Abstract
Rationale
Initial screening of new medications for potential efficacy (i.e. FDA early Phase 2), such as in aiding smoking cessation, should be efficient in identifying which drugs do, or do not, warrant more extensive (and expensive) clinical testing.
Objectives
This focused review outlines our research on development, evaluation, and validation of an efficient crossover procedure for sensitivity in detecting medication efficacy for smoking cessation. First-line FDA-approved medications of nicotine patch, varenicline, and bupropion were tested, as model drugs, in 3 separate placebo-controlled studies. We also tested specificity of our procedure in identifying a drug that lacks efficacy, using modafinil.
Results
This crossover procedure showed sensitivity (increased days of abstinence) during week-long “practice” quit attempts with each of the active cessation medications (positive controls) vs. placebo, but not with modafinil (negative control) vs. placebo, as hypothesized. Sensitivity to medication efficacy signal was observed only in smokers high in intrinsic quit motivation (i.e. already preparing to quit soon) and not smokers low in intrinsic quit motivation, even if monetarily reinforced for abstinence (i.e., given extrinsic motivation).
Conclusions
A crossover procedure requiring less time and fewer subjects than formal trials may provide an efficient strategy for a go/no-go decision whether to advance to subsequent Phase 2 randomized clinical trials with a novel drug. Future research is needed to replicate our results and evaluate this procedure with novel compounds, identify factors that may limit its utility, and evaluate its applicability to testing efficacy of compounds for treating other forms of addiction.
Keywords: Medication screening, Pharmacotherapy, Nicotine dependence, Addiction
Introduction
Despite passage of a half century since the first U.S. Surgeon General’s Report on health risks of tobacco smoking (USPHS 1964), persistent cigarette smoking by nearly 50 million Americans is still the greatest preventable cause of mortality in the U.S. at 20% of all deaths, or over 400,000 per year (Rostron 2013; USDHHS 2010). Worldwide, these prevalence and mortality numbers are ten-fold higher and rising (Giovino et al. 2012), foreshadowing continuing severe public health problems for decades to come. Despite most smokers stating a desire to quit, only a fraction actually do try to quit each year (e.g. Wewers et al. 2003). Moreover, 5% or fewer of those quit attempts are successful at one year (CDCP 2011), even though many quit attempts involve medication use (Hughes et al. 2009). Without efficient development of more effective medications, disappointing quit rates are likely to continue (e.g. Hughes 2011). This overview summarizes work from 2004-present in developing and validating a novel, efficient procedure to provide an early signal for medication efficacy (Perkins et al. 2006; 2008; 2010; 2013b).
In brief, our current procedure involves a within-subject comparison in the number of abstinent days during separate week-long “practice” quit attempts (e.g. Carpenter et al. 2011) under active medication vs. placebo conditions, testing smokers high in quit interest (wanting to quit soon, generally within the next 3 months). We tell them the study is aimed at assessing “short-term effects of medication,” and that participation may help them “learn how to be successful when you quit smoking permanently.” The initial quit week is when medication efficacy may be most critical, since abstinence with medication over the first 1–2 weeks of a quit attempt strongly predicts later cessation outcome (e.g., Kenford et al. 1994; Wileyto et al. 2004; Ferguson et al. 2009; Ashare et al. 2013), as also discussed in detail under Further Considerations. Because the duration of attempting to quit during our procedure is brief for each medication condition, our daily outcome measure for cessation, assessed at brief visits each weekday, is no smoking at all in the prior 24 hr. Importantly, we use the stringent criterion of CO<5 ppm as the objective biochemical validation of abstinence, to allow detection of even a brief lapse (Perkins et al. 2013a; see also Wileyto et al. 2004). Also, study sessions are scheduled for afternoons to allow assessment of mid-day CO (rather than CO just after waking; e.g. Perkins et al. 2009). Participants receive approximately $15/visit, which is intentionally kept modest so that their interest in participation is not overly swayed by monetary reimbursement, rather than the optional free treatment to help make a permanent quit attempt provided after the study to attract those with high quit interest. (The total amount of reimbursement varied among our past studies because of the variable number of required visits to complete each study and the randomization of subjects in two studies to presence vs. absence of additional monetary reinforcement for each quit day, as explained below in the detailed description of each.)
The rationale for a new early screening procedure and limitations of existing procedures are discussed next, followed by details of research conducted to develop, test, and validate our procedure. Further research showing the predictive validity of our primary outcome measure of week-long days quit, a consideration of our procedure’s limitations, and future directions of this approach are then provided.
Rationale for a New Procedure in Early Human Testing of Cessation Medications
Development of new medications for smoking cessation has been hampered by the need to identify promising new compounds (e.g., Gorodetzky and Grudzinkas 2005) and inefficiencies in their evaluation (Bough et al. 2013; Lerman et al., 2007). Accelerating this success may require more efficient detection of efficacy for cessation in these novel medications, to better guide preparations for their formal testing in clinical trials. Efficient early screening is mainly defined here by obtaining a valid answer as to the likely clinical efficacy of a medication versus a comparison (i.e. placebo, or current standard treatment) using the smallest sample of participants and/or shortest duration of testing possible (Kola 2008; Lesko 2007; Streiner 2007). Dozens of drugs have been tested for efficacy in smoking cessation over the last 20 years, but few of these have shown success (Benowitz and Peng 2000; Foulds et al. 2006; Harmey et al. 2012; Hughes et al. 2004; Schnoll and Lerman 2006; Polosa and Benowitz 2011). Such “failed” clinical trials (i.e. those in which a drug shows no efficacy) constitute unproductive uses of time and resources that ideally should be devoted to more promising candidate drugs.
The costs and time involved in medication development increase with each subsequent step in the evaluation process up to regulatory approval by governmental regulatory agencies, such as the U.S. Food and Drug Administration (FDA), the British Medicines and Healthcare Products Regulatory Agency (MHRA), or similar organizations (e.g., DiMasi et al. 2003; USDHHS 2004; see also McCormick and Olsen 2013). In brief, the FDA phases of human research to obtain approval for new medications are as follows (Vocci 1996). Preclinical animal studies with a compound showing likely safety characteristics and potential translational findings in experimental models (Miczek and de Wit 2008) are followed by FDA Phase 1 testing of safety and tolerability in humans (Butz and Morelli 2008), and treatment efficacy is usually not a focus. Drugs successful in Phase 1 can proceed to Phase 2, which first provides initial tests of therapeutic efficacy in highly-controlled studies of otherwise healthy patients. These studies are often labeled “Phase 2a”, or “early Phase 2”, to differentiate the first evaluations of evidence for efficacy in a select group of participants from the somewhat more extensive single clinical trials with more representative patients typical of “Phase 2b”, or “late Phase 2” (Sheiner 1997). Success in Phase 2 leads to Phase 3 confirmation of that evidence for treatment efficacy in large, heterogeneous patient samples often studied in multi-site clinical trials, prior to FDA approval of the medication for commercial marketing. Phase 4 follows FDA approval of the new medication and usually involves post-marketing surveillance of possible adverse effects, exploration of new treatment indications, etc.
Fewer than 10% of all new compounds entering Phase 1 human testing for any treatment indication eventually succeed in becoming FDA approved and marketed (USDHHS 2004), perhaps comparable to the low success rate in novel drugs evaluated for smoking cessation, as suggested above. Therefore, the efficient use of resources to speed delivery of medications to patients needing them partially requires that drugs unlikely to be effective be so identified as early as possible and dropped from further consideration (Kola 2008; Paul et al. 2010). Efficient Phase 2a evaluation of initial efficacy is critical since continuing into Phase 2b, and certainly Phase 3, of drug development involves committing substantial resources and time to test the drug’s clinical efficacy in large numbers of patients (e.g., Kroboth et al. 1991; Sheiner 1997), in this case smokers wanting to quit permanently. Note that the medication development issues to be outlined here involve only research on detecting evidence of clinical efficacy for smoking cessation, not smoking reduction (e.g. Levy et al. 2007; Stead and Lancaster 2007), during early Phase 2 testing. Not discussed are numerous other factors that can influence commercial decisions to proceed with efforts to bring a new medication to market (e.g. its safety, or its efficacy compared to existing treatments; DiMasi et al. 2003; Kola 2008; see also Lerman et al. 2007). Moreover, not included here but warranting research attention are strategies to encourage far more smokers to use existing efficacious medications to quit smoking, as well as methods to foster their appropriate use as directed (e.g. Cummings and Hyland 2005; Mahtani et al. 2011; Shiffman 2007).
Inefficiencies are also common in development of new medications to treat other drug dependence problems (e.g., Amato et al. 2011; Koob et al. 2009; Pierce et al. 2012) and have prompted FDA to establish the Critical Path Initiative to foster greater innovation in medication development procedures (Woodcock and Woosley 2008). The Executive Summary of the initial FDA report calling for this Initiative stated: “Not enough applied scientific work has been done to create new tools to get fundamentally better answers about how the safety and effectiveness of new products can be demonstrated, in faster time frames, with more certainty, and at lower costs….As a result, the vast majority of investigational products that enter clinical trials fail.” (USDHHS 2004). Later, this report notes “…a striking feature of this path is the difficulty, at any point, of predicting ultimate success with a novel candidate…..[and] inability to predict these failures before human testing or early in clinical trials dramatically escalates costs.” Among the Initiative’s recommended changes is validation of new biomarkers and surrogate endpoints to help streamline early clinical trials and improve the cost-effectiveness of medication development (e.g., Lesko 2007; Paul et al. 2010). In the current paper, we describe our decade of effort to develop, test, and validate an efficient new early Phase 2 testing procedure to evaluate initial evidence of efficacy in novel medications to treat smoking cessation, which may have applicability in similar initial evaluations of medications to treat other substances of abuse.
Limitations of Current Early Phase 2 Testing
A key question in enhancing early Phase 2 research efficiency is how to determine, in the shortest time and/or with the fewest subjects, whether a novel medication has evidence of treatment efficacy to inform the conduct of subsequent clinical trials. Ideally, positive results for an active novel medication (e.g. versus placebo) in early Phase 2 should support proceeding to the larger late Phase 2 assessments of the medication for cessation in randomized clinical trials, while negative results in early Phase 2 should indicate the novel medication may not be sufficiently efficacious as to warrant costs and time of late Phase 2 testing. The most common early Phase 2 approach, small (or “pilot”) randomized clinical trials of smokers trying to make a permanent quit on active vs. placebo, can be valid in predicting that medication’s efficacy in large Phase 3 trials, if sufficiently powered (e.g. N=190 in Ferry and Burchette 1994). Yet, they often may be inconclusive due to a lack of statistical power for between-groups comparisons resulting from their typically small sample sizes (often fewer than 50 per group; e.g. Benowitz and Peng 2000; Scholl and Lerman 2006; Farid and Abate 1998). Also, they can still be a time-consuming and expensive proposition for testing a novel compound of totally unknown efficacy, especially if the trial is not so small (Paul et al. 2010).
A second typical, though secondary, approach for trying to detect initial efficacy in a novel cessation medication (and often incorporated into Phase 1 studies of safety testing) is to assess brief responses on measures indirectly related to cessation among volunteer smokers after short-term exposure to active vs. placebo medication, often using within-subjects comparisons. These measures usually focus on whether the drug blunts abstinence-induced symptoms, such as withdrawal or craving, or briefly attenuates positive effects of acute smoking by assessing reduction in ad lib cigarette consumption or in the reinforcing effects of smoke intake (e.g. Lerman et al. 2007). However, as outlined next, changes in these measures are not strongly predictive of cessation outcome (i.e. quit initiation or duration), perhaps partly because the subjects in these tests tend to be non-quitting smokers being paid to participate, who are more numerous and easy to recruit than smokers actively trying to quit (Wewers et al. 2003). As a consequence, such research using known effective medications as model drugs very often does not provide results clinically consistent with the cessation efficacy of those drugs in formal randomized trials (Perkins et al. 2006).
In studies of abstinence-induced symptoms, for example, nicotine gum was shown in one recent study to have no effect on relieving craving or withdrawal during 6 hr abstinence in non-quitting smokers (Brown et al. 2013). Also, smokers paid to undergo 3 days of enforced abstinence for the study showed craving relief but no withdrawal relief from 21 mg nicotine vs placebo patch (Teneggi et al. 2002), or showed only limited withdrawal relief but no craving relief from 300 mg bupropion vs placebo (Shiffman et al. 2000). These symptoms can help determine medication efficacy for aiding cessation in smokers attempting to quit permanently (e.g., Lerman et al. 2002; Shiffman et al. 2006; Foulds et al. 2013) but may have limited validity for detecting medication efficacy in those abstaining temporarily just for study purposes.
Regarding attenuation of smoking’s positive effects, a comparable inpatient study of non-quitting smokers found no significant decrease in ad libitum smoking due to using two 21-mg nicotine patches per day, or 42 mg in total, compared to use of placebo patches (Benowitz et al. 1998). Similarly, acute bupropion dose-dependently increased ad libitum smoking over 3 hr in non-quitting smokers, contrary to its efficacy for cessation and as also found in this study with the comparison drug of amphetamine (Cousins et al. 2001). Fewer studies of varenicline effects vs. placebo in non-quitting smokers have been reported, but one recent between-groups study found no significant effects of varenicline or bupropion vs. placebo in delaying smoking onset during a 50-min cigarette access period following instructed overnight abstinence in 62 non-quitters (McKee et al. 2012). Latency to smoke was delayed by either medication among the subgroup of 27 reporting they typically smoked within 5 mins of waking (McKee et al. 2012). However, more research is needed to determine whether delaying acute smoking latency in this manner relates to success with cessation, or rather with tobacco reduction (e.g. Levy et al. 2007; Stead and Lancaster 2007). Other research has shown little influence of varenicline on smoking topography or craving over 21 days in non-quitters (e.g. Ashare et al. 2012).
If these results, showing modest or no medication effects on reducing withdrawal or smoking behavior in non-quitting smokers, had comprised the initial tests of efficacy in early Phase 2 screening of these medications for cessation, they may have discouraged proceeding with further clinical testing (i.e. late Phase 2, or Phase 3). Thus, these current early Phase 2 approaches generally involve under-powered and/or expensive small clinical trials with quitting smokers that may often be valid but impractical for testing novel drugs of totally unknown efficacy, or brief lab-based studies of acute drug effects in non-quitting smokers that are practical but often invalid (Perkins et al. 2006).
Overview of Procedure Development
We have long proposed that screening of novel cessation medications may be more efficient if initial human studies for early Phase 2 efficacy testing optimally combine the practical advantages of the lab tests with the validity of clinical trials (e.g. Perkins et al., 2006; Lerman et al., 2007), a strategy that guided the development of our procedure. We proceeded under the assumption that a dependent measure that more closely approximated the clinical outcome of interest would produce findings with greater clinical validity, as recommended for preclinical studies to justify Phase 1 human testing (see Miczek and de Wit 2008). During each of our studies, we have used smoking abstinence as the main index of efficacy and focused on testing smokers with high quit motivation, design features which are typical of clinical trials but not of lab studies. Yet, to achieve adequate statistical power with only a modest sample (i.e. greater efficiency), we have employed a within-subject, cross-over design (Cleophas 1993; Cohen 1988; Fletcher et al. 1990), typical in lab studies but not in clinical trials. Further enhancing power is our use of the quantitative dependent measure of number of days quit per medication period, rather than the dichotomous measure of quit/not quit at one follow-up assessment point (Cohen 1988). The high predictive clinical significance of days quit during brief abstinence attempt periods is described below (in Validation of Days Quit Efficacy Measure under the Future Directions section; see also Ashare et al. 2013).
In this development research, we used FDA-approved cessation medications as model drugs (i.e., positive controls) to determine the sensitivity of our early Phase 2 procedure in detecting efficacy (Koob et al. 2009). Each of our three primary studies to test this procedure is described in more detail below. Using diagnostic test terminology (e.g. Glaros and Kline 1988), we have demonstrated that our procedure has: 1) sensitivity in confirming efficacy in all three first-line cessation medications (NRT patch, varenicline, bupropion), and 2) specificity in verifying lack of efficacy in a medication known to be ineffective for cessation (modafinil). The ultimate purpose of this procedure is to detect evidence of treatment efficacy in novel compounds for smoking cessation, and perhaps for treating other addictions and behavioral problems. Its over-riding goal is not to replace traditional randomized late Phase 2 clinical trials but to improve the efficiency of the early Phase 2 testing which informs the decision of whether the time and costs of such trials are warranted (Perkins et al. 2006). This procedure may accelerate medication development by increasing the efficiency by which promising drugs do, and unpromising drugs do not, proceed to these larger Phase 2 clinical trials. It may be particularly useful in evaluating medications already FDA-approved for other indications (and thus already successful in Phase 1) but suspected of efficacy for smoking cessation (i.e. “repurposing”; Harmey et al. 2012), as occurred with bupropion (Ferry 1999). Potential strengths and limitations of our procedure and its potential alternative applications are addressed in the final sections of this paper.
Crossover Procedure Validation Studies
Sensitivity to Transdermal Nicotine Patch Efficacy
We began this effort by evaluating effects of nicotine versus placebo patch on ability to abstain during a brief quit period, as a function of participants’ level of quit motivation (Perkins et al. 2008). Our goal was to test whether the signal for medication efficacy is greater in smokers who are high in quit interest versus most typical smokers, who are low in current quit interest. We used two ways to vary quit motivation: 1) “intrinsic” motivation due to their pre-existing high (N=47) or low (N=93) quit interest (i.e. whether they were already intending to quit permanently soon, within 1 month, or not intending to quit soon, within the next 6 months), and 2) “extrinsic” motivation via randomization to presence (n=71) vs. absence (n=69) of monetary reinforcement for daily abstinence ($12/day), similar to contingency management for cessation (e.g. Stitzer et al. 1986). The objective of this first study was to identify the optimum sample for demonstrating sensitivity to a medication’s efficacy, and thus of primary interest was the interaction of each quit motivation variable with medication condition (nicotine vs. placebo) on number of quit days. We hypothesized that smokers high in “intrinsic” quit motivation would exhibit greater differences in effects of active vs. placebo medication on number of days quit, compared to those low in “intrinsic” quit motivation (Perkins et al. 2006). On the other hand, efforts to increase “extrinsic” motivation via monetary reinforcement alone have long been demonstrated to increase quitting (Stitzer et al. 1986), even in smokers not otherwise motivated to quit (i.e. those with low intrinsic quit interest; Romanowich and Lamb 2010a). If such extrinsic quit motivation increased sensitivity to a medication’s efficacy for cessation, our early Phase 2 cross-over procedure likely could be completed more quickly and easily, maximizing testing efficiency; a subject sample of non-quitting smokers monetarily reinforced daily for quitting would likely be far easier to recruit compared to smokers high in intrinsic quit interest (Wewers et al. 2003).
Because no medication “run-up” is needed for transdermal nicotine (e.g. Mulligan et al. 1990), all subjects smoked normally during weeks 1 and 3 and were instructed to try to meet quit criteria for 4 days (Tues-Fri) each during weeks 2 and 4 on one patch condition or the other (in counter-balanced order). As noted, abstinence was defined at daily visits by the criteria of CO < 5 ppm and self-report of no smoking at all in the prior 24 hr. The commonly used CO cutoffs for abstinence of 8 ppm or 10 ppm (SRNT 2002) have lower sensitivity for detecting recent smoking (Javors et al. 2005; Perkins et al. 2013a), which clearly impacts outcome results for any test of a medication’s efficacy for abstinence. Study visits were brief, mostly entailing assessment of CO and self-report measures of withdrawal, craving, adverse effects, etc. These afternoon visits were scheduled a few days per week in weeks 1 and 3 during ad lib smoking, and on all 5 weekdays to verify daily quit status during weeks 2 and 4. Subjects applied the nicotine (21 mg) or placebo patch every Mon-Fri a.m., with patches available under blind conditions and in counter-balanced order between subjects across weeks. (Quit days for formal study analyses were assessed Tues-Fri because the first day of patch use was Mon, which did not allow enough time to test patch effects on meeting the 24-hr quit criteria by the Mon p.m. session.) To allow comparable testing of efficacy for cessation in both crossover conditions, week 3 required resumption of ad lib smoking as a “washout” period following the first patch condition in week 2 and prior to the second patch condition in week 4.
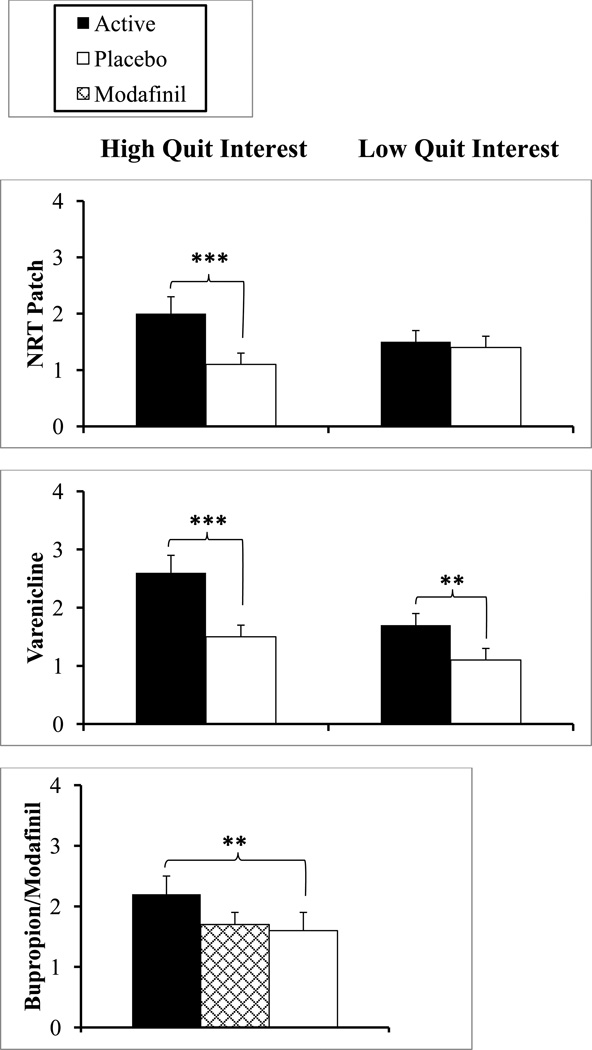
Results, in Figure 1, showed that nicotine (vs. placebo) patch during one week’s use increased days of abstinence in smokers who were high, but not low, in intrinsic quit interest (Perkins et al. 2008), with a significant interaction of nicotine × intrinsic quit interest (p<.005). Specifically, nicotine patch roughly doubled the number of abstinent days versus placebo patch during these short-term quit attempts in smokers with high intrinsic quit interest (p < .001; partial eta squared effect size of .317), while those low in intrinsic quit interest showed no difference between patch conditions. In contrast, there was no interaction of nicotine × extrinsic quit interest (monetary reinforcement for abstinence), despite a main effect of money in enhancing number of quit days (p<.005), similar to studies of monetary reinforcement alone, without medication (e.g. Kollins et al. 2010; Lamb et al. 2010). The triple interaction of nicotine × intrinsic interest × extrinsic interest also was not significant.
Figure 1.
Mean (SEM) days of abstinence during brief quit attempts on active medication versus placebo in 3 separate crossover studies, testing smokers high or low in intrinsic quit interest in the NRT and Varenicline studies but only smokers high in quit interest in the Bupropion/Modafinil study.
** p≤.01, *** p<.001 for difference from placebo.
After completing the study, all participants were offered brief cessation counseling to help make a permanent quit attempt as a benefit of study participation. To help us validate their high vs low intrinsic quit interest, we also assessed self-report of recent smoking behavior after two weeks post-study in all participants, regardless of original quit interest. During this brief follow-up phone contact on their current smoking, they were asked if they had “not quit or cut down”, “cut down but did not quit”, or “quit”, and then for how long if they said they had “quit”. Only if this quit lasted at least 24 hr was it considered a true post-study quit attempt, which occurred in 62% of those high, compared to 18% of those low, in intrinsic quit interest as assessed at study screening, consistent with expectations. Responses at this point were expected to be valid since there was no longer any contingency for being quit and subjects had already received payment for study participation, ending study contact. Those with high intrinsic quit interest who were unable to quit for 24 hr post-study may have required greater cessation assistance to be successful. (Even if those identified as such had not truly been high in intrinsic quit interest at screening, despite ability to qualify for the study if low in quit interest, their inclusion here would be expected to dilute the effect of intrinsic quit interest on sensitivity to nicotine vs placebo patch on the study quit days.)
Thus, in this first test, high intrinsic quit motivation (pre-existing quit interest) enhanced nicotine patch efficacy over placebo (i.e. increased sensitivity), while extrinsic quit motivation (money for each quit day) did not. Furthermore, other results of this initial study showed the feasibility of our cross-over design for this research: 1) there were no significant order effects of nicotine and placebo patch on abstinence, 2) no subject declined to resume smoking during week 3, between the patch conditions, and 3) the total participant drop-out rate was below 20%. Feasibility of this procedure is also addressed in Future Considerations, below, along with more discussion of the association of number of study days quit with post-study quit status.
Sensitivity to Varenicline Efficacy
Results of this first study, with nicotine patch, were informative in validating our early Phase 2 procedure. However, the importance of recruiting smokers high in intrinsic quit motivation to detect efficacy in a potentially broad array of novel medications required cross-validation of these findings with another medication. Consequently, our follow-up study validated the sensitivity of this procedure with varenicline versus placebo (Perkins et al. 2010), using a similar within-subjects, cross-over design and the comparison of subgroups high vs. low in intrinsic and extrinsic quit interest. As in our patch study, intrinsic quit motivation comprised smokers already high (n=57) vs. smokers low (n=67) in current quit interest, and extrinsic motivation involved subgroups randomized to presence (n=61) vs. absence (n=63) of monetary reinforcement for daily abstinence. The only difference in this study was that an extra week was needed for adequate dose run-up with each medication condition (including placebo, to maintain double-blind). Thus, all subjects engaged in two 3-week phases (six weeks in all), each phase involving a week of ad lib smoking without medication (baseline), a week of ad lib smoking during medication run-up, and a week of attempting to quit while on the full medication dose (1.0 mg varenicline b.i.d) or placebo. Brief visits for each study phase were scheduled a few days in each of the first two weeks and every weekday of the “quit week” (week 3), and the primary dependent measure was the number of days abstinent out of five (Mon-Fri), verified daily by CO<5 ppm.
Similar to our patch study, results showed a significant interaction of varenicline × intrinsic quit interest on number of quit days (p=.05), with greater sensitivity to varenicline in smokers high vs. low in intrinsic quit interest (effect sizes of .373 and .121, respectively), as also shown in Figure 1. (Note in Figure 1 that varenicline did show some efficacy in those with low quit interest, perhaps demonstrating evidence of its robustness for aiding cessation; Gonzales et al. 2006). The interaction of varenicline × extrinsic quit interest (reinforcement) was not significant (p>.10), as with the triple interaction of varenicline × intrinsic interest × extrinsic interest. Among all subjects, varenicline (vs placebo) increased continuous abstinence on all 5 days throughout the quit week (21.0% vs. 8.1%, p<.001). However, medication order effects were found, as those who received varenicline in the first phase quit more days overall (i.e. with each medication condition) than those who received placebo first. Yet, the varenicline × medication order interaction was not significant (p>.20), because the increase in quit days due to varenicline vs. placebo was similar regardless of when varenicline was received, lessening the impact of drug order on interpreting medication efficacy. Also very consistent with the patch study, post-study validation of intrinsic quit interest during the follow-up phone contact showed that quitting for at least 24 hr was found in 61% of those high, versus 11% of those low, in intrinsic quit interest.
Therefore, we replicated with varenicline our key finding with nicotine patch (Perkins et al. 2008), that sensitivity to effects of medication vs placebo on increasing days of abstinence depended on testing smokers with high intrinsic quit motivation but not high extrinsic motivation (daily monetary reinforcement for quitting). An important implication is that the results of efforts to test medications in non-treatment seeking smokers who are low in intrinsic quit motivation may have lower predictive validity with respect to cessation efficacy in the target population, even if monetary reinforcement for quitting is utilized. Our findings may also be relevant for acute laboratory modeling of lapse behavior in those not high in quit interest, such as tests of medication or other effects on ability to delay smoking initiation over 1–2 hr after overnight abstinence when every few mins of refraining from smoking is monetarily reinforced (e.g. Mueller et al. 2009; McKee et al. 2012).
Sensitivity to Bupropion Efficacy and Specificity Testing with Modafinil
Using this procedure, our study with varenicline replicated our patch results by demonstrating the influence of these between-subjects factors of intrinsic and extrinsic quit motivation on enhancing and not enhancing, respectively, sensitivity to the medication’s efficacy for quitting. Therefore, we concluded that the optimum subject sample for our early Phase 2 cross-over procedure should comprise smokers high in intrinsic quit interest, and that monetary reinforcement for quitting did not matter here. Subsequently, our third study (Perkins et al. 2013b) further validated our refined model, testing only smokers high in intrinsic quit interest, by assessing both its sensitivity with the third FDA-approved cessation medication, bupropion, as well as its specificity for detecting failure of an ineffective medication to aid quitting (Glaros and Kline 1988), using modafinil. Modafinil is FDA-approved to improve wakefulness but has been shown to be ineffective for smoking cessation (Schnoll et al. 2008) and for acute relief of withdrawal (Schnoll et al. 2008; Sofuoglu et al. 2008). The specificity of an early Phase 2 medication screening procedure is as important as its sensitivity because an efficient procedure must also be able to determine whether a novel compound does not warrant further evaluation in larger randomized clinical trials.
To simultaneously assess sensitivity and specificity, we conducted a double cross-over design (e.g. Koch et al. 1989; Parodi et al. 1986) with three medication conditions--bupropion, modafinil, and placebo—each phase involving 3 weeks for baseline, dose run-up, and quit week assessment on medication (9 weeks in total). As with our varenicline study (Perkins et al. 2010), medication conditions were presented double-blind and in counter-balanced order across weeks (6 possible orders). No between-subjects factors were compared, as all 45 participants were smokers high in intrinsic quit interest, those shown in our prior studies to be more sensitive to medication efficacy in our procedure. (We provided all with $12/day for each quit day to maintain their motivation for participation, given the study duration of 9 weeks, but this reinforcement did not differ between subjects or across the three study phases.) Relative to the placebo condition, bupropion did (p=.01; effect size of .167), but modafinil did not (p=.60), increase number of days abstinent (see Figure 1), both as hypothesized. Also, bupropion (vs placebo) increased ability to maintain continuous abstinence on all 5 days throughout the quit week (24.4% vs. 8.9%, p<.05), while modafinil did not (13.3%). Among those able to initiate quitting during each week, bupropion prevented any lapses in 45.2%, compared to 19.2% for placebo and 22.2% for modafinil. Finally, in follow-up phone contact, post-study quit for at least 24 hr was reported in 58% of these subjects, all high in quit interest, while only 4% reported no change in their smoking.
Further Considerations
Validation of Days Quit Efficacy Measure
Notably, to further validate the primary outcome measure of efficacy in this crossover procedure, data from our prior studies suggest that number of days quit during these week-long assessments may be clinically meaningful (unpublished data). Specifically, we examined the association between number of days quit during these brief attempts with success or failure in subsequently being able to quit (permanently) in the post-study follow-up assessments. To eliminate the influence of monetary reinforcement for days quit, we included only non-reinforced subjects high in quit interest (N=54) from our first two studies, testing nicotine patch or varenicline. Total days abstinent during the two study quit weeks (including placebo) were compared between those able vs. those unable to quit for 24 hrs or longer in the permanent attempt when contacted several days after their target quit date. FTND was included as a covariate, since dependence could explain quit ability during both the study quit weeks and the post-study quit attempt. In the ANCOVA, we found more days quit during the short-term study weeks among those 36 who later quit post-study, compared to those 18 who were unable to quit post-study, with respective means (SE) of 4.1±0.5 vs 0.6±0.7 study days quit, F (1,51)=14.55, p<.001. These results suggest that number of days quit during week-long assessments in this crossover test is a potentially meaningful clinical measure in predicting successful initiation of a permanent quit attempt.
These findings are consistent with Ashare et al. (2013), a very recent analysis of three separate randomized clinical trials (i.e. not crossovers) of those making a permanent quit attempt on FDA-approved medications (NRT patch or spray, or bupropion). In that analysis, number of quit days during the first week of these formal clinical trials very significantly predicted quit status (7-day point prevalence) at the end of treatment 8–10 weeks later and at the 6-month follow-up assessment of quit status (with OR’s>1.4, p<.001; Ashare et al. 2013). Our crossover results are also consistent with other studies showing the first 1–2 weeks of a quit attempt predict long-term success (e.g. Kenford et al. 1994; Perkins et al. 2001; Wileyto et al. 2004; Ferguson et al. 2009; Romanowich & Lamb 2010b), partly why we chose a week-long quit period for assessment under each medication condition in this cross-over procedure.
Feasibility
Our three studies also suggest this crossover design is a feasible approach. The study with varenicline, to replicate the NRT patch results for high/low intrinsic and extrinsic quit interest, showed some evidence of medication carryover effects, in that varenicline resulted in more days quit when it preceded rather than followed the placebo condition (Perkins et al. 2010). Yet, varenicline still significantly increased quit days over placebo under either medication order in this crossover procedure. The other medications we tested, NRT patch in study 1 and bupropion in study 3, did not show significant order effects in the counter-balancing of active vs placebo conditions. Among the smokers high in intrinsic quit interest, who show greater sensitivity to medication efficacy in our studies, as noted, we also found reasonably low rates of drop-out after entering the study. Out of the 192 high quit interest smokers entered in all three studies, 43 dropped out (22% of all entered), with drop-out rates rising as the study duration lengthened (15%, 21%, and 31% for the 4 weeks of NRT, 6 weeks of varenicline, and 9 weeks of bupropion/modafinil, respectively). Moreover, despite the requirement of daily visits to assess quit status each weekday during the quit weeks, fewer than 1% of these visits (15 out of 1621 scheduled visits) were missed by the total of 149 high quit interest smokers who completed the three studies (unpublished).
Potential Limitations
The breadth of our early Phase 2 procedure across the vast array of potential new medications, or other treatments, for smoking cessation and other addictive problems is uncertain and warrants further study. This cross-over design may have less utility in initial tests of efficacy for novel compounds with certain pharmacokinetic or pharmacodynamics characteristics. For example, our procedure may have practical limitations for early assessment of efficacy in medications requiring a long dose run-up period or a long washout period, which would also be required for a placebo condition to maintain blinding. Such kinetic factors could exacerbate the chances of significant carryover effects in a crossover design, which may require procedural alterations in the study to lessen their impact (e.g. Fleiss 1989). Novel drugs with obvious psychoactive or subjective effects would hamper the attempt to maintain blinding to placebo or other comparison medication conditions. Among other limitations may be the relatively few smokers who truly want to quit “soon” (e.g. within the next 3 months), thus demonstrating high quit interest, relative to the number who express a general desire to quit but have no time frame for making a concerted cessation attempt (Wewers et al. 2003).
Discussion and Conclusions
The greater efficiency of our current early Phase 2 crossover procedure is such that fewer than 50 smokers were needed in our third study (and fewer than 60 in the subgroups of those high in quit interest in the first two studies) to demonstrate medication effects on aiding cessation. By contrast, a between-groups early Phase 2 test of initial medication efficacy in a randomized trial with the same statistical power to compare active (i.e. bupropion) and placebo conditions would require more than 100 smokers in each of the two medication conditions (see Fleiss 1986; Perkins and Lerman 2011). This is due to the typically high within-subject correlation of days quit responses to active medication versus placebo, which in this third study was r=.59, both for bupropion and for modafinil, greatly reducing the error variance between conditions. Consequently, the sample size for a two-group randomized trial of similar power as in our crossover trial would be 2/(1-r) × the sample size for our within-S design (N=45), or 2/(1-.59) × 45, which (for this study) is 220 subjects (Fleiss 1986; see also Perkins and Lerman 2011).
In sum, the consistency of our procedure for sensitivity in detecting cessation efficacy is shown by all 3 FDA-approved first-line medications (NRT, varenicline, bupropion) increasing “days quit” over placebo. We demonstrated specificity of the procedure for detecting lack of efficacy by showing that the negative control of modafinil does not increase days quit over placebo (Perkins et al. 2013b). Although replication of our results with this innovative early Phase 2 procedure is needed, we believe these findings provide compelling data to support the notion that efficacy of novel medications for smoking cessation can be detected in a cross-over study in which smokers high in intrinsic quit interest make brief “practice” quit attempts with each condition (i.e. active and placebo). Further, this efficacy can be identified with a sample size of smokers high in quit interest that is much smaller, and a duration of participation shorter, than those in traditional randomized clinical trials, which by definition demonstrates that this procedure has greater efficiency.
The next step is to apply our procedure in the manner for which it is ultimately intended, to screen truly novel medications for smoking cessation, in comparison with placebo and/or a known effective medication to provide a comparator. The double crossover design in our most recent study (Perkins et al. 2013b), with two different active medications along with a placebo, could be used to determine superiority (or equivalence) of efficacy for a novel drug vs. an existing standard treatment drug (Streiner 2007) or to compare two active doses of a novel compound to determine the optimum dose for comparison with placebo in a randomized (and larger) late Phase 2 clinical trial. A potentially similar approach that could be more practical by reducing subject burden may be to conduct separate cross-over early Phase 2 studies comparing each of two active medications with a placebo, to determine non-equivalence (e.g. Song et al. 2008). Our procedure may also be an efficient method to compare efficacy of combination vs. single medications to inform the likely results of subsequent randomized trials of combination treatments (Loh et al. 2012; Issa et al. 2012; Shiltz et al. 2011).
Finally, warranting consideration is the possibility a similar procedure could increase sensitivity and specificity in early Phase 2 tests of novel compounds to treat other drug abuse problems, such as alcohol dependence (Jupp and Lawrence 2009; Ray et al. 2010) and perhaps others (e.g. Koob 2009; Pierce et al. 2012). Findings with such examinations could thereby increase the efficiency of initial screening of new medications for treating these drug dependencies. Conceivably, this crossover approach could be further adapted to evaluate efficacy in new behavioral treatments or other non-medication interventions, perhaps to aid development and refinement of these treatment procedures prior to large, randomized trials of their utility.
Acknowledgment
This paper, and the research it describes, were supported by NIH Grant CA143187. Dr. Perkins has served as a consultant for Embera Neurotherapeutics that is unrelated to the current research. Dr. Lerman has served as a consultant for GlaxoSmithKline, Pfizer, and Astra Zeneca. She has received research funding, unrelated to the current research, from Pfizer and Astra Zeneca. The authors thank K.N. Roy Chengappa, Carolyn Fonte, Joshua Karelitz, and Maxine Stitzer for their assistance in this research.
Contributor Information
Kenneth A. Perkins, Western Psychiatric Institute and Clinic, University of Pittsburgh School of Medicine, 3811 O'Hara Street, Pittsburgh, PA 15213, USA
Caryn Lerman, Center for Interdisciplinary Research on Nicotine Addiction, Department of Psychiatry and Annenberg School for Communication, Abramson Cancer Center of the University of Pennsylvania, 3535 Market Street - Suite 4100, Philadelphia PA 19104 USA.
References
- Ashare R, Tang KZ, Mesaros AC, Blair IA, Leone F, Strasser AA. Effects of 21 days of varenicline versus placebo on smoking behaviors and urges among non-treatment seeking smokers. J Psychopharmacol. 2012;26:1383–1390. doi: 10.1177/0269881112449397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashare R, Wileyto EP, Perkins KA, Schnoll R. The first seven days of a quit attempt predict relapse: validation of a measure for screening medications for nicotine dependence. J Addiction Med. 2013;7:249–254. doi: 10.1097/ADM.0b013e31829363e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato L, Davoli M, Vecchi S, Ali R, Ferrell M, Faggiano F, Foxcroft D, Ling W, Minnozi S, Chengzheng Z. Cochrane systematic reviews in the field of addiction: what’s there and what should be. Drug Alc Dep. 2011;113:96–103. doi: 10.1016/j.drugalcdep.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Peng MW. Non-nicotine pharmacotherapy for smoking cessation. CNS Drugs. 2000;13:265–285. [Google Scholar]
- Bough KJ, Lerman C, Rose JE, McClernon FJ, Kenny PJ, Tyndale RF, David SP, Stein EA, Uhl GR, Conti DV, Green C, Amur S. Biomarkers for smoking cessation. Clin Pharmacol Ther. 2013;93:526–536. doi: 10.1038/clpt.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J, Hajek P, McRobbie H, Locker J, Gillison F, McEwen A, Beard E, West R. Cigarette craving and withdrawal symptoms during temporary abstinence and the effect of nicotine gum. Psychopharmacology. 2013;229:209–218. doi: 10.1007/s00213-013-3100-2. [DOI] [PubMed] [Google Scholar]
- Butz RF, Morelli G. Innovative strategies for early clinical R&D. iDrugs. 2008;11:36–41. [PubMed] [Google Scholar]
- Carpenter MJ, Hughes JR, Gray KM, Wahlquist AE, Saladin ME, Alberg AJ. Nicotine therapy sampling to induce quit attempts among smokers unmotivated to quit. Arch Intern Med. 2011;171:1901–1907. doi: 10.1001/archinternmed.2011.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDCP) Quitting smoking among adults—United States, 2001–2010. Morbidity Mortality Weekly Report. 2011;60:1513–1519. [PubMed] [Google Scholar]
- Cleophas TJM. Cross-over studies: a modified analysis with more power. Clin Pharmacol Ther. 1993;53:515–520. doi: 10.1038/clpt.1993.64. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the social sciences. 2nd ed. Hillsdale NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Cummings KM, Hyland A. Impact of nicotine replacement therapy on smoking behavior. Ann Rev Public Health. 2005;26:583–599. doi: 10.1146/annurev.publhealth.26.021304.144501. [DOI] [PubMed] [Google Scholar]
- DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Economics. 2003;22:151–185. doi: 10.1016/S0167-6296(02)00126-1. [DOI] [PubMed] [Google Scholar]
- Farid P, Abate MA. Buspirone use for smoking cessation. Annals Pharmacother. 1998;32:1362–1364. doi: 10.1345/aph.17175. [DOI] [PubMed] [Google Scholar]
- Ferguson SG, Gitchell JG, Shiffman S, Sembower MA. Prediction of abstinence at 10 weeks based on smoking status at 2 weeks during a quit attempt. Clin Ther. 2009;31:1957–1965. doi: 10.1016/j.clinthera.2009.08.029. [DOI] [PubMed] [Google Scholar]
- Ferry L. Non-nicotine pharmacotherapy for smoking cessation. Prim Care. 1999;26:653–669. doi: 10.1016/s0095-4543(05)70122-6. [DOI] [PubMed] [Google Scholar]
- Ferry LH, Burchette RJ. Efficacy of bupropion for smoking cessation in non-depressed smokers. J Addict Dis. 1994;13:249. [Google Scholar]
- Fleiss JL. The Design and Analysis of Clinical Experiments. New York: Wiley; 1986. [Google Scholar]
- Fleiss JL. A critique of recent research on the two-treatment crossover design. Controlled Clin Trials. 1989;10:237–243. doi: 10.1016/0197-2456(89)90065-2. [DOI] [PubMed] [Google Scholar]
- Fletcher DJ, Lewis SM, Matthews JNS. Factorial designs for crossover clinical trials. Stat Med. 1990;9:1121–1129. doi: 10.1002/sim.4780091002. [DOI] [PubMed] [Google Scholar]
- Foulds J, Russ C, Yu C-R, Zou KH, Galaznik A, Franzon M, Berg A, Hughes JR. Effects of varenicline on individual nicotine withdrawal symptoms: a combined analysis of eight randomized, placebo-controlled trials. Nic Tobacco Res. 2013;15:1849–1857. doi: 10.1093/ntr/ntt066. [DOI] [PubMed] [Google Scholar]
- Foulds J, Steinberg MB, Williams JM, Ziedonis D. Developments in pharmacotherapy for tobacco dependence: past, present and future. Drug Alc Rev. 2006;25:59–71. doi: 10.1080/09595230500459529. [DOI] [PubMed] [Google Scholar]
- Giovino GA, Mirza SA, Samet JM, Gupta PC, Jarvis MJ, Bahla N, Doll R, et al. Tobacco use in 3 billion individuals from 16 countries: an analysis of nationally representative cross-sectional household surveys. Lancet. 2012;380:668–679. doi: 10.1016/S0140-6736(12)61085-X. [DOI] [PubMed] [Google Scholar]
- Glaros AG, Kline RB. Understanding the accuracy of tests with cutting scores: the sensitivity, specificity, and predictive value model. J Clinical Psychol. 1988;44:1013–1023. doi: 10.1002/1097-4679(198811)44:6<1013::aid-jclp2270440627>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR. Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs. sustained-release bupropion and placebo for smoking cessation. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Gorodetzky CW, Grudzinkas C. Involving the pharmaceutical and biotech communities in medication development for substance abuse. Pharmacol Ther. 2005;108:109–118. doi: 10.1016/j.pharmthera.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Harmey D, Griffin PR, Kenny PJ. Development of novel pharmacotherapeutics for tobacco dependence: progress and future directions. Nic Tobacco Res. 2012;14:1300–1318. doi: 10.1093/ntr/nts201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR. The hardening hypothesis: is the ability to quit decreasing due to increasing nicotine dependence?. A review and commentary. Drug Alc Dep. 2011;117:111–117. doi: 10.1016/j.drugalcdep.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Marcy TW, Naud S. Interest in treatments to stop smoking. J Substance Abuse Treatment. 2009;36:18–24. doi: 10.1016/j.jsat.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Stead L, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2004;(4):CD000031. doi: 10.1002/14651858.CD000031. [DOI] [PubMed] [Google Scholar]
- Issa JS, Abe TO, Moura S, Santos PCJL, Pereira AC. Effectiveness of coadministration of varenicline, bupropion, and serotonin reuptake inhibitors in a smoking cessation program in the real-life setting. Nic Tobacco Res. 2013;15:1146–1150. doi: 10.1093/ntr/nts230. [DOI] [PubMed] [Google Scholar]
- Javors MA, Hatch JP, Lamb RJ. Cut-off levels for breath carbon monoxide as a marker for cigarette smoking. Addiction. 2005;100:159–167. doi: 10.1111/j.1360-0443.2004.00957.x. [DOI] [PubMed] [Google Scholar]
- Jupp B, Lawrence AJ. New horizons for therapeutics in drug and alcohol abuse. Pharmacol Ther. 2009;125:138–168. doi: 10.1016/j.pharmthera.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Kenford SL, Fiore MC, Jorenby DE, Smith SS, Wetter D, Baker TB. Predicting smoking cessation. Who will quit with and without the nicotine patch. JAMA. 1994;271:589–594. doi: 10.1001/jama.271.8.589. [DOI] [PubMed] [Google Scholar]
- Koch GG, Amara IA, Brown BW, Colton T, Gillings DB. A two-period crossover design for the comparison of two active treatments and placebo. Stat Med. 1989;8:487–504. doi: 10.1002/sim.4780080412. [DOI] [PubMed] [Google Scholar]
- Kola I. The state of innovation in drug development. Clin Pharm Ther. 2008;83:227–230. doi: 10.1038/sj.clpt.6100479. [DOI] [PubMed] [Google Scholar]
- Kollins SH, McClernon FJ, Van Voorhees EV. Monetary incentives promote smoking abstinence in adults with Attention Deficit Hyperactivity Disorder (ADHD) Exper Clin Psychopharmacol. 2010;18:221–228. doi: 10.1037/a0019565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Lloyd GK, Mason BJ. Development of pharmacotherapies for drug addiction: a Rosetta Stone approach. Nature Rev Drug Disc. 2009;8:500–515. doi: 10.1038/nrd2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroboth PD, Schmith VD, Smith RB. Pharmacodynamic modeling: application to new drug development. Clin Pharmacokin. 1991;20:91–98. doi: 10.2165/00003088-199120020-00001. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Kirby KC, Morral AR, Balbicka G, Iguchi MY. Shaping smoking cessation in hard-to-treat smokers. J Consult Clin Psychol. 2010;78:62–71. doi: 10.1037/a0018323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C, Roth D, Kaufmann V, Audrain J, Hawk L, Liu A, Niaura R, Epstein L. Mediating mechanisms for the impact of bupropion in smoking cessation treatment. Drug Alc Depend. 2002;67:219–223. doi: 10.1016/s0376-8716(02)00067-4. [DOI] [PubMed] [Google Scholar]
- Lerman C, LeSage MG, Perkins KA, O’Malley SS, Siegel SJ, Benowitz NL, Corrigall WA. Translational research in medication development for nicotine dependence. Nature Rev Drug Dev. 2007;6:746–762. doi: 10.1038/nrd2361. [DOI] [PubMed] [Google Scholar]
- Lesko LJ. Paving the critical path: how can clinical pharmacology help achieve the vision? Clin Pharm Ther. 2007;81:170–177. doi: 10.1038/sj.clpt.6100045. [DOI] [PubMed] [Google Scholar]
- Levy DE, Thorndike AN, Biener L, Rigotti NA. Use of nicotine replacement therapy to reduce or delay smoking but not to quit: prevalence and association with subsequent cessation efforts. Tob Control. 2007;16:384–389. doi: 10.1136/tc.2007.021485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh W-Y, Piper ME, Schlam TR, Fiore MC, Smith SS, Jorenby DE, Cook JW, Bolt DM, Baker TB. Should all smokers use combination smoking cessation pharmacotherapy? Nic Tobacco Res. 2012;14:131–141. doi: 10.1093/ntr/ntr147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahtani KR, Heneghan CJ, Glasziou PP, Perera R. Reminder packaging for improving adherence to self-administered long-term medications. Cochrane Database Syst Rev. 2011;(9):CD005025. doi: 10.1002/14651858.CD005025.pub3. [DOI] [PubMed] [Google Scholar]
- McCormick J, Olsen J. The European Union: politics and policies. 5th ed. Boulder CO: Westview Press; 2013. [Google Scholar]
- McKee SA, Weinberger AH, Shi J, Tetrault J, Coppola S. Developing and validating a human laboratory model to screen medications for smoking cessation. Nic Tobacco Res. 2012;14:1362–1371. doi: 10.1093/ntr/nts090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, de Wit H. Challenges for translational psychopharmacology research—some basic principles. Psychopharmacology. 2008;199:291–301. doi: 10.1007/s00213-008-1198-4. [DOI] [PubMed] [Google Scholar]
- Mills EJ, Wu P, Lockhart I, Thorlund K, Puhan M, Ebbert JO. Comparisons of high-dose and combination nicotine replacement therapy, varenicline, and bupropion for smoking cessation: a systematic review and multiple treatment meta-analysis. Annals Med. 2012;44:588–597. doi: 10.3109/07853890.2012.705016. [DOI] [PubMed] [Google Scholar]
- Mueller ET, Landes RD, Kowal BP, Yi R, Stitzer ML, Burnett CA, Bickel WK. Delay of smoking gratification as a laboratory model of relapse: effects of incentives for not smoking, and relationship with measures of executive function. Behav Pharmacol. 2009;20:461–473. doi: 10.1097/FBP.0b013e3283305ec7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan SC, Masterson JG, Devane JG, Kelly JG. Clinical and pharmacokinetic properties of a transdermal nicotine patch. Clin Pharmacol Ther. 1990;47:331–337. doi: 10.1038/clpt.1990.36. [DOI] [PubMed] [Google Scholar]
- Parodi O, Simonetti I, Michelassi C, Carpeggiani C, Biagini A, LAbbate A, Maseri A. Comparison of verapamil and propranolol therapy for angina pectoris at rest: a randomized, multiple-crossover, controlled trial in the coronary care unit. Amer J Cardiology. 1986;57:899–906. doi: 10.1016/0002-9149(86)90727-7. [DOI] [PubMed] [Google Scholar]
- Paul SM, Mytelka DS, Dunwiddie CT, Persinger CC, Munos BH, Lindborg SR, Schacht AL. How to improve R&D productivity: the pharmaceutical industry’s grand challenge. Nature Rev Drug Discov. 2010;9:203–214. doi: 10.1038/nrd3078. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Briski J, Fonte C, Scott J, Lerman C. Severity of tobacco abstinence symptoms varies by time of day. Nic Tobacco Res. 2009;11:84–91. doi: 10.1093/ntr/ntn003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Conklin CA, Levine MD. Cognitive-behavioral Therapy for Smoking Cessation: A Practical Guide to the Most Effective Treatments. New York: Routledge; 2008. [Google Scholar]
- Perkins KA, Karelitz JL, Jao NC. Optimal carbon monoxide criteria to confirm 24-hr smoking abstinence. Nic Tobacco Res. 2013a;15:578–582. doi: 10.1093/ntr/nts205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Lerman C. Early human screening of medications to treat drug addiction: Novel paradigms and the relevance of pharmacogenetics. Clin Pharmacol Ther. 2011;89:460–463. doi: 10.1038/clpt.2010.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Lerman C, Fonte C, Mercincavage M, Stitzer ML, Chengappa KRN, Jain A. Cross-validation of a new procedure for early screening of smoking cessation medications in humans. Clin Pharmacol Ther. 2010;88:109–114. doi: 10.1038/clpt.2010.65. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Lerman C, Karelitz JL, Jao NC, Chengappa KNR, Sparks GM. Sensitivity and specificity of a procedure for early human screening of novel smoking cessation medications. Addiction. 2013b;108:1962–1968. doi: 10.1111/add.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Lerman C, Stitzer ML, Fonte CA, Briski JL, Scott JA, Chengappa KNR. Development of procedures for early human screening of smoking cessation medications. Clin Pharmacol Ther. 2008;84:216–221. doi: 10.1038/clpt.2008.30. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Marcus MD, Levine MD, D’Amico D, Miller A, Broge M, Ashcom J, Shiffman S. Cognitive-behavioral therapy to reduce weight concerns improves smoking cessation outcome in weight-concerned women. J Consult Clin Psychol. 2001;69:604–613. [PubMed] [Google Scholar]
- Perkins KA, Stitzer M, Lerman C. Medication screening for smoking cessation: a proposal for new methodologies. Psychopharmacology. 2006;184:628–636. doi: 10.1007/s00213-005-0105-5. [DOI] [PubMed] [Google Scholar]
- Pierce RC, O’Brien CP, Kenny PJ, Vanderschuren LJMJ. Rational development of addiction pharmacotherapies: successes, failures, and prospects. Cold Spring Harbor perspectives in medicine. 2012;2:1–8. doi: 10.1101/cshperspect.a012880. article number a012880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polosa R, Benowitz NL. Treatment of nicotine addiction: present therapeutic options and pipeline developments. Trends Pharmacol Sci. 2011;32:281–289. doi: 10.1016/j.tips.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE, Tartter M. Application of human laboratory models to pharmacotherapy development for alcohol dependence. Curr Pharmaceutical Design. 2010;16:2149–2158. doi: 10.2174/138161210791516422. [DOI] [PubMed] [Google Scholar]
- Romanowich P, Lamb RJ. Effects of escalating and descending schedules of incentives on cigarette smoking in smokers without plans to quit. J Applied Behav Analysis. 2010a;43:357–367. doi: 10.1901/jaba.2010.43-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanowich P, Lamb RJ. The relationship between in-treatment abstinence and post-treatment abstinence in a smoking cessation treatment. Exper Clin Psychopharmacol. 2010b;18:32–36. doi: 10.1037/a0018520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostron B. Smoking-attributable mortality by cause in the United States : revising the CDC’s data and estimates. Nic Tobacco Res. 2013;15:238–246. doi: 10.1093/ntr/nts120. [DOI] [PubMed] [Google Scholar]
- Schnoll RA, Lerman C. Current and emerging pharmacotherapies for treating tobacco dependence. Expert Opinion on Emerging Drugs. 2006;11:429–444. doi: 10.1517/14728214.11.3.429. [DOI] [PubMed] [Google Scholar]
- Schnoll RA, Wileyto EP, Pinto A, Leone F, Gariti P, Siegel S, Perkins KA, Dackis C, Heitjan, Berritini W, Lerman C. A placebo-controlled trial of modafinil for nicotine dependence. Drug Alc Depend. 2008;98:86–93. doi: 10.1016/j.drugalcdep.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheiner LB. Learning versus confirming in clinical drug development. Clin Pharmacol Ther. 1997;61:275–291. doi: 10.1016/S0009-9236(97)90160-0. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Use of more nicotine lozenges leads to better success in quitting smoking. Addiction. 2007;102:809–814. doi: 10.1111/j.1360-0443.2007.01791.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Ferguson SG, Gwaltney CJ, Balabanis MH, Shadel WG. Reduction of abstinence-induced withdrawal and craving using high-dose nicotine replacement therapy. Psychopharmacology. 2006;184:637–644. doi: 10.1007/s00213-005-0184-3. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Johnston JA, Khayrallah M, Elash CA, Gwaltney CJ, Paty JA, Evoniuk G, DeVeaugh-Geiss J. The effect of bupropion on nicotine craving and withdrawal. Psychopharmacology. 2000;148:33–40. doi: 10.1007/s002130050022. [DOI] [PubMed] [Google Scholar]
- Shiltz D, Paniagua A, Hastings JE. A retrospective comparison of varenicline monotherapy versus the combination of varenicline and bupropion or bupropion and nicotine patches in a VA tobacco cessation clinic. J Smoking Cessation. 2011;6:65–73. [Google Scholar]
- Sofuoglu M, Waters AJ, Mooney M. Modafinil and nicotine interactions in abstinent smokers. Hum Psychopharmacol. 2008;23:21–30. doi: 10.1002/hup.878. [DOI] [PubMed] [Google Scholar]
- Song F, Harvey I, Lilford R. Adjusted indirect comparison may be less biased than direct comparison for evaluating new pharmaceutical interventions. J Clin Epid. 2008;61:455–463. doi: 10.1016/j.jclinepi.2007.06.006. [DOI] [PubMed] [Google Scholar]
- SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nic Tobacco Res. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Stead LF, Lancaster T. Interventions to reduce harm from continued tobacco use. Cochrane Database Syst Rev. 2007;(3):CD005231. doi: 10.1002/14651858.CD005231.pub2. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Rand CS, Below GE, Mead AM. Contingent payment procedures for smoking reduction and cessation. J Appl Beh Analysis. 1986;19:197–202. doi: 10.1901/jaba.1986.19-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streiner DL. Alternatives to placebo-controlled trials. Can J Neurol Sci. 2007;34(suppl 1):S37–S41. doi: 10.1017/s0317167100005540. [DOI] [PubMed] [Google Scholar]
- Teneggi V, Tiffany ST, Squassante L, Milleri S, Ziviani L, Bye A. Smokers deprived of cigarettes for 72 h: effect of nicotine patches on craving and withdrawal. Psychopharmacology. 2002;164:177–187. doi: 10.1007/s00213-002-1176-1. [DOI] [PubMed] [Google Scholar]
- US Dept of Health, Education and Welfare (USDHEW) Smoking and Health: Report of the Advisory Committee to the Surgeon General of the Public Health Service. Washington: USDHEW, PHS, Center for Disease Control; 1964. 1964. PHS Publication No. 1103. [Google Scholar]
- US Dept of Health and Human Services (USDHH) Innovation or Stagnation: Challenge and Opportunity on the Critical Path to New Medical Products. (FDA Critical Path Initiative whitepaper.) Bethesda MD: USDHHS; 2004. [Google Scholar]
- US Dept of Health and Human Services (USDHHS) How Tobacco Smoke Causes Disease: The Biological and Behavioral Basis for Smoking-Attributable Disease. (A Report of the Surgeon General.) Atlanta: USDHHS; 2010. [Google Scholar]
- Vocci FJ. Development of medications for addictive disorders. In: Schuster CR, Kuhar M, editors. Handbook of Experimental Pharmacology, vol. 118: Pharmacological Aspects of Drug Dependence, Towards an Integrated Neurobehavioral Approach. Berlin: Springer Verlag; 1996. pp. 473–485. [Google Scholar]
- Wewers ME, Stillman FA, Harman AM, Shopland DR. Distribution of daily smokers by stage of change: current population survey results. Preventive Med. 2003;36:710–720. doi: 10.1016/s0091-7435(03)00044-6. [DOI] [PubMed] [Google Scholar]
- Wileyto EP, Patterson F, Niaura R, Epstein LH, Brown RA, Audrain-McGovern J, Hawk LW, Lerman C. Do small lapses predict relapse to smoking behavior under bupropion treatment? Nic Tobacco Res. 2004;6:357–367. doi: 10.1080/1462220042000202463. [DOI] [PubMed] [Google Scholar]
- Woodcock J, Woosley R. The FDA Critical Path Initiative and its influence on new drug development. Ann Rev Med. 2008;59:1–12. doi: 10.1146/annurev.med.59.090506.155819. [DOI] [PubMed] [Google Scholar]