Abstract
Japanese encephalitis virus (JEV) is the most common cause of viral encephalitis in Asia, and it is increasingly a global public health concern due to its recent geographic expansion. While commercial vaccines are available and used in some endemic countries, JEV continues to be a public health problem, with 50,000 cases reported annually. Research with virulent JEV in mouse models to develop new methods of prevention and treatment is restricted to BSL-3 containment facilities, confining these studies to investigators with access to these facilities. We have developed an adult small animal peripheral challenge model using interferon-deficient AG129 mice and the JEV live-attenuated vaccine SA14-14-2, thus requiring only BSL-2 containment. A low dose of virus (10 PFU/0.1 ml) induced 100% morbidity in infected mice. Increased body temperatures measured by implantable temperature transponders correlated with an increase in infectious virus and viral RNA in serum, spleen and brain as well as an increase in pro-inflammatory markers measured by a 58-biomarker multi-analyte profile (MAP) constructed during the course of infection. In the future, the MAP measurements can be used as a baseline for comparison in order to better assess the inhibition of disease progression by other prophylactic and therapeutic agents. The use of the AG129/JEV SA14-14-2 animal model makes vaccine and therapeutic studies feasible for laboratories with limited biocontainment facilities.
Keywords: Flavivirus, Japanese encephalitis virus, interferon-deficient mice, AG129 mouse model, flavivirus pathogenesis, viral encephalitis, JEV SA14-14-2 vaccine strain
Introduction
Japanese encephalitis virus (JEV) is a mosquito-borne virus from the genus Flavivirus in the family Flaviviridae historically found in southern and eastern Asia. It has become an increasing public health concern due to its recent geographic emergence into Pakistan, western Indonesia, Papua New Guinea and northern Australia [1,2]. JE is the most common viral encephalitis in Asia. According to the WHO, 50,000 cases of JE are reported annually, although this number may be inaccurate due to inadequate laboratory-based surveillance and reporting. Of the cases reported, 25–30% result in death, while 50% result in permanent neurologic sequelae. Most of the cases occur in children under the age of 15 in rural areas.
Several JEV vaccines are available, including inactivated whole virus formulations derived from infected mouse brains and cell cultures, and live attenuated virus preparations produced in primary hamster kidney and Vero cell cultures. No approved therapeutic drug is available for the treatment of JE. Vaccines from mouse brain tissue-derived inactivated virus manufactured in several Asian countries are expensive and require administration of several doses in a short time period to achieve adequate immunity [3]. The inactivated IXIARO vaccine, based on the attenuated vaccine strain SA14-14-2 grown in Vero cells, was licensed for use in 2009 in Europe, the United States and Australia. It provides antiviral immunity after 2 doses without adverse effects [4–7]. Even with available effective JEV vaccines, the virus continues to cause large disease outbreaks throughout Asia. In view of this, effective therapeutic agents, in addition to safe and affordable vaccines, are needed, and authentic animal models in which to test them are necessities.
Research with virulent JEV in mouse models to develop new methods for prevention and treatment is restricted to BSL-3 containment facilities. These animal models use neonate or weanling out-bred mice and intracerebral viral inoculation because susceptibility to wild-type (WT) JEV infection has been shown to be dependent on mouse age and route of inoculation [8]. These constraints on animal research greatly reduce the ability of researchers to evaluate JEV vaccine candidates and therapeutic agents.
We have previously developed interferon-deficient AG129 mouse models for assessment of prophylaxis and therapy of the viscerotropic flaviviruses In this study, we have developed a new small animal model for an encephalitic flavivirus, using peripheral challenge of AG129 mice with live-attenuated JEV vaccine, SA14-14-2, which requires only BSL-2/ABSL-2 containment. We also have previously used these mice to develop BSL-2/ABSL-2 infection models for Venezuelan equine encephalitis (VEE) and West Nile (WN)/DEN chimeric virus infections [9–12]. The use of this animal model and intraperitoneal inoculation of virus makes JEV animal challenge studies a feasible option for laboratories with limited biocontainment. We present an in-depth characterization of the AG129/JEV SA14-14-2 model, detailing the viral disease progression, to provide measurements that in the future can be used as a baseline for studying vaccines and anti-viral therapeutic agents against JEV in this model.
Materials and Methods
JEV vaccine strain SA14-14-2 was originally obtained from the Arbovirus Diseases Branch, DVBD reference collection (Fort Collins, CO). Stock virus was grown in C6/36 cells to a titer of 2.25 × 108 PFU/ml. Vero cells were used in plaque assays and grown in MEM: DMEM supplemented with 10% FBS, 2mM l-glutamine, 0.15% sodium bicarbonate, 100 U/ml penicillin G sodium, and 100 µg/ml streptomycin at 37°C in 5% CO2.
Animal Studies
AG129 mice deficient in interferon α/β and γ receptors were originally obtained from B&K Universal (Hull, United Kingdom) and bred in-house. Mice were handled as specified by the Division of Vector-Borne Diseases Institutional Animal Care and Use Committee recommendations (Protocol #12-026). JEV vaccine strain SA14-14-2 was evaluated for neuroinvasiveness in 6–8 week old AG129 mice. Nine groups of mice (n=12) were inoculated intraperitoneally (i.p.) with 10-fold dilutions of virus (106 to 10−3 PFU/0.1 ml). Before challenge, baseline body temperatures measured by implantable temperature transponders (BMDS) and weights were recorded daily for 3–4 days. After challenge mice were monitored 4 times per day for an increase followed by a decrease in normal temperature, weight loss and other signs of morbidity (hunched posture, neurological signs). Mice that showed signs of morbidity were euthanized immediately.
To determine growth rate of JEV SA14-14-2 in tissues, 6 week-old AG129 mice (n=5) were infected with 10 PFU/0.1 ml i.p., and mice were euthanized for sample collection at 6 time points (24, 48, 66, 78, 90 and 102 hpi). Blood was collected via cardiac puncture and placed immediately into microtainer tubes for serum collection. Brain and spleen were harvested post-mortem, rinsed with PBS, manually chopped into sections, and placed in pre-weighed MagNA Lyser Green Bead tubes (Roche Diagnostics) containing either 500 µl of BA1 for plaque assay, 500 µl RNA Later (Qiagen) for viral RNA quantitation or 500 µl TE buffer (2mM EDTA, 50 mM Tris-HCl, pH 7.4) containing protease inhibitors [1 mg/ml of Pefabloc SC and 1 µg/ml each of aprotinin, antipain, leupeptin and pepstatin (Roche)] for rodent multi-analyte profile (MAP) analysis (Myriad RBM). Tissue samples were weighed and homogenized for two 30s cycles at 4000 rpm using a Roche MagNA Lyser (Roche Diagnostics). Homogenized samples were centrifuged for 5 minutes at 10,000 rpm at room temperature and stored at −80°C along with serum samples prior to analysis.
Statistical analysis of body temperatures in infected mice
A semiparametric, mixed model was fit to the longitudinal temperature data of the mice. The model includes treatment-specific temperature curves, a component to model the diurnal pattern of body temperature, and mouse-specific curves. Each of the components was characterized by a second degree penalized spline with a truncated power basis. The solution to the fit and estimated variances were obtained by computing the best, linear, unbiased predictors of the penalized spline’s representation as a linear, mixed model [13].
Plaque assay
Viral plaque titrations of serum and tissue samples from 6 time points were performed under double agarose overlay in six-well plates of confluent Vero cells [14]. Serum and tissue samples homogenized in BA-1 diluent were serially diluted in the same medium. Each dilution (100 µl) was added to a confluent monolayer of cells and incubated at 37°C for one h with gentle rocking. Cells were overlaid with 3 ml of LE agarose in YE-LAH medium as described previously [14]. Plates were incubated for 4 days before 3 ml of a second overlay containing 0.008% neutral red in the same medium was added to each well. Plaques were counted 24 and 48 h later.
RNA extraction from tissues and sera and in vitro-transcribed RNA controls
Viral RNA from 140 µl of serum samples was extracted from virus supernatant with a QIAmp Viral RNA kit (Qiagen), and total RNA was purified from 100 µl of tissue homogenate using the RNeasy Mini kit (Qiagen) following the manufacturer’s protocols. The JEV consensus primers and probe for quantitative RT-PCR were developed and kindly provided by Dr. Barbara W. Johnson, Diagnostic and Reference Lab, Arbovirus Diseases Branch, Division of Vector-Borne Diseases, CDC (Supplemental Table 1). To prepare in vitro transcribed RNA from the 5' untranslated region (UTR) of the genome for a copy number control, the JEV consensus primers (JEVF and JEVR) with the T7 promoter sequence (TAATACGACTCACTATAGGGAGA) added to the 5' end of JEVF primer were used to amplify a 105 bp segment of cDNA. The same size segment of RNA was transcribed from the cDNA using the mMessage mMachine kit (Life Technologies) according to the manufacturer’s protocol. RNA was quantified using the RNA Nano kit in the Agilent Bioanalyzer, and RNA copy numbers/ml were calculated based on spectrophotometry readings.
Quantitative reverse-transcription polymerase chain reaction (qRT-PCR)
A 5 µl aliquot of each purified RNA sample was added to master mix from iScript One-step RT-PCR kit (Bio-Rad) containing primers and probe described above: 25 pmol of probe JEVP, 100 pmol of forward primer JEVF and 100 pmol of reverse primer JEVR (Supplemental Table 1). JEV-specific RNA extracted from serum and tissue samples was analyzed in triplicate by qRT-PCR using the homologous RNA standards on a Bio-Rad IQ5 Real-time PCR detection system under the following conditions: 50°C for 30 min, 95°C for 15 min, followed by 45 cycles of 94°C for 15 sec, and 55°C for 30 sec with continuous fluorescence data collection.
Rodent multi-analyte profile (MAP)
Serum and tissue samples homogenized in TE buffer containing protease inhibitors from each time point were pooled (n = 5 samples/pool) and frozen at −80°C. Pooled samples were shipped to Myriad RBM for analysis of 58 biomarkers on the RodentMAP® version 3.0 platform which uses microspheres infused with fluorescent dyes and coated with reagents that bind target analytes in serum or tissue homogenates. The least detectable dose (LDD) is defined as 3 standard deviations above mean background measured for each analyte in each multiplex.
Results
Determination of effective challenge dose of JEV SA14-14-2 in AG129 mice
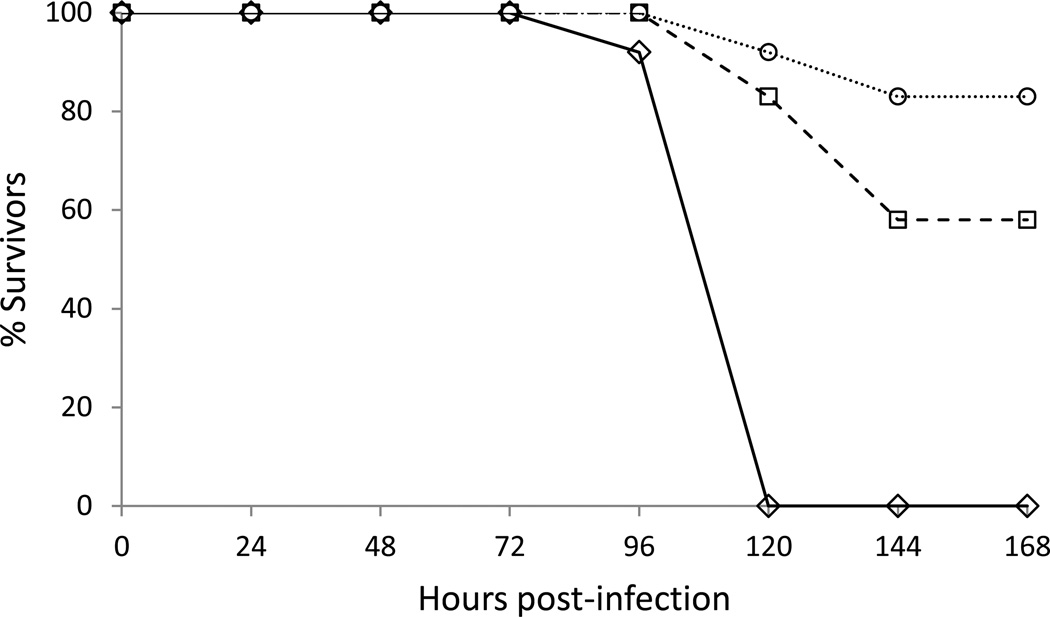
Previously, we determined that AG129 mice lacking an intact IFN response were susceptible to virulent DENVs and WNV, and VEEV and YFV vaccine strains. In order to determine if these mice were susceptible to JEV vaccine strain SA14-14-2, mice were challenged via i.p. inoculation with varying doses of virus ranging from 106 to 10 PFU/0.1 ml. The onset of morbidity occurred in a dose-dependent manner with mice receiving 106 PFU/0.1 ml of virus showing outward signs of morbidity earliest. Sick individuals, as indicated by an increase followed by rapid decrease in baseline body temperatures and neurological signs, were euthanized as early as 66 hpi (data not shown). Mice receiving 10 PFU/0.1 ml of JEV SA14-14-2 began to show outward signs of morbidity at 96 hpi (Fig. 1). The mean survival time (MST) of mice challenged peripherally with JEV SA14-14-2 was shown to be dose-dependent with shortened MSTs correlating with higher-titer inocula. Mice inoculated with 10 PFU/0.1 ml of virus had a MST of 106 h (SD±7.9). Mice inoculated with 1 or 0.1 PFU/0.1 ml of virus had MSTs of 130 h (P = 0.016) and 122 h (P=0.094), respectively (Table 1). At 144 hpi mice challenged with 1 or 0.1 PFU/0.1 ml of virus had survival rates of 58% and 83%, respectively (Fig. 1).
Figure 1. Dose-dependent virulence of JEV SA14-14-2 in AG129 mice.
Mice (n=12) were inoculated i.p. with 10 (◊), 1(□) and 0.1 (○) PFU of JEV SA14-14-2.
Table 1.
Mean survival times (MST) of AG129 mice infected with JEV SA14-14-2.
| Challenge dose (PFU/0.1ml) |
% morbidity (survivors/total) |
MST (hours) ±SD |
|---|---|---|
| 10 | 100 (0/12) | 106 ± 7.9 |
| 1 | 42 (7/12) | 130 ± 17.1 |
| 0.1 | 17 (10/12) | 122 ± 8.5 |
Determination of clinical sign onset with effective challenge dose (10 MD50)
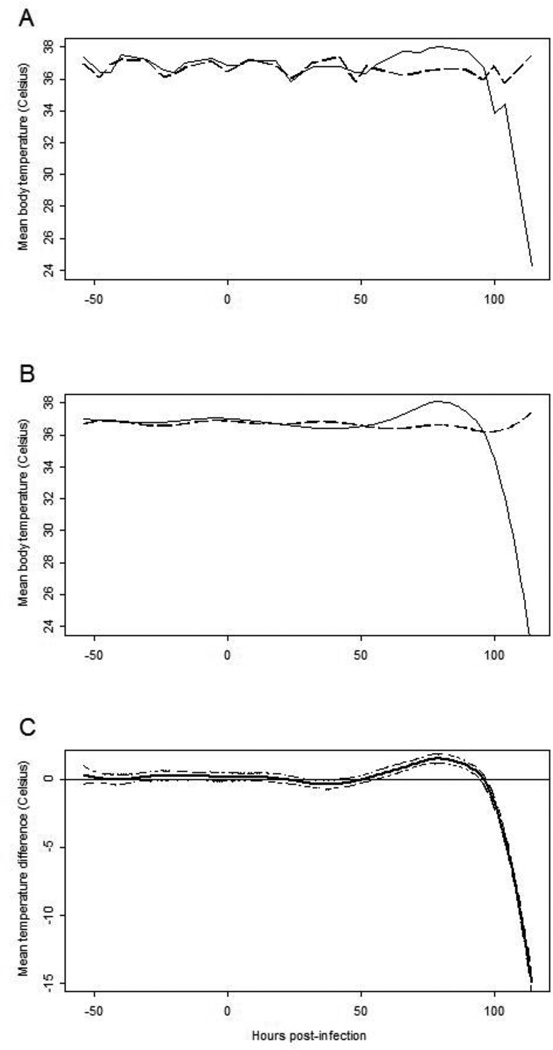
In order to determine an accurate measurement of the time of onset of clinical signs, baseline body temperatures were measured by implantable temperature transponders for 3–4 days prior to infection. After challenge, body temperatures of infected mice were monitored 4 times per day in addition to weight change and neurological signs of disease. Body temperatures of infected mice rose observably between 72 and 96 hpi (Fig. 2A and 2B). Statistical analysis suggests this increase in the mean temperature of infected mice begins by 57 hpi as indicated by the upper bound of a 95% confidence for the mean temperature curve of infected mice (Fig. 2C).
Figure 2. Body temperatures of uninfected and JEV SA14-14-2 infected AG12 mice.
Panel A: Mean of observable body temperatures of infected (solid line) and uninfected (dashed line) mice. Panel B: Estimated mean body temperatures from model with mouse-specific and diurnal effects removed for infected (solid) and uninfected (dashed) mice. Panel C: Difference in estimated mean body temperatures (infected minus uninfected) with 95% confidence bound (dashed lines).
Determination of viral load in tissues from JEV SA14-14-2-infected AG129 mice
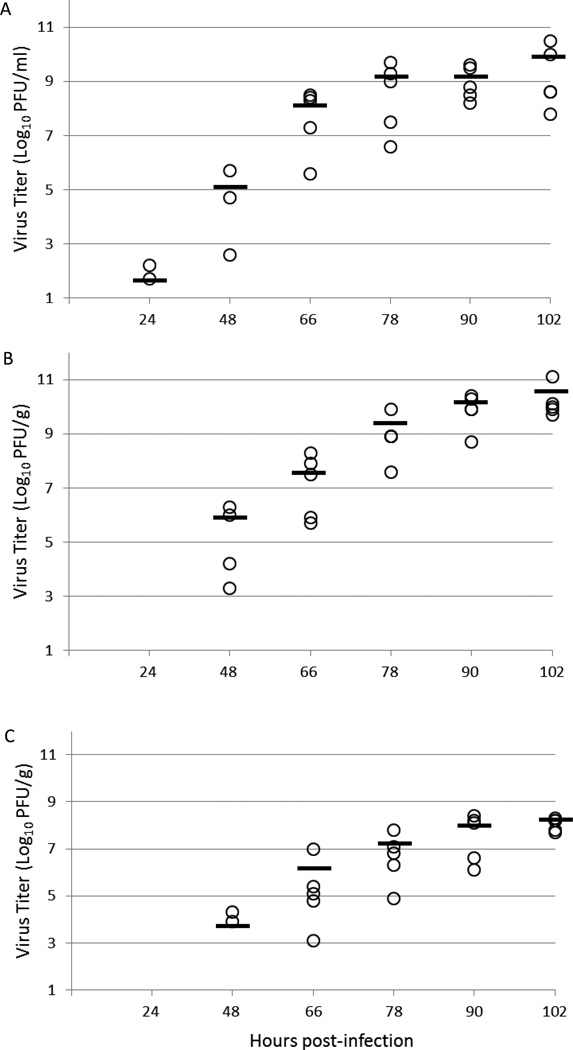
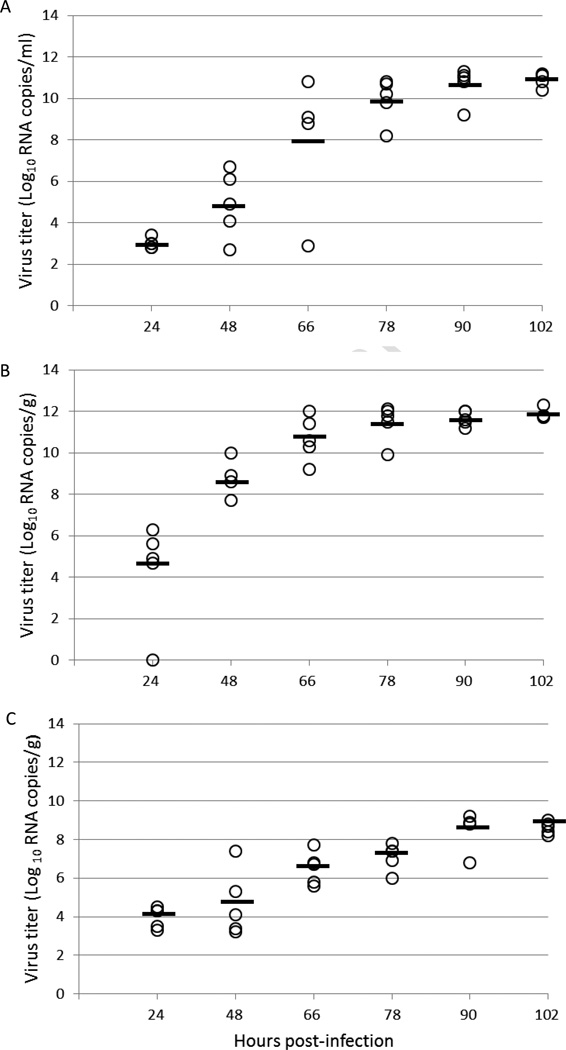
An analysis of viral replication in the sera, spleens and brains of infected animals was performed by viral plaque assay and qRT-PCR. Infectious virus and viral RNA were first detected in sera of mice infected with 10 PFU/0.1 ml of virus at 24 hpi by plaque assay and qRT-PCR with average titers of 1.6 log10 PFU/ml and 3.0 log10 RNA copies/ml, respectively. Virus and RNA titers peaked in sera at 102 hpi with average titers of 9.9 log10 PFU/ml and 10.9 log10 RNA copies/ml (Fig. 3A and 4A). Infectious virus in the spleen was first detected at 48 hpi by plaque assay with an average titer of 5.8 log10 PFU/g; however, viral RNA could be detected at 24 hpi with an average titer of 4.3 log10 RNA copies/g. Infectious virus and viral RNA titers peaked in the spleen at 102 hpi with average titers of 10.5 log10 PFU/g and 11.9 log10 RNA copies/g, respectively (Fig. 3B and 4B). Infectious virus in the brain was also detected by plaque assay at 48 hpi with an average titer of 3.7 log10 PFU/g, while viral RNA was detectable in the brain at 24 hpi with an average titer of 4.0 log10 RNA copies/g. Infectious virus and viral RNA rose steadily in the brain and reached highest titers at the last time point with average titers of 8.1 log10 PFU/g of infectious virus and 8.6 log10 RNA copies/g of viral RNA (Fig. 3C and 4C). These findings of infectious virus titer and presence of viral RNA in the tissues correspond to morbidity onset of animals determined by increased body temperature, calculated to peak at 57 hpi.
Figure 3. Infection virus titers in various tissues of AG129 mice infected with JEV SA14-14-2 expressed as PFU/gram (or ml).
Panels: A, serum; B, spleen; C, brain. Plaque assays of tissue samples taken at 6 different time points during infection were performed in duplicate.
Figure 4. Virus RNA in various tissues of AG129 mice infected with JEV SA14-14-2 expressed as genomic copies/gram (or ml).
Panels: A, serum; B, spleen; C, brain. Virus RNA titers of tissue samples taken at 6 different time points during infection were measured by quantitative RT-PCR. Each assay was performed in triplicate.
Analyte biomarker profile to assess inflammation or tissue damage as a result of JEV SA14-14-2 infection in AG129 mice
A 58 analyte pro- and anti-inflammatory and tissue damage biomarker profile was constructed with pooled samples of serum and tissue homogenate supernatants from brain and spleen of JEV SA14-14-2-infected AG129 mice throughout the course of infection. Overall, spleen tissue homogenates showed 4-fold or greater increases in concentration in the largest numbers of analytes (47 of 58) compared to mock-infected animals. Analyte concentrations that increased in the spleen tended to peak at 78 hpi, decrease slightly by 90 hpi and increase again by 102 hpi. Overall analyte concentrations in serum (26 of 58) and brain tissue homogenates (16 of 58) also increased when compared to samples taken from mock-infected animals. Analyte concentrations in the brain and serum that were shown to increase peaked at 90 hpi and remained high until the last time point at 102 hpi. Particular analytes known to increase in JEV infection in humans that were also shown to have a marked increase in SA14-14-2-infected AG129 mice included IL-6, macrophage inflammatory protein (MIP)-1α, MIP-1β, monocyte chemotactic protein (MCP)-1, T-cell specific protein RANTES (RANTES), tumor necrosis factor (TNF)α, and matrix metalloproteinase (MMP)-9.
Table 2 summarizes those analytes from sera and tissues of AG129 mice that exhibited a 4-fold or greater change in concentrations as a result of JEV SA14-14-2 infection compared to mock-infected animals. Only those analytes associated with inflammation or tissue damage are included. A more detailed report of the concentrations of each analyte in the different tissue samples at each time point is presented in Supplemental Table 2.
Table 2.
Analytes associated with inflammation or tissue damage with a four-fold or greater change in concentration as a result of JEV SA14-14-2 infection in AG129 mice.
| Serum | Brain | Spleen | ||
|---|---|---|---|---|
| Pro-inflammatory | ||||
| CRP | C-Reactive Protein Mouse | ↑ | ↑ | ↑ |
| CD40 | CD40 | ↑ | ||
| CD40L | CD40 Ligand | ↑ | ||
| Fibrinogen | ↑ | ↑ | ||
| GM-CSF | Granulocyte-Macrophage Colony Stimulating Factor | ↑ | ||
| KC/GRO | Growth-Regulated Alpha Protein | ↑ | ↑ | ↑ |
| IFNγ | Interferon gamma | ↑ | ↑ | |
| IP-10 | Interferon gamma induced protein | ↑ | ↑ | ↑ |
| IL-1α | Interleukin-1 alpha | ↑ | ↑ | |
| IL-1β | Interleukin-1 beta | ↑ | ||
| IL-6 | Interleukin-6 | ↑ | ↑ | ↑ |
| IL-11 | Interleukin-11 | ↑ | ||
| IL-12p70 | Interleukin-12 subunit p70 | ↑ | ||
| IL-18 | Interleukin 18 | ↑ | ||
| MDC | Macrophage-derived chemokine | ↑ | ↑ | ↑ |
| MIP-1 α | Macrophage Inflammatory protein-1 alpha | ↑ | ||
| MIP-1 β | Macrophage Inflammatory protein-1 beta | ↑ | ↑ | |
| MIP-1 γ | Macrophage Inflammatory protein-1 gamma | ↑ | ↑ | |
| MIP-2 | Macrophage inflammatory protein-2 | ↑ | ↑ | ↑ |
| MIP-3β | Macrophage inflammatory protein 3 beta | ↑ | ||
| MCP-1 | Monocyte chemotactic protein-1 | ↑ | ↑ | ↑ |
| MCP-3 | Monocyte chemotactic protein-3 | ↑ | ↑ | ↑ |
| MCP-5 | Monocyte chemotactic protein-5 | ↑ | ↑ | ↑ |
| OSM | Oncostatin-M | ↑ | ||
| RANTES | T-cell Specific Protein RANTES | ↑ | ↑ | ↑ |
| TNFα | Tumor Necrosis Factor alpha | ↑ | ||
| Anti-inflammatory | ||||
| IL-10 | Interleukin-10 | ↑ | ↑ | |
| TMP-1 | Tissue Inhibitor of Metalloproteinases | ↑ | ↑ | ↑ |
| Tissue damage | ||||
| IL-7 | Interleukin-7 | ↑ | ↑ | |
| MMP-9 | Matrix Metalloproteinase-9 | ↑ | ||
| MPO | Myeloperoxidase | ↑ | ↑ | ↑ |
| Myoglobin | ↓ | |||
| PAI-1 | Plasminogen Activator Inhibitor-1 | ↑ | ↑ | ↑ |
Discussion
JEV continues to be a public health concern as the most common cause of viral encephalitis in Asia and due to its continued geographic expansion into Pakistan, western Indonesia, Papua New Guinea and Australia [1,2,15]. New vaccines and treatments are being developed to combat the spread of the virus, and appropriate small animal models are needed in order to test these anti-viral agents. In this study, we report the use of the AG129 interferon-deficient mouse as an animal model for JEV using the vaccine strain SA14-14-2. We have previously shown these mice to be susceptible to both viscerotropic and encephalitic flaviviruses and alphaviruses including DENV, YFV, WNV and VEEV [9–12]. A low dose of JEV SA14-14-2 (10 PFU/0.1 ml) induced 100% morbidity in infected mice with a mean survival time of 106 hpi (±7.9). Increased body temperatures of infected mice beginning at a calculated time point of 57 hpi correlated with a marked increase in titers of infectious virus and viral RNA in the serum and tissues. With the RodentMAP® version 3.0 platform a 58-analyte profile was developed to determine baseline concentrations of important biomarkers during infection. The MAP showed a marked increase in pro-inflammatory cytokines in serum and tissue samples, especially in the spleen.
Vaccines developed for prophylaxis of JEV infection originally were made from infected mouse brain tissue or primary hamster kidney cells [16,17]. The JEV vaccine strain SA14-14-2 was developed in China and has been used for prophylaxis in that country since 1988 [16,18–21]. Recently, a vaccine consisting of inactivated JEV SA14-14-2 grown in Vero cells was developed and licensed in the U.S., Europe, Japan and Australia [4–6,22,23]. The mechanism of attenuation of JEV SA14-14-2 is attributed to several amino acid (AA) substitutions in both the E protein and the nonstructural proteins and nucleotide substitutions in UTRs of the genome [24–29]. Overall there are 29 amino acid differences between WT SA14 and vaccine strain SA14-14-2 [25]. The mutation in the E protein at AA 138 from a glutamic acid (E) to a lysine (K) results in an increased dependence on glycosaminoglycans like heparin for virus-cell attachment and entry [28,29]. This mutation was also shown to increase viral sensitivity to IFNα by preventing the virus from blocking IFN signaling [27]. The type I IFN response restricts flaviviral spread and dissemination to the CNS [30,31]. JEV SA14-14-2 may lack neurovirulence due to inducing the maturation of dendritic cells that activate anti-viral cytokines such as IFN and TNFα [18,32]. JEV SA14-14-2 infection activates dendritic cells and other monocytes while WT virus suppresses their maturation and function [32–34]. Due to its increased susceptibility to IFN in the mouse model we wanted to determine if this virus could cause morbidity in interferon-deficient AG129 mice. In this study JEV SA14-14-2 produced 100% morbidity in 6–8 week old AG129 mice with doses as low as 10 PFU per mouse. The lack of an effective IFN response in AG129 mice is most likely the reason this highly attenuated virus with increased sensitivity to IFN was able to infect and disseminate to the CNS in infected mice in this study.
Since AG129 mice are highly sensitive to viral infection, they are an excellent animal model to study other live-attenuated vaccine candidates developed for flaviviruses, and any potential vaccine that does not cause disease in these animals may be considered a very safe candidate due to the high sensitivity of AG129 mice to viral infection [10,11]. In fact, a WNV/DENV2 chimera vaccine featuring the structural proteins of WNV in the DENV2 backbone was shown to be completely attenuated in this animal model while still producing an active infection that resulted in production of neutralizing antibody to WNV [11]. This animal model has also been shown to be valuable for initial testing of potential therapeutics to flaviviruses. We found that the humanized MAb 2C9-cIgG was able to protect AG129 mice from lethal YFV-17D challenge at a dose of ≥1.27 µg/mouse when administered 24 hours prior to infection [35].
Rodent models to study pathogenesis of JEV are available but require neonate or weanling animals in BSL-3 facilities. Age and route of inoculation are key factors for susceptibility to flaviviruses in mice [8]. Up to 3 to 4 weeks of age, immunocompetent mice are susceptible to a low dose peripheral challenge with flaviviruses, but after this time signs of viremia and infection are not apparent [28]. This may be because JEV has a greater affinity for and replicates more efficiently in immature neurons [36]. Other rodent models for JEV challenge have included β2m−/−, C57BL/6, C3H/HeN, BALB/c, ICR, Swiss mice and suckling hamsters by intracerebral or peripheral challenge [37–41]. Interestingly, the β2 microglobulin-deficient β2m−/− mice are deficient in CD8+T cells and are more susceptible to viral infection due to a reduced IFNγ response [42]. The AG129/JEV SA14-14-2 model of infection developed in this study is a safer and more broadly applicable model since it allows for studies to be conducted under reduced biocontainment (BSL-2/ABSL-2) in adult mice with i.p. inoculation that more closely resembles the route of natural infection compared to other animal models with WT virus inoculated intracerebrally.
Using the RodentMAP® version 3.0 platform we constructed a comprehensive pro-inflammatory biomarker profile of JEV SA14-14-2 infection of AG129 mice. We previously used this technology to construct a similar profile of the AG129/YF17D-204 infection model. During infection with YF17D-204, the greatest fluctuation in analytes over the course of infection occurred in the brain. Significant increases in pro-inflammatory analytes in the serum and liver were detected in a bi-phasic pattern [12]. In contrast, we found a sustained increase in pro-inflammatory analytes, particularly in the spleen, in the AG129/SA14-14-2 model.
Analytes identified as important in human JEV infections include TNFα, IL-6, IL-8 and RANTES [43,44]. Winter et al. [43] correlated an increase of IL-6, IL-8, and TNFα in CSF along with an increase in RANTES in the plasma of JEV infected patients with a negative disease outcome. In this study we saw similar results with an increase in IL-6 and RANTES in the serum, brain and spleen, along with an increase in TNFα in the spleen. TNFα is a pro-inflammatory cytokine produced mainly by activated macrophages and to some extent CD4+T cells, natural killer (NK) cells and neurons. It is important in stimulating the acute phase reaction to infection along with IL-1, IL-6 and IL-8. An increase in TNFα through TLR-3 signaling can lead to decreased integrity of the blood-brain barrier, allowing virus particles and virus-infected immune cells access to the CNS [45,46]. Exposure to pro-inflammatory cytokines over long periods of time in combination with low levels of anti-inflammatory cytokines such as IL-10 and IL-4 is linked to more severe cases of encephalitis [47]. Interestingly, after vaccination of humans with JEV SA14-14-2 live attenuated vaccine, levels of IL-8 in the serum along with MCP-1, MIP-1α and MIP-1β and IL-6 to a lesser extent were significantly higher than those not receiving the vaccine, and TNFα and IFNγ were minimally detectable in the vaccinated population [48]. In the AG129/SA14-14-2 model we also found an increase in MCP-1 in serum, brain and spleen and an increase in MIP-1α in the spleen and MIP-1β in serum and spleen. The strong increase of pro-inflammatory cytokines, especially in the spleen, after infection with JEV SA14-14-2 combined with the inability of AG129 mice to utilize an IFN response against viral infection appear to be the main reason for the attenuated virus’s ability to cause disease in this mouse model.
The AG129/SA14-14-2 animal model results in a lethal encephalitic infection that mimics the progression of disease from virus infection in the peripheral tissues to the CNS and the upregulation of pro-inflammatory analytes associated with fatal human infections. Since this model uses the JEV vaccine strain, research is not limited to high biocontainment facilities, allowing for the evaluation of vaccines and therapeutics to JEV under reduced containment. Compared to models utilizing virulent JEV strains, the AG129/SA14-14-2 animal model is a safe and effective tool to investigate the pathogenesis of JE viral disease and evaluate future vaccine candidates and therapeutic agents.
Supplementary Material
Highlights.
An animal model using mice that lack interferon and a vaccine strain of JEV was developed.
A low dose of virus was able to induce 100% morbidity in infected mice.
Fever in mice correlated with the dissemination of infectious virus in serum and tissues.
Increased pro-inflammatory analytes in serum and tissues correlated with disease progression.
The model allows for the ability to effectively evaluate JEV vaccine and therapeutic candidates.
Acknowledgements
This research was supported by NIH/NIAID grant U54AI-065357 to the Rocky Mountain Regional Center of Excellence for Biodefense and Emerging Infectious Disease Research and the Centers for Disease Control and Prevention.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.van den Hurk AF, Ritchie SA, Mackenzie JS. Ecology and geographical expansion of Japanese encephalitis virus. Annu Rev Entomol. 2009;54:17–35. doi: 10.1146/annurev.ento.54.110807.090510. [DOI] [PubMed] [Google Scholar]
- 2.Mackenzie JS, Williams DT. The zoonotic flaviviruses of southern, south-eastern and eastern Asia, and Australasia: the potential for emergent viruses. Zoonoses Public Health. 2009;56(6–7):338–356. doi: 10.1111/j.1863-2378.2008.01208.x. [DOI] [PubMed] [Google Scholar]
- 3.Beasley DW, Lewthwaite P, Solomon T. Current use and development of vaccines for Japanese encephalitis. Expert Opin Biol Ther. 2008;8(1):95–106. doi: 10.1517/14712598.8.1.95. [DOI] [PubMed] [Google Scholar]
- 4.Tauber E, Kollaritsch H, Korinek M, et al. Safety and immunogenicity of a Vero-cell-derived, inactivated Japanese encephalitis vaccine: a non-inferiority, phase III randomised controlled trial. Lancet. 2007;370(9602):1847–1853. doi: 10.1016/S0140-6736(07)61780-2. [DOI] [PubMed] [Google Scholar]
- 5.Tauber E, Kollaritsch H, von Sonnenburg F, et al. Randomized, double-blind, placebo-controlled phase 3 trial of the safety and tolerability of IC51, an inactivated Japanese encephalitis vaccine. J Infect Dis. 2008;198(4):493–499. doi: 10.1086/590116. [DOI] [PubMed] [Google Scholar]
- 6.Schuller E, Klade CS, Wolfl G, Kaltenbock A, Dewasthaly S, Tauber E. Comparison of a single, high-dose vaccination regimen to the standard regimen for the investigational Japanese encephalitis vaccine, IC51: a randomized, observer-blind, controlled Phase 3 study. Vaccine. 2009;27(15):2188–2193. doi: 10.1016/j.vaccine.2008.12.062. [DOI] [PubMed] [Google Scholar]
- 7.Dubischar-Kastner K, Kaltenboeck A, Klingler A, Jilma B, Schuller E. Safety analysis of a Vero-cell culture derived Japanese encephalitis vaccine, IXIARO (IC51), in 6 months of follow-up. Vaccine. 2010;28(39):6463–6469. doi: 10.1016/j.vaccine.2010.07.040. [DOI] [PubMed] [Google Scholar]
- 8.Clark DC, Brault AC, Hunsperger E. The contribution of rodent models to the pathological assessment of flaviviral infections of the central nervous system. Arch Virol. 2012;157(8):1423–1440. doi: 10.1007/s00705-012-1337-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spotts DR, Reich RM, Kalkhan MA, Kinney RM, Roehrig JT. Resistance to alpha/beta interferons correlates with the epizootic and virulence potential of Venezuelan equine encephalitis viruses and is determined by the 5' noncoding region and glycoproteins. J Virol. 1998;72(12):10286–10291. doi: 10.1128/jvi.72.12.10286-10291.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson AJ, Roehrig JT. New mouse model for dengue virus vaccine testing. J Virol. 1999;73(1):783–786. doi: 10.1128/jvi.73.1.783-786.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calvert AE, Huang CY, Kinney RM, Roehrig JT. Non-structural proteins of dengue 2 virus offer limited protection to interferon-deficient mice after dengue 2 virus challenge. J Gen Virol. 2006;87(Pt 2):339–346. doi: 10.1099/vir.0.81256-0. [DOI] [PubMed] [Google Scholar]
- 12.Thibodeaux BA, Garbino NC, Liss NM, Piper J, Blair CD, Roehrig JT. A small animal peripheral challenge model of yellow fever using interferon-receptor deficient mice and the 17D-204 vaccine strain. Vaccine. 2012;30(21):3180–3187. doi: 10.1016/j.vaccine.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruppert D, Wand MP, Carroll RJ. Semiparametric Regression. Cambridge, United Kingdom: Cambridge University Press; 2003. [Google Scholar]
- 14.Huang CY, Butrapet S, Pierro DJ, et al. Chimeric dengue type 2 (vaccine strain PDK-53)/dengue type 1 virus as a potential candidate dengue type 1 virus vaccine. J Virol. 2000;74(7):3020–3028. doi: 10.1128/jvi.74.7.3020-3028.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solomon T, Dung NM, Kneen R, Gainsborough M, Vaughn DW, Khanh VT. Japanese encephalitis. J Neurol Neurosurg Psychiatry. 2000;68(4):405–415. doi: 10.1136/jnnp.68.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halstead SB, Thomas SJ. New vaccines for Japanese encephalitis. Curr Infect Dis Rep. 2010;12(3):174–180. doi: 10.1007/s11908-010-0098-z. [DOI] [PubMed] [Google Scholar]
- 17.Yu YX, Wu PF, Ao J, Liu LH, Li HM. Selection of better immunogenic and highly attenuated live vaccine virus strain of Japanese encephalitis. I. Some biological characteristics of SA14-14-2 mutant. Chinese Journal of Microbiology and Immunology. 1981;1:77–85. [Google Scholar]
- 18.Eckels KH, Yu YX, Dubois DR, Marchette NJ, Trent DW, Johnson AJ. Japanese encephalitis virus live-attenuated vaccine, Chinese strain SA14-14-2; adaptation to primary canine kidney cell cultures and preparation of a vaccine for human use. Vaccine. 1988;6(6):513–518. doi: 10.1016/0264-410x(88)90103-x. [DOI] [PubMed] [Google Scholar]
- 19.Kumar R, Tripathi P, Rizvi A. Effectiveness of one dose of SA 14-14-2 vaccine against Japanese encephalitis. N Engl J Med. 2009;360(14):1465–1466. doi: 10.1056/NEJMc0808664. [DOI] [PubMed] [Google Scholar]
- 20.Hennessy S, Liu Z, Tsai TF, et al. Effectiveness of live-attenuated Japanese encephalitis vaccine (SA14-14-2): a case-control study. Lancet. 1996;347(9015):1583–1586. doi: 10.1016/s0140-6736(96)91075-2. [DOI] [PubMed] [Google Scholar]
- 21.Yu YX, Zhang GM, Guo YP, Ao J, Li HM. Safety of a Live-Attenuated Japanese Encephalitis-Virus Vaccine (Sa14-14-2) for Children. American Journal of Tropical Medicine and Hygiene. 1988;39(2):214–217. doi: 10.4269/ajtmh.1988.39.214. [DOI] [PubMed] [Google Scholar]
- 22.Eder S, Dubischar-Kastner K, Firbas C, et al. Long term immunity following a booster dose of the inactivated Japanese Encephalitis vaccine IXIARO(R), IC51. Vaccine. 2011;29(14):2607–2612. doi: 10.1016/j.vaccine.2011.01.058. [DOI] [PubMed] [Google Scholar]
- 23.Heinz FX, Stiasny K. Flaviviruses and flavivirus vaccines. Vaccine. 2012;30(29):4301–4306. doi: 10.1016/j.vaccine.2011.09.114. [DOI] [PubMed] [Google Scholar]
- 24.Ni H, Chang GJ, Xie H, Trent DW, Barrett AD. Molecular basis of attenuation of neurovirulence of wild-type Japanese encephalitis virus strain SA14. J Gen Virol. 1995;76(Pt 2):409–413. doi: 10.1099/0022-1317-76-2-409. [DOI] [PubMed] [Google Scholar]
- 25.Arroyo J, Guirakhoo F, Fenner S, Zhang ZX, Monath TP, Chambers TJ. Molecular basis for attenuation of neurovirulence of a yellow fever Virus/Japanese encephalitis virus chimera vaccine (ChimeriVax-JE) J Virol. 2001;75(2):934–942. doi: 10.1128/JVI.75.2.934-942.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chambers TJ, Droll DA, Jiang X, Wold WS, Nickells JA. JE Nakayama/JE SA14-14-2 virus structural region intertypic viruses: biological properties in the mouse model of neuroinvasive disease. Virology. 2007;366(1):51–61. doi: 10.1016/j.virol.2007.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang JJ, Liao CL, Liao JT, Lee YL, Lin YL. A Japanese encephalitis virus vaccine candidate strain is attenuated by decreasing its interferon antagonistic ability. Vaccine. 2009;27(21):2746–2754. doi: 10.1016/j.vaccine.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Lee E, Lobigs M. Mechanism of virulence attenuation of glycosaminoglycan-binding variants of Japanese encephalitis virus and Murray Valley encephalitis virus. J Virol. 2002;76(10):4901–4911. doi: 10.1128/JVI.76.10.4901-4911.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nitayaphan S, Grant JA, Chang GJ, Trent DW. Nucleotide sequence of the virulent SA-14 strain of Japanese encephalitis virus and its attenuated vaccine derivative, SA-14-14-2. Virology. 1990;177(2):541–552. doi: 10.1016/0042-6822(90)90519-w. [DOI] [PubMed] [Google Scholar]
- 30.King NJ, Getts DR, Getts MT, Rana S, Shrestha B, Kesson AM. Immunopathology of flavivirus infections. Immunol Cell Biol. 2007;85(1):33–42. doi: 10.1038/sj.icb.7100012. [DOI] [PubMed] [Google Scholar]
- 31.Diamond MS, Roberts TG, Edgil D, Lu B, Ernst J, Harris E. Modulation of Dengue virus infection in human cells by alpha, beta, and gamma interferons. J Virol. 2000;74(11):4957–4966. doi: 10.1128/jvi.74.11.4957-4966.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, Xu M, Chen L, et al. Evaluation of murine bone marrow-derived dendritic cells loaded with inactivated virus as a vaccine against Japanese encephalitis virus. Vaccine. 2009;27(43):6004–6010. doi: 10.1016/j.vaccine.2009.07.078. [DOI] [PubMed] [Google Scholar]
- 33.Cao S, Li Y, Ye J, et al. Japanese encephalitis Virus wild strain infection suppresses dendritic cells maturation and function, and causes the expansion of regulatory T cells. Virol J. 2011;8:39. doi: 10.1186/1743-422X-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sooryanarain H, Sapkal GN, Gore MM. Pathogenic and vaccine strains of Japanese encephalitis virus elicit different levels of human macrophage effector functions. Arch Virol. 2012;157(10):1905–1918. doi: 10.1007/s00705-012-1386-8. [DOI] [PubMed] [Google Scholar]
- 35.Thibodeaux BA, Garbino NC, Liss NM, et al. A humanized IgG but not IgM antibody is effective in prophylaxis and therapy of yellow fever infection in an AG129/17D-204 peripheral challenge mouse model. Antiviral Res. 2012;94(1):1–8. doi: 10.1016/j.antiviral.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogata A, Nagashima K, Hall WW, Ichikawa M, Kimura-Kuroda J, Yasui K. Japanese encephalitis virus neurotropism is dependent on the degree of neuronal maturity. J Virol. 1991;65(2):880–886. doi: 10.1128/jvi.65.2.880-886.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor JL, Schoenherr C, Grossberg SE. Protection against Japanese encephalitis virus in mice and hamsters by treatment with carboxymethylacridanone, a potent interferon inducer. J Infect Dis. 1980;142(3):394–399. doi: 10.1093/infdis/142.3.394. [DOI] [PubMed] [Google Scholar]
- 38.Lin YL, Liao CL, Yeh CT, et al. A highly attenuated strain of Japanese encephalitis virus induces a protective immune response in mice. Virus Res. 1996;44(1):45–56. doi: 10.1016/0168-1702(96)01343-3. [DOI] [PubMed] [Google Scholar]
- 39.Ishag HZ, Li C, Huang L, et al. Griffithsin inhibits Japanese encephalitis virus infection in vitro and in vivo. Arch Virol. 2013;158(2):349–358. doi: 10.1007/s00705-012-1489-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larena M, Regner M, Lee E, Lobigs M. Pivotal role of antibody and subsidiary contribution of CD8+ T cells to recovery from infection in a murine model of Japanese encephalitis. J Virol. 2011;85(11):5446–5455. doi: 10.1128/JVI.02611-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larena M, Prow NA, Hall RA, Petrovsky N, Lobigs M. JE-ADVAX vaccine protection against Japanese encephalitis virus mediated by memory B cells in the absence of CD8(+) T cells and pre-exposure neutralizing antibody. J Virol. 2013;87(8):4395–4402. doi: 10.1128/JVI.03144-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klingel K, Schnorr JJ, Sauter M, Szalay G, Kandolf R. beta2-microglobulin-associated regulation of interferon-gamma and virus-specific immunoglobulin G confer resistance against the development of chronic coxsackievirus myocarditis. Am J Pathol. 2003;162(5):1709–1720. doi: 10.1016/s0002-9440(10)64305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winter PM, Dung NM, Loan HT, et al. Proinflammatory cytokines and chemokines in humans with Japanese encephalitis. J Infect Dis. 2004;190(9):1618–1626. doi: 10.1086/423328. [DOI] [PubMed] [Google Scholar]
- 44.Ravi V, Parida S, Desai A, Chandramuki A, Gourie-Devi M, Grau GE. Correlation of tumor necrosis factor levels in the serum and cerebrospinal fluid with clinical outcome in Japanese encephalitis patients. J Med Virol. 1997;51(2):132–136. [PubMed] [Google Scholar]
- 45.Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, Flavell RA. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med. 2004;10(12):1366–1373. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- 46.Daffis S, Samuel MA, Suthar MS, Gale M, Jr, Diamond MS. Toll-like receptor 3 has a protective role against West Nile virus infection. J Virol. 2008;82(21):10349–10358. doi: 10.1128/JVI.00935-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Biswas SM, Kar S, Singh R, et al. Immunomodulatory cytokines determine the outcome of Japanese encephalitis virus infection in mice. J Med Virol. 2010;82(2):304–310. doi: 10.1002/jmv.21688. [DOI] [PubMed] [Google Scholar]
- 48.Zhang JS, Zhao QM, Zuo SQ, Jia N, Guo XF. Cytokine and chemokine responses to Japanese encephalitis live attenuated vaccine in a human population. Int J Infect Dis. 2012;16(4):e285–e288. doi: 10.1016/j.ijid.2011.12.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.