kow of 1 – 3.5 (with an optimal log Kow of 1.8) have the greatest plant uptake potential because lipid and aqueous solubility are balanced (Pilon-Smits, 2005). In addition to hydrophobicity, molecular ionization has also been shown to influence plant accumulation, such as of herbicides (Sterling, 1994). Charged molecules may have a reduced potential for plant uptake, since ionization may reduce their ability to permeate cell membranes (Trapp, 2004). However, the role of ionization is poorly understood and exceptions have been noted (Eggen and Lillo, 2012).
To date only a handful of studies have considered plant uptake of PPCP/EDCs (Boxall et al., 2006; Herklotz et al., 2010). While these studies have clearly shown the ability for plants to take up PPCP/EDCs, the state of knowledge is limited to a few compounds or plant types. Due to the analytical challenges of detecting chemicals at trace levels in plant matrices, most studies also relied on the use of artificially high concentrations, with a few exceptions (Calderón-Preciado et al., 2011; Holling et al., 2012; Shenker et al., 2011). In this study, we comparatively determined the accumulation of four commonly occurring PPCP/EDCs, i.e., bisphenol A (BPA), diclofenac (DCL), naproxen (NPX), or nonylphenol (NP), at relevant environmental levels into two leafy vegetables, lettuce and collards, and examined the composition and distribution of accumulated residues. These compounds have been frequently detected in reclaimed water (Anderson et al., 2010) and surface water (Benotti et al., 2008), and have different ionization states at neutral pH. To achieve realistically low concentrations while affording quantitative measurement, 14C-labeled compounds were used. Results were used to infer effects of plant type and compound characteristics on plant accumulation and estimate probable human intakes.
2. Materials and methods
2.1. Chemicals
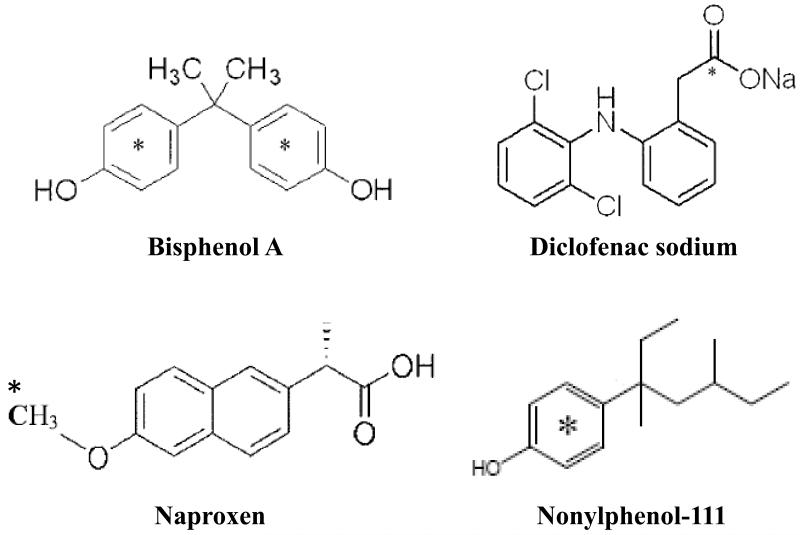
14C-Labeled bisphenol A (BPA) (4,4′-(propane-2,2-diyl)diphenol), diclofenac sodium (DCL) ((o-(2,6-dichloroanilino)phenyl)acetic acid monosodium salt), and naproxen (NPX) ((S)-6-methoxy-a-methyl-2-naphthaleneacetic acid) with 99% chemical purity were purchased from American Radiolabeled Chemicals (Saint Louis, MO). The specific activities were 200, 55, and 55 mCi/mmol, respectively. 14C-Labeled nonylphenol-111 (NP) (4-[1-ethyl-1,3-dimethylpentyl]phenol) (specific activity 75 mCi/mmol) was kindly provided by Dr. Rong Ji at Nanjing University in Nanjing, China. Other chemicals were ACS grade or better (Fisher Scientific, West Chester, PA). Chemical structures, including location of the 14C label, are shown in Figure 1.
Fig. 1.
Chemical structures of PPCP/EDCs used in this study. *Location of 14C label.
2.2. Hydroponic Cultivation Experiment
Seedlings of lettuce (Lactuca sativa cv. Nevada, Batavia lettuce) and collards (Brassica oleracea) were purchased at three weeks post seeding from Certified Plant Growers (Temecula, CA) through a local nursery. Glass jars with 2 L capacity and polyvinyl screw-top lids were washed with soap and deionized water, rinsed with methanol, and enclosed in opaque plastic sheeting to avoid photodegradation. Hydroponic nutrient solution was made with constituents and concentrations as in Seyfferth et al. (2008). Briefly, nutrient concentrations and a pH of 7 were controlled with HEDTA, HCl, NaOH, and 2-(N-morpholino)ethanesulfonic acid (MES) and nutrients were supplied at the following concentrations (μM): NO3−, 4900; Ca, 1900; K, 1080; Mg, 500; S, 500; Cl, 191; Si, 187; NH4+, 100; P, 80; Fe, 20; B, 10; Zn, 8; Cu, 2; Mn, 0.6; Mo, 0.1; Ni, 0.1.
Experimental treatments were created, in triplicate, for each 14C-PPCP/EDC with lettuce or collards plants. A spiked, no plant control for each PPCP/EDC and a non-spiked control with a collards plant were also included in triplicate. The experiments were conducted in a growth chamber programmed for a 16 h light/8 h dark cycle, with constant 65% relative air humidity and a gradual increase and then decrease of photosynthetic photon flux density that peaked daily at 350 μmol/m2s. Growth chamber air was freely exchangeable with ambient air. Plant seedlings were removed from packaging and soil, rinsed with deionized water, and placed in jars of continuously aerated nutrient solution, one plant per 2 L jar. Plants were suspended in the nutrient solution by means of a non-reactive foam collar around the stem that secured the plant in an opening in the lid. After 3 d, the jars and nutrient solution were exchanged with clean jars and fresh nutrient solution to restore nutrient levels and reduce microbial load in the solution. After 6 d of acclimation under the prescribed conditions, plants of similar size for each species were randomly chosen and transferred into new jars containing nutrient solution spiked with 14C-BPA, DCL, NPX or NP at, respectively, 46.4, 237.4, 178.2, or 110.4 ng/L (about 1.7×105 dpm/jar). These concentrations are representative of concentrations measured in reclaimed water (Anderson et al., 2010). Every 3 d after the initial treatment, all plants were transferred into clean jars with fresh, spiked nutrient solution that replicated their initial nutrient and PPCP/EDC conditions. Plants were grown for a total of 21 d in spiked solutions, a total growth time that represents commercial growth to a “market size”.
2.3. Plant Sampling and Analysis
Following 21 d of hydroponic cultivation, plants were sacrificed for analysis of 14C accumulation and distribution. Each whole plant was rinsed with DI water, and then separated into roots, stems, new leaves, and original leaves. Original leaves were designated as leaves present on the seedling at the beginning of the experiment. Individual plant samples were placed in pre-weighed metal screen pouches, weighed to determine wet weight, and dried at 50 °C for 60 h. After drying, each plant sample was weighed to measure the dry weight, and then chopped and mixed in a stainless steel coffee grinder. The grinder was rinsed between samples with DI water and methanol to prevent cross contamination. Multiple 150 mg subsamples of each plant sample were analyzed until standard deviation of the subsamples was below 20%, due to notable variation in plant tissue activity. Subsamples were combusted on an OX-500 Biological Oxidizer (R.J. Harvey, Hillsdale, NJ) at 900 °C for 4 min, and the evolved 14CO2 was trapped in 15 mL of Harvey Carbon-14 cocktail (R.J. Harvey, Tappan, NY). The 14C was measured on a Beckman LS 5000TD Liquid Scintillation Counter (LSC) (Fullerton, CA). Recovery was 91-96% for spiked standards, which was used to correct for the actual activity. The activity and weight of the subsamples were used to determine the total radioactivity accumulated in different tissues of each plant.
Analysis of 14C by combustion provided information on total residue in plant tissues. To better understand the nature of the residue, plant samples were solvent extracted using a method modified from Wu et al. (2012). The fractions of 14C in solvent-extractable and non-extractable forms were separately determined. Briefly, 400 mg subsamples of the dried, ground plant matter were freeze-dried for 12 h, weighed, and extracted in polypropylene tubes by sequential sonication (20 min) and centrifugation (20 min, 3500 rpm) with 20 mL methyl tert-butyl ether (MTBE) and then again with 20 mL acetonitrile. The combined extracts were evaporated under nitrogen to less than 1 mL, and mixed with 5 mL methanol and 20 mL water. A 6 mL aliquot of the extract was taken for analysis by LSC to determine the fraction of activity as extractable residue. Selected 150 mg subsamples of the solvent-extracted plant matter were combusted on the Biological Oxidizer as described above to determine the fraction of 14C present as non-extractable residue.
2.4. Nutrient Solution Sampling and Analysis
When nutrient solution and jars were exchanged, the volume of remaining nutrient solution in each jar was gravimetrically determined. A 9 mL aliquot of the solution was mixed with 13 mL Ultima Gold scintillation cocktail (Fisher Scientific, West Chester, PA) and the 14C was quantified by LSC. Water loss from evaporation during each 3 d period was found to be negligible in the no-plant control containers. It is likely that microbial activity in the nutrient solution may have resulted in transformation of the spiked 14C-compounds and that plants may have accumulated both parent PPCP/EDCs and transformation products. To discern the contribution of transformation products to plant accumulation, the used nutrient solution from day 21 was preserved with 2 g sodium azide and 100 mg ascorbic acid, extracted, and fractionated using high performance liquid chromatography (HPLC).
Solutions from 14C-BPA, DCL, or NPX treatments were first filtered through a Whatman #4 filter paper and then passed through a HLB (150 mg 6cc, Waters, Milford, MA) solid phase extraction (SPE) cartridge (Vanderford and Snyder, 2006). Before use, the cartridges were sequentially conditioned with 5 mL each of MTBE, methanol (MeOH), and water. The filtered solution was drawn through the conditioned HLB cartridges under vacuum and followed by 50 mL deionized water. A subsample of the filtrate that passed through the cartridge was collected for analysis by LSC to quantify 14C that was not retained by the cartridge. The cartridges were dried with nitrogen gas, and then sequentially eluted with 5 mL of MeOH:MTBE (10:90) and 5 mL MeOH. The collected eluent was dried under nitrogen to 100 μL. The concentrated eluent was transferred to an HPLC vial equipped with a 250 μL insert. The condensing vial was rinsed with 130 μL of methanol, and the rinsate and 20 μL of non-labeled parent standard (25 mg/L) were added to the HPLC vial. Preliminary experiments showed that the recovery of this extraction procedure from the initial solution to HPLC analysis was 81.5 ± 7.1% for BPA, 85.8 ± 2.5% for DCL, and 74.0 ± 1.9% for NPX.
Nutrient solutions from the 14C-NP treatment were extracted by a simple liquid-liquid extraction method. Each nutrient solution sample was shaken with 50 mL hexane for 30 min, and then the upper layer of the sample was transferred to a centrifuge tube and centrifuged at 3500 rpm for 30 min to reduce emulsification. The hexane phase was transferred to a 15 mL glass tube, concentrated under nitrogen to 300 μL, and transferred to an HPLC vial. The condensing vial was rinsed with 180 μL of methanol, and the rinsate and 20 μL of non-labeled NP standard (20 mg/L) were added to the HPLC vial. The recovery of this extraction method from the initial solution to HPLC analysis for NP was determined to be 66.8 ± 12.0%.
An aliquot (10 μL for BPA, DCL, and NPX; 20 μL for NP) of the finalized sample was injected into an Agilent 1100 Series HPLC equipped with a Dionex Acclaim 120 C18 RP column (4.6 × 250 mm). Column temperature was maintained at 35 °C. Mobile phase was created from ultra-pure water with 0.2% acetic acid (mobile phase A; ultra-pure water for NP) and acetonitrile (mobile phase B). Flow rate and mobile phase mix were 1.25 mL/min and 60:40 (A/B) for BPA, 1.25 mL/min and 47:53 (A/B) for DCL, 1.60 mL/min and 60:40 (A/B) for NPX, and 1.0 mL/min and 20:80 (A/B) for NP. Ultraviolet detection was set at 280, 284, 278, and 280 nm, respectively. Retention times were 13.3, 11.6, 13.1, and 11.0 min, respectively, for BPA, DCL, NPX, and NP. The column eluent was fractionally collected in 1 min increments into 7 mL glass tubes using an automated fraction collector (LKB Bromma 2112 Redirac, Bromma, Sweden) and the 14C in each elution sample was measured by LSC. The distribution of 14C in the HPLC eluent as a function of run time was used to infer the fractions of parent and transformation products in the nutrient solution.
2.5. QA/QC and Data Analysis
All experimental treatments were performed in triplicate, with untreated blanks to ensure quality control. Statistical analysis of data was performed with software R (R Development Core Team, 2008. R Foundation for Statistical Computing) using multi-way ANOVA and post-hoc Tukey’s Honestly Significant Difference test. Significance level was assigned at p ≤ 0.05.
3. Results and discussions
3.1. PPCP/EDC Removal from Nutrient Solution
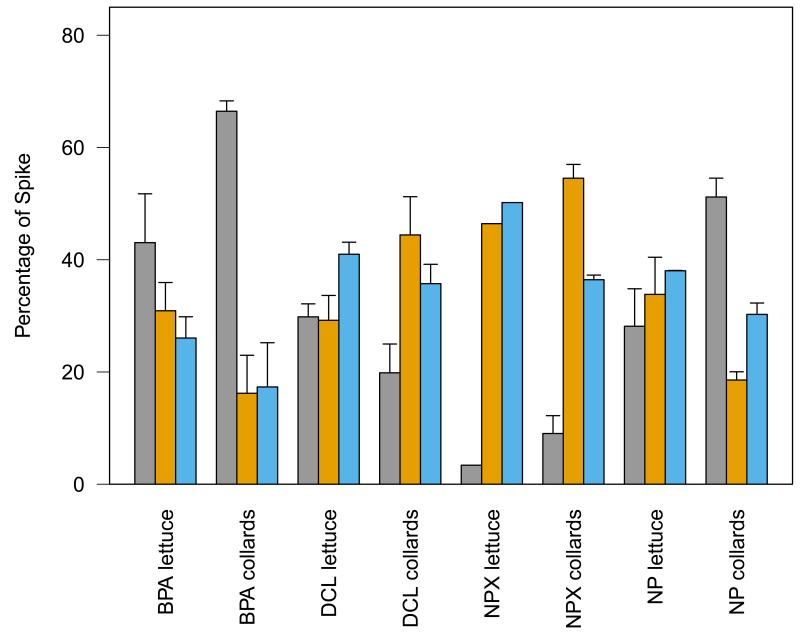
Young plants of lettuce and collards were grown for 21 d in nutrient solution containing one of the four 14C-labeled PPCP/EDCs. No significant differences in plant mass were observed between treatments at the end of the experiment. During the experiment, three plants died (two from the NPX-lettuce treatment and one from the NP-lettuce treatment). Figure 2 shows the mean mass balance for the systems at the end of the experiment, depicting the fractions of the spiked 14C present in plant tissues, in the used nutrient solution, and as unaccounted activity. The unaccounted activity reflected the 14C that was not found in the nutrient solution at the time of solution renewal or in the plant tissues after harvest and may include losses via unidentified processes, such as volatilization, microbial mineralization in the nutrient solution (and the subsequent release of activity as 14CO2), or stomatal release. Activity in each fraction varied across compounds and to a lesser degree across plant species, suggesting specificity to uptake.
Fig. 2.
Mass balance of 14C-bisphenol A, 14C-diclofenac, 14C-naproxen, and 14C-nonylphenol spiked into hydroponic systems growing lettuce or collards plants for 21 d. Distribution of spike is between plant tissue ( ), used nutrient solution (
), used nutrient solution ( ), and unaccounted activity (
), and unaccounted activity ( ), as a percentage of total spike ± standard error. The naproxen-lettuce treatment lacks standard error due to plant death.
), as a percentage of total spike ± standard error. The naproxen-lettuce treatment lacks standard error due to plant death.
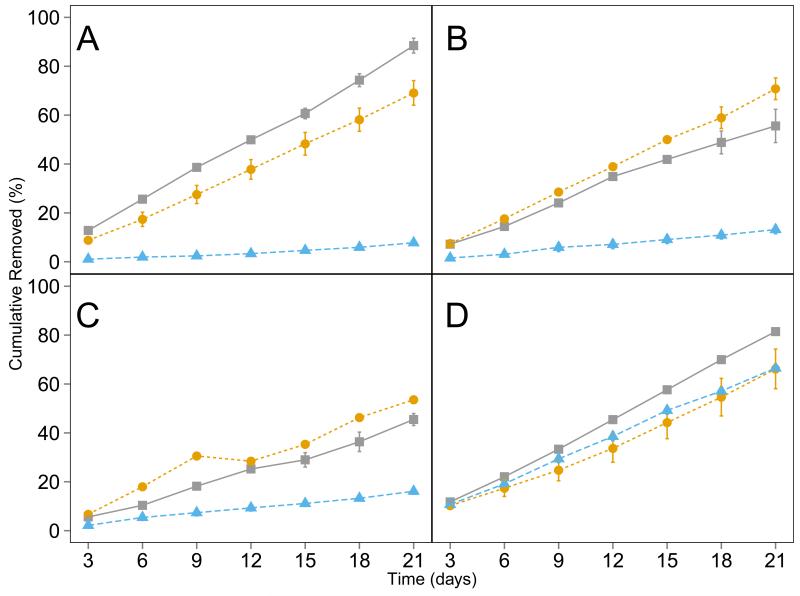
Figure 3 shows the cumulative 14C dissipation from the nutrient solution as calculated from the difference in activity in the solution at the beginning and end of each 3 d interval of solution renewal, representing 14C loss from plant uptake and other processes. Dissipation followed the decreasing order of BPA > NP > DCL > NPX for all treatments and occurred at a similar rate throughout the 21 d cultivation. The presence of plants significantly (p < 0.05) increased the dissipation of PPCP/EDCs from the nutrient solution, except for NP. For example, the initial concentration of 14C-DCL in the nutrient solution was 105.3 ± 0.3 dpm/mL, but it decreased to only 32.8 ± 1.9 dpm/mL after 3 d in the presence of lettuce, while 91.2 ± 3.2 dpm/mL remained in the no-plant control. Lettuce and collards treatments had different levels of chemical dissipation in the nutrient solution. For example, the overall dissipation of BPA in the lettuce treatment was 69.1 ± 8.7%, as compared to 88.4 ± 5.3% in the collards treatment (Figure 3). Different compounds also dissipated at different rates. For instance, in the presence of collards, the cumulative loss was 88.4 ± 5% for BPA, 55.6 ± 11.8% for DCL, and 45.5 ± 4.3% for NPX.
Fig. 3.
Cumulative removal of 14C-bisphenol A (A), 14C-diclofenac (B), 14C-naproxen (C), or 14C-nonylphenol (D) from hydroponic nutrient solution growing lettuce ( ) collards (
) collards ( ), or no-plants (
), or no-plants ( ) for 21 d. Cumulative removal is expressed as percentage of total spike ± standard error. The naproxen-lettuce treatment lacks standard error due to plant death.
) for 21 d. Cumulative removal is expressed as percentage of total spike ± standard error. The naproxen-lettuce treatment lacks standard error due to plant death.
The dissipation of NP in the solutions with plants was found to be similar to that in the no-plant control, especially for the lettuce treatment (Figure 3D). The loss of NP from the no-plant control was likely associated with volatilization, as continuous aeration was used to maintain the oxygen level in the nutrient solution throughout the experiment. The Henry’s Law constant for NP is 1.09 ×10−4 atm m3 mol−1 (European Chemicals Bureau, 2002), suggesting a tendency for volatilization. An additional experiment was carried out in an air-tight container without aeration. The loss of NP in the solution was found to be insignificant, as all of the spiked 14C was found in the solution (104.5 ± 4.7%), and a solvent rinse of the system showed little sorption of 14C-NP on the container wall (<5.1% of the spiked amount). Doucette et al. (2005) found that in a hydroponic set up, about 13% of the spiked NP was lost to volatilization in the absence of plants. The increased volatilization losses in the current study were likely due to specific aeration and temperature conditions used. Despite volatilization losses, significant amounts of 14C were detected in plant tissues, suggesting that both collards and lettuce accumulated NP (Figure 2).
Noureddin et al. (2004) studied the uptake of 5 mg/L BPA from hydroponic solution by water convolvulus (Ipomoea aquatic) and found that approximately 75% of the spiked BPA was removed after 3 d. This removal was comparable to that observed for BPA with lettuce (70%) in this study, but was smaller than that with collards (88%). Calderón-Preciado et al. (2012) evaluated hydroponic uptake of triclosan, hydrocinnamic acid, tonalide, ibuprofen, naproxen, and clofibric acid by lettuce (Lactuca sativa L) and spath (Spathiphyllum spp.) and showed that the removal of NPX from solution was about 70% for lettuce and 10% for spath after 3 d. In comparison, Matamoros et al. (2012) observed less than 10% removal of NPX after 3 d of hydroponic growth with wetland plants (Salvinia molesta, Lemna minor, Ceratophyllum demersum, and Elodea canadensis), while 46% removal of NPX was measured in the collards treatment in the present study. Matamoros et al. also showed that DCL did not dissipate appreciably in treatments with wetland plants, which was in contrast to the high removal of DCL by leafy vegetables observed in this study (70.8 ± 7.7% and 55.6 ± 11.8% for lettuce and collards, respectively). It is likely that the smaller plant mass and the use of non-aerated nutrient solution in the earlier study contributed to the limited plant uptake. The range of variation suggests that plant species, along with other factors such as plant mass and environmental conditions, affect the actual accumulation of PPCP/EDCs into plant tissues.
3.2. Accumulation in Plant Tissues
Plant tissues were collected after 21 d of cultivation, rinsed with deionized water, and separated into roots, stems, new leaves, and original leaves for analysis of both extractable and non-extractable 14C. Table 1 shows concentrations of 14C in plant tissues, expressed as parent-equivalents. In agreement with the dissipation trends in solution, plant accumulation followed the decreasing order of BPA > NP > DCL > NPX. Concentrations based on dry plant mass ranged from 0.22 ± 0.03 to 12.12 ± 1.91 ng/g in leaves and stems. Statistical analysis showed that the accumulation in leaves and stems was not significantly different between lettuce and collards, or among the different compounds. In contrast, roots accumulated significantly more (p < 0.05) 14C than all the other plant tissues, with concentrations that ranged from 71.08 ± 12.12 to 926.89 ±212.89 ng/g.
Table 1.
Concentrations of PPCP/EDCs in plant tissues, calculated by dividing the mean measured 14C (expressed as parent-equivalents) by dry-weight plant mass (ng/g ± standard error).
| Plant Structure | Bisphenol A | Diclofenac | Naproxen | Nonylphenol |
|---|---|---|---|---|
| Lettuce | ||||
| New Leaves | 0.22 ± 0.03 | 3.71 ± 1.80 | 3.15a | 1.18 ± 0.04 |
| Original Leaves | 0.36 ± 0.07 | 9.05 ± 4.08 | 2.81a | 2.59 ± 0.30 |
| Stem | 0.30 ± 0.08 | 5.10 ± 1.53 | 5.02a | 4.31 ± 2.54 |
| Roots | 441.7 ± 138.9 | 872.9 ± 98.2 | 330.2a | 926.9 ± 212.8 |
| Collards | ||||
| New Leaves | 1.42 ± 0.37 | 7.48 ± 0.99 | 4.50 ± 0.78 | 3.80 ± 0.99 |
| Original Leaves | 3.05 ± 0.51 | 7.75 ± 0.68 | 8.14 ± 1.77 | 6.95 ± 0.97 |
| Stem | 2.39 ± 0.66 | 12.0 ± 5.2 | 12.1 ± 1.9 | 3.79 ± 1.26 |
| Roots | 199.6 ± 42.6 | 229.6 ± 35.7 | 71.1 ± 12.1 | 339.2 ± 19.2 |
Naproxen-lettuce treatment lacks standard error due to plant death.
Accumulation of 14C in plant tissues exhibited several apparent trends. In whole collards plants, significantly greater accumulation was found for the neutral compounds BPA (66.5 ± 3.2% of spike) and NP (51.2 ± 5.8%) than the anionic compounds DCL (19.8 ± 8.9%) and NPX (9.0 ± 5.8%), suggesting that the charge state of PPCP/EDCs may greatly influence plant uptake (Trapp, 2004). Similar effects have been frequently observed for anionic herbicides, and are attributed to exclusion of negatively charged molecules by cell membranes (Sterling, 1994). Between lettuce and collards, lettuce significantly accumulated less PPCP/EDC when all test compounds were pooled (0.007), although the interaction effect for individual compounds was not significant (p > 0.11). Accumulation of BPA or NP in plant roots was significantly higher for collards than lettuce (when comparing portion of spike accumulated), while portion of DCL accumulated into lettuce and collards roots was not significantly different. Analysis of tissue extracted with solvent showed that essentially all of the 14C was non-extractable; only the root samples from NP-collards treatment contained a detectable fraction of 14C in extracts (1.5% of total tissue 14C). Combustion of extracted plant tissues confirmed that almost all 14C remained as non-extractable residue, one possible endpoint for xenobiotics taken up by plants (Sandermann, 1992).
Only a few studies have examined the plant uptake of some of the same PPCP/EDCs considered in this study. Wu et al. (2012) grew iceberg lettuce (Lactuca sativa L.) and spinach (Spinacia oleracea) for 21 d in hydroponic solution initially spiked with a suite of 19 PPCPs, including DCL and NPX, each at 500 ng/L and found no detectable residues of DCL or NPX, except for NPX in spinach at 0.04 ng/g. Calderón-Preciado et al. (2011) analyzed apple tree leaves and alfalfa from fields irrigated with water containing BPA, DCL, and NPX. DCL was detected at 0.354 ng/g in apple leaves and 0.198 ng/g in alfalfa; NPX was detected at 0.043 ng/g and 0.04 ng/g, respectively. The low concentrations found in these studies generally agree with the findings of this study, but there is some variation in the tendency for specific compounds to accumulate. This variation may be partly attributed to the different analytical approaches. In other studies, uptake of PPCP/EDCs by plants was evaluated using non-labeled compounds, and accumulation was measured by targeted chromatographic analysis for the extractable parent compound. The use of 14C-labeled compounds in the current study should have provided “worst-case” estimates of human exposure, as the concentrations included non-extractable residue and likely also included transformation products. Transformation products may be an important component of potential risk since the metabolites of some PPCP/EDCs have higher biological activity than their parents (Celiz et al., 2009) and studies have shown that a large portion of PPCP/EDCs that are taken up by plants may be transformed in vivo (Macherius et al., 2012).
A translocation factor (TF), which was the total 14C in stems, new leaves, and original leaves divided by the 14C in roots, was calculated (Table 2). The derived TFs were consistently very small, demonstrating the poor translocation of these PPCP/EDCs from roots to upper tissues after uptake. The TF values followed the decreasing order of NPX > DCL > NP > BPA, the opposite observed for plant accumulation. Lettuce displayed lower TFs than collards for the same PPCP/EDCs. For example, the mean TF for BPA was only 0.010 ± 0.003 for lettuce, but was 0.051 ± 0.008 for collards. The much greater accumulation of PPCP/EDCs in roots, as compared to leaves, has been observed in previous studies. For instance, Herklotz et al. (2010) found that the levels in leaves were 0.00952 – 0.00503 of those in roots for cabbage grown in nutrient solution spiked with carbamazepine, salbutamol, sulfamethoxazole, and trimethoprim. Doucette et al. (2005) reported that the accumulation of NP in leaves was 0.0233 – 0.0167 of that in the roots of crested wheatgrass grown in solution. The poor translocation of the selected PPCP/EDCs from roots to leaves may be attributed to several factors. The compounds considered in this study have moderately high hydrophobicity with log Kow (in their neutral forms) from 3.35 to 4.48 (Soares et al., 2008; Staples et al., 1998; Tsantili-Kakoulidou et al., 1997). Translocation of organic compounds within plants generally decreases with increasing hydrophobicity (Trapp and Legind, 2011). Also, roots have higher lipid content than most other plant tissues, and neutral compounds have been shown to be preferentially distributed in tissues with high lipid content (Collins et al., 2011). In addition, the rapid conversion of 14C residue to the non-extractable form, as discussed above, may be another important factor for the negligible transfer from roots to other plant tissues.
Table 2.
Translocation factor (TF) of 14C from root tissue to above-ground tissue (stems, original leaves, and new leaves), calculated by dividing the sum of 14C in above-ground tissue by 14C in root tissue.
| Bisphenol A | Diclofenac | Naproxen | Nonylphenol | |
|---|---|---|---|---|
| Lettuce TF | 0.010 ± 0.003 | 0.059 ± 0.005 | 0.182a | 0.025 ± 0.009 |
| Collards TF | 0.051 ± 0.008 | 0.131 ± 0.040 | 0.511 ± 0.051 | 0.079 ± 0.019 |
Naproxen-lettuce treatment lacks standard error due to plant death.
3.3. Transformation in Nutrient Solution
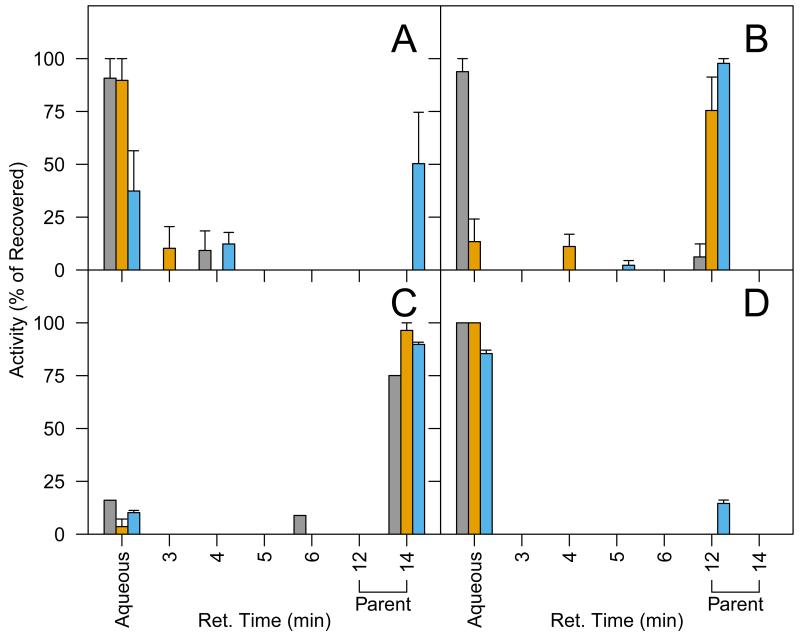
The use of 14C labeling, while giving unique information such as the total chemical accumulation in plant tissues, did not provide insights on the chemical composition of the accumulated residue. It is likely that some of the PPCP/EDCs were transformed in the nutrient solution before they were taken up by plants. The used nutrient solution from hydroponic cultivation was subjected to fractionation on HPLC to characterize the portions of 14C existing as parent compound and transformation products (Figure 4). It is evident that different PPCP/EDCs were transformed to different degrees in the nutrient solution and the presence of plants generally enhanced the transformation.
Fig. 4.
Composition of 14C in used nutrient solution originally spiked with 14C-bisphenol A (A), 14C-diclofenac (B), 14C-naproxen (C), or 14C-nonylphenol (D) and then used for cultivation of lettuce ( ), collards (
), collards ( ), or no-plants (
), or no-plants ( ). Activity was detected in aqueous phases of the extraction process, at the HPLC retention time of the parent compound, and at earlier retention times than the parent compound. Activity is expressed as a percent of the total activity recovered from these stages ± standard error.
). Activity was detected in aqueous phases of the extraction process, at the HPLC retention time of the parent compound, and at earlier retention times than the parent compound. Activity is expressed as a percent of the total activity recovered from these stages ± standard error.
In the no-plant control of DCL and NPX, the majority of 14C was in the form of the parent compound (97.8 ± 2.2% and 89.8 ± 1.0% of 14C recovered from nutrient solution, respectively), while the percentage of 14C in the SPE aqueous filtrate or eluted on HPLC prior to the parent compound was very small (Figure 4). The presence of lettuce or collards did not increase the transformation of DCL or NPX, with the exception of the DCL-collards treatment, where 93.8 ± 6.2% of the recovered activity was detected in the SPE aqueous filtrate. In contrast, BPA and NP were extensively transformed, even in the absence of plants, and transformation was accelerated in the presence of a plant. For example, 50.3 ± 24.3% of the recovered 14C was identified as the parent in the BPA no-plant control, but collards and lettuce treatments had no detectable BPA. In the presence of a plant, 14C was detected in the aqueous filtrate (89.7 – 90.7% of recovered activity) and in HPLC eluent prior to the retention time for BPA (9.3 – 10.3%). Extensive transformation of NP was also observed; all of the 14C from lettuce or collards cultivation was found in the aqueous phase of the extraction (Figure 4).
The fraction of activity in aqueous phases may be attributed to transformation products that were not retained by the HLB cartridge or solvent phase during solvent extraction (for NP). Preliminary experiments showed that an average of 93.6% of 14C-BPA, 84.5% of 14C-DCL, and 92.0% of 14C-NPX were recovered from the HLB cartridges and 97.8% of the spiked 14C-NP was recovered in the solvent phase, while the activity in aqueous phases were below detection. Therefore, 14C in the SPE aqueous filtrate for BPA, DCL, and NPX, or in the aqueous phase for NP, was likely from polar transformation products containing the 14C label. The detection of transformation products in used solution suggests that some of the 14C found in plant tissues may be from transformation products formed in the nutrient solution prior to plant uptake.
3.4. Human Exposure Implications
The demonstrated accumulation of PPCP/EDCs into leafy vegetables suggests a potential risk to humans through dietary uptake. To assess whether the concentrations detected in plant tissues in this study may present a potential human health risk, an individual’s annual exposure was estimated using values from the U.S. Environmental Protection Agency (2011) for average daily consumption of leafy vegetables (0.54 gwet weight/kgbody weight-day) (Table 4). The annual exposure values ranged from 0.32 × 10−3 mg for BPA-lettuce to 2.14 × 10−2 mg for DCL-collards for an average, 70 kg individual residing in the United States. To place these amounts in context, the values were then converted to either medical dose or 17β-estradiol (E2) equivalents. Both DCL and NPX are commonly available non-steroidal anti-inflammatory pharmaceuticals. Based on typical doses and the observed plant concentrations, an average individual would consume the equivalent of much less than one dose of these medicines in a year due to consumption of leafy vegetables, representing a very minor exposure to these PPCPs. However, it should be noted that DCL has proven ecotoxicity (Triebskorn et al., 2004) and NPX has shown toxicity in mixture with other pharmaceuticals (Cleuvers, 2004), so a simple estimation may not encompass all possible human health effects. Both BPA and NP are industrial products known to have endocrine disrupting activity. Bonefeld-Jørgensen et al. (2007) calculated the Relative Potency of these compounds as compared to 17β-estradiol (E2), an endogenous estrogen hormone, at activating estrogenic receptors. In Table 4, the exposure values of BPA and NP were estimated as E2 equivalents by dividing by their Relative Potency (BPA, 1.0 × 10 −4; NP, 1.0 × 10−3). When the calculated E2 equivalents of BPA and NP are compared with the Lowest Observable Effect Concentration for E2 (2.72 ng/L), it is obvious that the even the highest expected annual exposure to these compounds by consuming leafy vegetables would not reach the LOEC. This rough calculation suggests that consumption of vegetables would be unlikely to influence an individual’s overall endocrine activity, though caution should be used when considering risk to susceptible population groups.
Moreover, it must be noted that the use of hydroponic cultivation likely resulted in greater plant accumulation of these PPCP/EDCs, in relation to soil cultivation, due to the absence of chemical sorption to soil organic matter and minerals. This likelihood, when coupled with the fact that most of the 14C in plant tissues was in the non-extractable form, implies that the actual plant accumulation of these PPCP/EDCs by leafy vegetables grown in uncontaminated fields irrigated with reclaimed water may be negligibly small. On the other hand, bio-solids have been shown to contain some PPCP/EDCs at much higher concentrations than treated wastewater and plant uptake from soil amended with may pose an enhanced human exposure risk. Also, given that many PPCP/EDCs may be preferentially distributed in plant roots as compared to above-ground tissues (e.g. Boxall et al., 2006), the potential risk may be significantly greater for root vegetables such as carrots, radishes, and onions. The occurrence of these and other PPCP/EDCs in leafy and root vegetables should be evaluated in the field under typical cultivation and management conditions.
Highlights.
Accumulation of bisphenol A, diclofenac, naproxen, and 4-nonylphenol by lettuce and collards was examined.
Plant accumulation had the order of BPA > NP > DCL > NPX.
Accumulation in roots was greater than in new leaves, original leaves, and stems.
Nearly all accumulated analyte was non-extractable.
Table 3.
Annual human exposure to PPCP/EDCs in leafy vegetables, calculated from the weighted concentration in leaves (wet weight) in this study and the mean intake of leafy vegetables for a 70 kg individual.
| Bisphenol A | Diclofenac | Naproxen | Nonylphenol | |
|---|---|---|---|---|
| Lettuce | ||||
| Tissue concentration (mg/kg) |
0.23 × 10−4 | 3.01 × 10−4 | 4.84 × 10−4 | 1.20 × 10−4 |
| Human exposurea (mg) |
0.32 × 10−3 | 4.15 × 10−3 | 6.67 × 10−3 | 1.65 × 10−3 |
| Medical dose equvalents b |
--- | <0.001 | <0.001 | --- |
| E2-equivalentsc (ng) | 0.032 | --- | --- | 1.65 |
| Collards | ||||
| Tissue concentration (mg/kg) |
3.31 × 10−4 | 1.55 × 10−4 | 9.95 × 10−4 | 7.78 × 10−4 |
| Human exposurea (mg) |
4.57 × 10−3 | 21.42 × 10−3 | 13.72 × 10−3 | 10.74 × 10−3 |
| Medical dose equivalentsb |
--- | <0.001 | <0.001 | --- |
| E2-equivalentsc (ng) | 0.457 | --- | --- | 10.74 |
Human exposure based on leafy vegetable intake of 0.54 gwet weight/kgbody weight-day (U.S. Environmental Protection Agency, 2011).
Dose of Diclofenac = 150 mg. Dose of Naproxen = 250 mg.
BPA Relative Potency to 17β-estradiol (E2) = 1.0 × 10−4. NP Relative Potency to E2 = 1.0 × 10−3 (Bonefeld-Jorgensen et al., 2007).
Acknowledgements
This research was supported by the NIH/NIEHS NRSA T32 institutional training grant (T32 ES018227) and USDA-NIFA Grant (2011-67019-21120). The authors also wish to thank Dr. Rong Ji at Nanjing University for providing 14C-labeled nonylphenol.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson P, Denslow N, Drewes JE, Olivieri A, Schlenk D, Snyder S. Monitoring strategies for chemicals of emerging concern (CECs) in recycled water. California State Water Resources Control Board; 2010. [Google Scholar]
- Avisar D, Lester Y, Ronen D. Sulfamethoxazole contamination of a deep phreatic aquifer. Sci. Total Environ. 2009;407:4278–4282. doi: 10.1016/j.scitotenv.2009.03.032. [DOI] [PubMed] [Google Scholar]
- Barnes KK, Kolpin DW, Furlong ET, Zaugg SD, Meyer MT, Barber LB. A national reconnaissance of pharmaceuticals and other organic wastewater contaminants in the United States — I) Groundwater. Sci. Total Environ. 2008;402:192–200. doi: 10.1016/j.scitotenv.2008.04.028. [DOI] [PubMed] [Google Scholar]
- Benotti MJ, Snyder SA. Pharmaceuticals and endocrine disrupting compounds: implications for ground water replenishment with recycled water. Ground Water. 2009;47:499–502. doi: 10.1111/j.1745-6584.2009.00587_4.x. [DOI] [PubMed] [Google Scholar]
- Benotti MJ, Trenholm RA, Vanderford BJ, Holady JC, Stanford BD, Snyder SA. Pharmaceuticals and endocrine disrupting compounds in U.S. drinking water. Environ. Sci. Technol. 2008;43:597–603. doi: 10.1021/es801845a. [DOI] [PubMed] [Google Scholar]
- Bonefeld-Jørgensen EC, Long M, Hofmeister MV, Vinggaard AM. Endocrine-disrupting potential of bisphenol A, bisphenol A dimethacrylate, 4-n-nonylphenol, and 4-n-octylphenol in vitro: new data and a brief review. Environ Health Perspect. 2007;115:69–76. doi: 10.1289/ehp.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxall ABA, Johnson P, Smith EJ, Sinclair CJ, Stutt E, Levy LS. Uptake of veterinary medicines from soils into plants. J. Agric. Food Chem. 2006;54:2288–2297. doi: 10.1021/jf053041t. [DOI] [PubMed] [Google Scholar]
- Briggs GG, Bromilow RH, Evans AA. Relationships between lipophilicity and root uptake and translocation of non-ionised chemicals by barley. Pestic. Sci. 1982;13:504. [Google Scholar]
- Calderón-Preciado D, Matamoros V, Bayona JM. Occurrence and potential crop uptake of emerging contaminants and related compounds in an agricultural irrigation network. Sci. Total Environ. 2011;412-413:14–19. doi: 10.1016/j.scitotenv.2011.09.057. [DOI] [PubMed] [Google Scholar]
- Calderón-Preciado D, Renault Q, Matamoros V, Cañameras N, Bayona JM. Uptake of organic emergent contaminants in spath and lettuce: an in vitro experiment. J. Agric. Food Chem. 2012;60:2000–2007. doi: 10.1021/jf2046224. [DOI] [PubMed] [Google Scholar]
- California State Water Resources Control Board . Recycled Water Policy. 2009. [Google Scholar]
- Celiz MD, Tso J, Aga DS. Pharmaceutical metabolites in the environment: Analytical challenges and ecological risks. Environmental Toxicology and Chemistry. 2009;28:2473–2484. doi: 10.1897/09-173.1. [DOI] [PubMed] [Google Scholar]
- Chefetz B, Mualem T, Ben-Ari J. Sorption and mobility of pharmaceutical compounds in soil irrigated with reclaimed wastewater. Chemosphere. 2008;73:1335–1343. doi: 10.1016/j.chemosphere.2008.06.070. [DOI] [PubMed] [Google Scholar]
- Cleuvers M. Mixture toxicity of the anti-inflammatory drugs diclofenac, ibuprofen, naproxen, and acetylsalicylic acid. Ecotoxicol. Environ. Safety. 2004;59:309–315. doi: 10.1016/S0147-6513(03)00141-6. [DOI] [PubMed] [Google Scholar]
- Collins CD, Martin I, Doucette W. Plant uptake of xenobiotics. In: Schröder P, Collins, Christopher D, editors. Organic Xenobiotics and Plants, Plant Ecophysiology. Springer; Netherlands, Dordrecht: 2011. pp. 3–16. [Google Scholar]
- Calderón-Preciado D, Jiménez-Cartagena C, Matamoros V, Bayona JM. Screening of 47 organic microcontaminants in agricultural irrigation waters and their soil loading. Water Res. 2011;45:221–231. doi: 10.1016/j.watres.2010.07.050. [DOI] [PubMed] [Google Scholar]
- Daughton CG, Ternes TA. Pharmaceuticals and Personal Care Products in the Environment: Agents of Subtle Change? Environ. Health Perspect. 1999;107:907–938. doi: 10.1289/ehp.99107s6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucette WJ, Wheeler BR, Chard JK, Bugbee B, Naylor CG, Carbone JP, Sims RC. Uptake of nonylphenol and nonylphenol ethoxylates by crested wheatgrass. Environ Toxicol Chem. 2005;24:2965–2972. doi: 10.1897/05-171r.1. [DOI] [PubMed] [Google Scholar]
- Eggen T, Lillo C. The antidiabetic II drug metformin in plants: uptake and translocation to edible parts of cereals, oily seeds, beans, tomato, squash, carrots and potatoes. J. Agric. Food Chem. 2012 doi: 10.1021/jf301267c. [DOI] [PubMed] [Google Scholar]
- European Chemicals Bureau . European Union Risk Assessment Report: 4-nonylphenol (branched) and nonylphenol. 2002. [Google Scholar]
- Herklotz PA, Gurung P, Vanden Heuvel B, Kinney CA. Uptake of human pharmaceuticals by plants grown under hydroponic conditions. Chemosphere. 2010;78:1416–1421. doi: 10.1016/j.chemosphere.2009.12.048. [DOI] [PubMed] [Google Scholar]
- Holling CS, Bailey JL, Vanden Heuvel B, Kinney CA. Uptake of human pharmaceuticals and personal care products by cabbage (Brassica campestris) from fortified and biosolids-Damended soils. Journal of Environmental Monitoring. 2012;14:3029. doi: 10.1039/c2em30456b. [DOI] [PubMed] [Google Scholar]
- Kinney CA, Furlong ET, Werner SL, Cahill JD. Presence and distribution of wastewater-derived pharmaceuticals in soil irrigated with reclaimed water. Environ. Toxicol. Chem. 2006;25:317–326. doi: 10.1897/05-187r.1. [DOI] [PubMed] [Google Scholar]
- Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: a national reconnaissance. Environ. Sci. Technol. 2002;36:1202–1211. doi: 10.1021/es011055j. [DOI] [PubMed] [Google Scholar]
- Macherius A, Eggen T, Lorenz W, Moeder M, Ondruschka J, Reemtsma T. Metabolization of the Bacteriostatic Agent Triclosan in Edible Plants and its Consequences for Plant Uptake Assessment. Environ. Sci. Technol. 2012;46:10797–10804. doi: 10.1021/es3028378. [DOI] [PubMed] [Google Scholar]
- Marwick C. Hormonally active agents throughout the environment. JAMA. 1999;282:722. [PubMed] [Google Scholar]
- Matamoros V, Nguyen LX, Arias CA, Salvadó V, Brix H. Evaluation of aquatic plants for removing polar microcontaminants: A microcosm experiment. Chemosphere. 2012;88:1257–1264. doi: 10.1016/j.chemosphere.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Noureddin MI, Furumoto T, Ishida Y, Fukui H. Absorption and metabolism of bisphenol A, a possible endocrine disruptor, in the aquatic edible plant, water convolvulus (Ipomoea aquatica) Biosci., Biotechnol., Biochem. 2004;68:1398–1402. doi: 10.1271/bbb.68.1398. [DOI] [PubMed] [Google Scholar]
- Pilon-Smits E. Phytoremediation. Annu. Rev. Plant Biol. 2005;56:15–39. doi: 10.1146/annurev.arplant.56.032604.144214. [DOI] [PubMed] [Google Scholar]
- Sandermann H., Jr. Plant metabolism of xenobiotics. Trends in Biochemical Sciences. 1992;17:82–84. doi: 10.1016/0968-0004(92)90507-6. [DOI] [PubMed] [Google Scholar]
- Seyfferth AL, Henderson MK, Parker DR. Effects of common soil anions and pH on the uptake and accumulation of perchlorate in lettuce. Plant Soil. 2008;302:139–148. [Google Scholar]
- Shenker M, Harush D, Ben-Ari J, Chefetz B. Uptake of carbamazepine by cucumber plants – a case study related to irrigation with reclaimed wastewater. Chemosphere. 2011;82:905–910. doi: 10.1016/j.chemosphere.2010.10.052. [DOI] [PubMed] [Google Scholar]
- Soares A, Guieysse B, Jefferson B, Cartmell E, Lester JN. Nonylphenol in the environment: a critical review on occurrence, fate, toxicity and treatment in wastewaters. Environ. Int. 2008;34:1033–1049. doi: 10.1016/j.envint.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Staples CA, Dome PB, Klecka GM, Oblock ST, Harris LR. A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere. 1998;36:2149–2173. doi: 10.1016/s0045-6535(97)10133-3. [DOI] [PubMed] [Google Scholar]
- Sterling TM. Mechanisms of herbicide absorption across plant membranes and accumulation in plant cells. Weed Sci. 1994;42:263–276. [Google Scholar]
- Trapp S. Plant uptake and transport models for neutral and ionic chemicals. Environ. Sci. Pollut. R. 2004;11:33–39. doi: 10.1065/espr2003.08.169. [DOI] [PubMed] [Google Scholar]
- Trapp S, Legind CN. Uptake of organic contaminants from soil into vegetables and fruits. In: Swartjes FA, editor. Dealing with Contaminated Sites. Springer; Netherlands, Dordrecht: 2011. pp. 369–408. [Google Scholar]
- Triebskorn R, Casper H, Heyd A, Eikemper R, Köhler H-R, Schwaiger J. Toxic effects of the non-steroidal anti-inflammatory drug diclofenac: Part II. Cytological effects in liver, kidney, gills and intestine of rainbow trout (Oncorhynchus mykiss) Aquat. Toxicol. 2004;68:151–166. doi: 10.1016/j.aquatox.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Tsantili-Kakoulidou A, Panderi I, Csizmadia F, Darvas F. Prediction of distribution coefficient from structure. 2. Validation of Prolog D, an expert system. J. Pharm. Sci. 1997;86:1173–1179. doi: 10.1021/js9601804. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency . Exposure Factors Handbook. 2011th ed National Center for Environmental Assessment; Washington, D.C.: 2011. [Google Scholar]
- Vanderford BJ, Snyder SA. Analysis of pharmaceuticals in water by isotope dilution liquid chromatography/tandem mass spectrometry. Environ. Sci. Technol. 2006;40:7312–7320. doi: 10.1021/es0613198. [DOI] [PubMed] [Google Scholar]
- Wu X, Conkle JL, Gan J. Multi-residue determination of pharmaceutical and personal care products in vegetables. J. Chromatogr. A. 2012;1254:78–86. doi: 10.1016/j.chroma.2012.07.041. [DOI] [PubMed] [Google Scholar]