Abstract
Our laboratory has shown that peripheral inflammatory pain induced by λ-carrageenan (CIP) can increase blood–brain barrier (BBB) permeability and alter tight junction (TJ) protein expression leading to changes in BBB functional integrity. However, the intracellular signaling mechanisms involved in this pathophysiologic response have not been elucidated. Transforming growth factor (TGF)-β signaling pathways are known to regulate vascular integrity and permeability. Therefore, we examined the function of TGF-β signaling at the BBB in rats subjected to CIP. During CIP, serum TGF-β1 and protein expression of the TGF-β receptor activin receptor-like kinase-5 (ALK5) were reduced. Brain permeability to 14C-sucrose was increased and expression of TJ proteins (i.e., claudin-5, occludin, zonula occluden (ZO-1)) were also altered after 3 h CIP. Pharmacological inhibition of ALK5 with the selective inhibitor SB431542 further enhanced brain uptake of 14C-sucrose, increased TJ protein expression (i.e., claudin-3, claudin-5, occludin, ZO-1), and decreased nuclear expression of TGF-β/ALK5 signaling molecules (i.e., Smad2, Smad3), which suggests a role for TGF-β/ALK5 signaling in the regulation of BBB integrity. Interestingly, administration of exogenous TGF-β1 before CIP activated the TGF-β/ALK5 pathway and reduced BBB permeability to 14C-sucrose. Taken together, our data show that TGF-β/ALK5 signaling is, in part, involved in the regulation of BBB functional integrity.
Keywords: blood–brain barrier, brain vascular permeability, inflammatory pain, tight junctions, transforming growth factor-β
Introduction
The blood–brain barrier (BBB) constitutes a remarkable physical and biochemical barrier between the brain and the circulation. Structurally, the BBB is composed of a monolayer of nonfenestrated endothelial cells surrounded by pericytes and perivascular astrocytes. These endothelial cells are interconnected by tight junctions (TJs), large multiprotein complexes that are maintained by astrocytic trophic factors (Janzer and Raff, 1987). Tight junctions form a continuous, almost impermeable barrier that limits paracellular flux of xenobiotics with the exception of small, lipid-soluble molecules (Abbott, 2005). The high BBB transendothelial electrical resistance (1,500 to 2,000 Ωcm2) further restricts the free flow of water and solutes (Butt et al, 1990). BBB TJs are formed by occludin and claudins (i.e., claudin-1, -3, and -5), transmembrane proteins linked to the cytoskeleton through interactions with accessory proteins (i.e., zonula occluden (ZO)-1, -2, and -3) (Hawkins and Davis, 2005). Zonula occluden proteins act as a scaffold for multiple intracellular signaling pathways and are involved in regulation of TJ function (Haskins et al, 1998). Although TJ proteins may dynamically interact with each other, occludin and claudins are primarily linked to the cytoskeleton through ZO-1 binding (Itoh et al, 1999).
It is established that the BBB may be compromised in response to various pathologies including multiple sclerosis, Alzheimer’s disease, human immunodeficiency virus encephalitis, cerebral ischemia, and meningitis (Hawkins and Davis, 2005). Our laboratory has also shown changes in BBB functional integrity during peripheral inflammatory pain (Huber et al, 2001, 2002, 2006; Brooks et al, 2005; Campos et al, 2008). For example, studies using a rodent model of inflammatory pain, where λ-carrageenan is injected into right hind paw (i.e., λ-carrageenan-induced inflammatory pain (CIP)), showed increased 14C-sucrose permeation across the BBB (Huber et al, 2001, 2002; Campos et al, 2008). These changes in permeability were paralleled by altered expression of TJ proteins including occludin, claudin-5, and ZO-1 (Huber et al, 2001, 2002; Campos et al, 2008). Taken together, these data suggest that inflammatory pain may compromise the BBB and thus render the brain more sensitive to central effects of drugs used in pain management (i.e., opioid analgesics).
Despite the observation that inflammatory pain alters BBB functional integrity, the intracellular signaling mechanisms involved have not been elucidated. One pathway that may be involved is the transforming growth factor-β (TGF-β) system, which regulates multiple cellular processes including vascular remodeling (Pepper, 1997). The TGF-βs are a family of pleiotropic cytokines that modulate cellular function by binding to a heterotetrameric complex of type I and type II serine/threonine kinase receptors (Derynck and Zhang, 2003). The type I receptors, also known as activin receptor-like kinases (ALKs), propagate intracellular signals through the phosphorylation of specific Smad proteins (i.e., receptor-regulated (R)-Smads). Phosphorylated (R)-Smads form complexes with the common Smad (i.e., Smad4) enabling them to be translocated to the nucleus and regulate the transcription of target genes (Derynck and Zhang, 2003).
In the majority of tissues, TGF-β signaling is mediated by ALK5 (Lebrin et al, 2005); however, studies in cultured human endothelial cells (Wu et al, 2006) and in isolated arterial endothelium from ALK1-deficient mice (Seki et al, 2003) have indicated that ALK1 is also involved in vascular TGF-β-mediated signaling. TGF-β regulates the endothelial activation state through a precise balance between ALK1 and ALK5 signaling processes (Goumans et al, 2002; Wu et al, 2006). Whereas the ALK1 pathway leads to endothelial activation characterized by cellular proliferation and increased permeability, ALK5-mediated signaling promotes vascular resolution that is demarcated by impaired cellular proliferation and decreased permeability (Lebrin et al, 2005; Wu et al, 2006). ALK1 and/or ALK5 signaling may also be involved in the regulation of TJ protein expression and, by extension, paracellular xenobiotic permeability. Studies using human glioblastoma cells cocultured with human brain endothelial cells showed that activation of TGF-β-mediated signaling altered the endothelial expression of occludin, claudin-1, and claudin-5 (Ishihara et al, 2008). In addition, claudin-5 expression was increased by pharmacological ALK5 inhibition in embryonic endothelial stem cells, suggesting the involvement of TGF-β/ALK5 signaling in the regulation of TJ proteins (Watabe et al, 2003).
In the present study, we investigated in vivo the function of TGF-β-mediated signaling on (1) TJ protein expression in rat brain microvessels and (2) substrate permeability at the BBB by measuring brain 14C-sucrose uptake using in situ brain perfusion. Both of these research objectives were evaluated in the context of CIP.
Materials and methods
Materials
Rabbit polyclonal antibodies against ALK1 (H-150), ALK5 (V-22), ZO-1 (H-300) and Glut1 (H-43) as well as goat polyclonal antibodies against claudin-3 (M-20) and lamin B (M-20) were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). The rabbit monoclonal antiphospho-Smad3 antibody and rabbit polyclonal antibodies against phospho-Smad1/5/8, phospho-Smad2, and Smad4 were obtained from Cell Signaling Technology (Danvers, MA, USA). The mouse monoclonal anticlaudin-5 antibody 4C3C2 and the mouse monoclonal antioccludin antibody OC-3F10 were purchased from Invitrogen Corporation (Camarillo, CA, USA). 14C-sucrose (633 mCi/mmol) was obtained from GE Healthcare Biosciences (Piscataway, NJ, USA). The selective ALK5 inhibitor SB431542, human recombinant TGF-β1, λ-carrageenan, sodium pentobarbital, and the murine monoclonal antiactin antibody AC40 were purchased from Sigma-Aldrich Inc. (St Louis, MO, USA).
Animals and Treatments
All animal experiments were approved by the University of Arizona Institutional Animal Care and Use Committee and conform to National Institutes of Health guidelines. Female Sprague–Dawley rats (200 to 250 g) were purchased from Harlan Sprague–Dawley (Indianapolis, IN, USA), housed under standard 12:12 h light/dark conditions, and provided with food and water ad libitum. Animals were randomly assigned to each treatment group. Rats were injected (100 µL, s.c.) with 3% λ-carrageenan (m/v in 0.9% saline) or with 0.9% saline (m/v) into the plantar surface of the right hind paw. For experiments designed to evaluate the involvement of ALK5-mediated signaling, SB431542 (1.5 mg/kg (1.0 mL/kg), i.p.), a selective ALK5 inhibitor, was dissolved in 100% dimethyl sulfoxide and administered 30 mins before footpad injection. For experiments examining the function of exogenous TGF-β1, human recombinant TGF-β1 (12.5 ng/kg (1.0 mL/kg), i.p.) was dissolved in 0.9% saline and injected 30 mins before treatment with saline or λ-carrageenan. After 3h exposure to λ-carrageenan or saline, animals were anaesthetized with sodium pentobarbital (64.8 mg/kg (1.0 mL/kg), i.p.) and prepared for microvessel isolation or for in situ brain perfusion.
Microvessel Isolation
After anesthesia, rats were decapitated and brains were removed. The meninges and choroid plexus were excised and the cerebral hemispheres were homogenized in 4 mL of microvessel isolation buffer (103 mmol/L NaCl, 4.7 mmol/L KCl, 2.5 mmol/L CaCl2, 1.2 mmol/L KH2PO4, 1.2 mmol/L MgSO4, 15 mmol/L HEPES, 0.1 mmol/L EGTA, 1 mmol/L sodium orthovanadate, 0.5 mmol/L phenylmethylsulfonyl fluoride, pH 7.4) containing protease inhibitor (PI) cocktail (Sigma-Aldrich Inc.). At this time, 8mL of 26% dextran (m/v) at 4°C was added to the homogenate. Samples were then gently vortexed, centrifuged (5,600g, 4°C) for 10 mins, and the supernatant was aspirated. Pellets were resuspended in 10 mL of fresh microvessel isolation buffer and passed through a 70 µm filter (Becton Dickinson, Franklin Lakes, NJ, USA). Filtered homogenates were pelleted by centrifugation at 3,000g for 10 mins. At this time, the supernatant was aspirated and the pellet, which was enriched in brain microvessels, was collected.
Crude Membrane Preparations
After brain microvessels were collected, the pellet was resuspended in 500 µL CellLytic (Sigma-Aldrich Inc.) containing PI cocktail. The samples were subjected to a 15 secs homogenization every 15 mins for 1 h while on ice and then centrifuged at 10,000g (30 mins, 4°C). The supernatants were collected and centrifuged at 100,000g (90 mins, 4°C). At this time, the supernatant was discarded and the pellet was resuspended in phosphate-buffered saline containing PI cocktail. The protein concentration of each sample was determined using the bicinchonic acid protein assay (Pierce Biotechnology, Rockford, IL, USA) and used for Western blot analysis.
Cytoplasmic and Nuclear Cell Fractions
Cytoplasmic and nuclear cell fractions were prepared as previously described (Zastre et al, 2009). Briefly, microvessels were washed, harvested in ice-cold phosphate-buffered saline, and pelleted by centrifugation (1,500g, 5 mins). The pellet was resuspended in cytoplasmic lysis buffer (10 mmol/L HEPES, 10 mmol/L KCl, 0.1 mmol/L EGTA, 1 mmol/L dithiothreitol, 1 mmol/L sodium orthovanadate, 0.5 mmol/L phenylmethylsulfonyl fluoride and 0.1% (v/v) PI cocktail), and incubated on ice for 15 mins to allow for cell swelling. NP-40 was added (0.15%, v/v) and the samples were gently mixed for 1 min. Microvessels were centrifuged for 5 mins to pellet-intact nuclei and the supernatant (i.e., cytoplasmic fraction) was collected. Pelleted nuclei were washed with phosphate-buffered saline and centrifuged for 5 mins. The pellet was then resuspended in ice-cold nuclear lysis buffer (20 mmol/L HEPES, 1.2 mol/L NaCl, 1 mmol/L EGTA, 1 mmol/L dithiothreitol, 1 mmol/L sodium orthovanadate, 0.5 mmol/L phenylmethylsulfonyl fluoride, 0.1% (v/v) PI cocktail and DNaseI) at 4°C for 30 mins. The nuclear extract was centrifuged for 5 mins, and the supernatant was collected and stored at −80°C. The protein concentration of each sample was obtained using the bicinchonic acid protein assay.
Western Blotting
For Western blotting, 20 µg aliquots of crude membrane preparations, cytoplasmic extracts, or nuclear extracts were mixed in XT sample loading buffer containing Tris(2-carboxyethyl) phosphine and heated for 10 mins at 70°C. The samples were then resolved on 10% sodium dodecyl sulfate–polyacrylamide gels (Bis-Tris Criterion XT; Bio-Rad, Hercules, CA, USA). The gels were electrotransferred onto a polyvinylidene difluoride (PVDF) membrane and protein transfer was verified by Ponceau S staining. The membranes were blocked for 1 h at room temperature in SuperBlock (Pierce Biotechnology) containing 0.05% (v/v) Tween 20. After six washes (5 mins each) with Tris-buffered saline (15 mmol/L Tris-HCl, 150 mmol/L NaCl, pH 7.6) containing 0.05% (v/v) Tween 20, the membrane was incubated at 4°C overnight with the appropriate primary antibody (Table 1). After a second wash, membranes were incubated for 1 to 2 h with antimouse (GE Healthcare Biosciences), antirabbit (GE Healthcare), or antigoat (Santa Cruz Biotechnology) horseradish-peroxidase-conjugated secondary antibodies in SuperBlock containing 0.05% (v/v) Tween 20 at room temperature. Protein bands were detected by enhanced chemiluminescence. Bands were quantitated and corrected for background using ImageJ densitometric software (Wayne Rasband, Research Services Branch, National Institute of Mental Health, Bethesda, MD, USA). Protein expression in crude membrane preparations and cytoplasmic extracts was normalized to expression of actin, which served as a loading control. Similarly, protein expression in nuclear extracts was normalized according to lamin B expression, which was used as a loading control for these samples.
Table 1.
Detailed antibody information
| Antigen | MW (kDa) | Clone | Type | Species | Epitope |
|---|---|---|---|---|---|
| Actin | 42 | AC-40 | MC | Mouse | C-terminal end, which is conserved in all actin isoforms |
| ALK1 | 53 | H-150 | PC | Rabbit | N-terminal extracellular domain |
| ALK5 | 53 | V-22 | PC | Rabbit | C-terminal cytoplasmic domain |
| Claudin-3 | 22 | M-20 | PC | Goat | C-terminal domain |
| Claudin-5 | 22–24 | 4C3C2 | MC | Mouse | Intracellular domain |
| Glut1 | 55 | H-43 | PC | Rabbit | Epitope corresponds to amino acids 218–260 of human Glut1 |
| Lamin B | 67 | M-20 | PC | Goat | C terminus |
| Occludin | 63 (α-band) 65 (β-band) | OC-3F10 | MC | Mouse | C terminus |
| p-Smad1/5/8 | 60 | Ser463/465 | PC | Rabbit | Residues surrounding Ser463 and Ser465 on Smad5 |
| p-Smad2 | 60 | Ser465/467 | PC | Rabbit | Smad2 dually phosphorylated at Ser465 and Ser467 |
| p-Smad3 | 52 | Ser423/425 | PC | Rabbit | Smad3 dually phosphorylated at Ser423 and Ser425 |
| Smad4 | 70 | NA | PC | Rabbit | Residues surrounding Pro278 |
| ZO-1 | 220 | H-300 | PC | Rabbit | Amino acids 1437–1736 of the C-terminal domain |
MC, monoclonal; PC, polyclonal.
ELISA Analysis
A Quantikine enzyme-linked immunosorbant assay (ELISA) kit (R&D Systems, Minneapolis, MN, USA) was used to determine the serum concentration of TGF-β1 from rats treated with λ-carrageenan or saline. A standard curve for TGF-β1 (0 to 2,500 pg/mL) was generated using recombinant rat TGF-β1 and the assay was performed according to the manufacturer’s instructions. Absorbance was read at 450 nm using a Synergy 2 microplate spectrophotometer (Biotek Instruments Inc., Winooski, VT, USA). Absorbance was also measured at 550nm to correct for optical imperfections in the assay plate. The concentration of serum TGF-β1 was expressed as pg/mL.
Edema Formation and Paw Withdrawal Latency
Hind paw edema and paw withdrawal latency in response to a thermal stimulus were determined as previously described (Campos et al, 2008). Briefly, hind paw edema was measured using a plethysmometer (model 7141; Ugo Basile, Comerio, Varese, Italy). Thermal allodynia through paw withdrawal latency was determined using a Plantar Analgesia Instrument (model 7375; Ugo Basile) that used an infrared heat stimulus (Montagne-Clavel and Oliveras, 1996). To ensure uniformity of testing, rats were prehabituated to the experimental apparatus for 20 mins before thermal allodynia measurements. Paw withdrawal latencies are defined as the time (sec) for the rat to remove its paw from the heat source. Three consecutive measurements were performed on the right hind paw with 5 mins intervals between measurements. Data were also collected from the left hind paw (i.e., contralateral, noninjected paw) to confirm that edema and thermal allodynia were localized to the injection site.
In Situ Brain Perfusion
In situ brain perfusion was performed as previously described by our group (Huber et al, 2006; Campos et al, 2008). Three hours after paw injection, animals were anesthetized and heparinized (10,000 U/kg, i.p.). Body temperature was maintained at 37°C using a heating pad. The common carotid arteries were cannulated with silicone tubing connected to a perfusion circuit. The perfusate was erythrocyte-free modified mammalian Ringer’s solution consisting of 117 mmol/L NaCl, 4.7 mmol/L KCl, 0.8 mmol/L MgSO4, 1.2 mmol/L KH2PO4, 2.5 mmol/L CaCl2, 10 mmol/L d-glucose, 3.9% (m/v) dextran (MW 60,000), and 1.0 g/L bovine serum albumin (type IV), pH 7.4, warmed to 37°C and oxygenated with 95% O2/5% CO2. Evans blue dye (55 mg/L) was added to the perfusate to serve as a visual marker of BBB integrity. Perfusion pressure and flow rate were maintained at 95 to 105 mm Hg and 3.1 mL/min, respectively. Both jugular veins were severed to allow for perfusate drainage. Using a slow-drive syringe pump (Harvard Apparatus, Holliston, MA, USA), we added 14C-sucrose (10 µCi/20 mL perfusate; purity confirmed by high-pressure liquid chromatography analysis) to the inflowing perfusion solution at a rate of 0.5 mL/min per cerebral hemisphere. After a 20-min perfusion, the rat was decapitated and the brain was removed. The meninges and choroid plexus were excised and the cerebral hemispheres were sectioned. TS2 tissue solubilizer (1 mL; Research Products International Corp., Mount Prospect, IL, USA) was added to the tissue samples, which were allowed to solubilize for 2 days at room temperature. To eliminate chemiluminescence, 100 µL of 30% glacial acetic acid was added, along with 2.0 mL Optiphase SuperMix liquid scintillation cocktail (PerkinElmer, Boston, MA, USA). Radioactivity was measured using a model 1450 Liquid Scintillation and Luminescence Counter (PerkinElmer). Results were reported as the ratio of radioactivity in the brain to that in the perfusate (RBr), which is equal to the total amount of radioisotope in the brain (C(Brain), d.p.m./g tissue) divided by the amount of radioisotope in the perfusate (C(Perfusate), d.p.m./mL).
Statistical Analysis
Densitometry analysis data are reported as mean ± s.d. from three separate experiments where each treatment group consists of pooled microvessels from three animals. ELISA and in situ brain perfusion data are reported as mean ± s.d. from six individual animals per treatment group. To determine statistical significance between treatment groups in ELISA and Western blot experiments, Student’s t-test was used for unpaired experimental data. To determine the significance of brain 14C-sucrose accumulation, the test of repeated-measures ANOVA and the post hoc multiple-comparison Bonferroni t-test was used. A value of P < 0.05 was accepted as statistically significant.
Results
Serum Concentrations of TGF-β1 During CIP
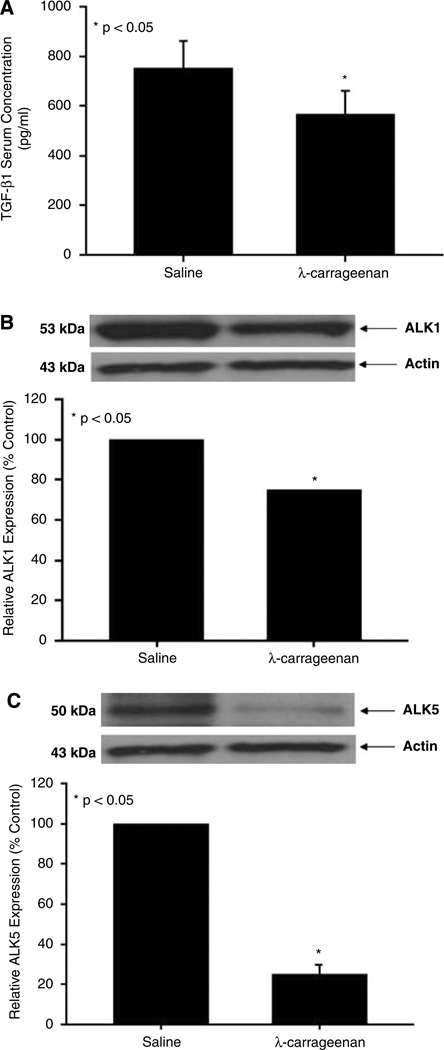
TGF-β1 is a cytokine that is involved in maintaining microvascular integrity through two highly regulated and balanced processes: (1) endothelial activation demarcated by increased permeability and (2) endothelial resolution, which is characterized by decreased permeability (Lebrin et al, 2005). Because our laboratory has showed that CIP conditions can increase BBB permeability to 14C-sucrose, a molecule that does not normally cross the BBB (Bhattacharjee et al, 2001), we hypothesized that TGF-β1-mediated signaling may be involved in this pathophysiologic response. Therefore, we measured TGF-β1 serum concentrations in rats administered saline or λ-carrageenan for 3 h. Enzyme-linked immunosorbent assay analysis detected a significant decrease (1.3-fold, P < 0.05) in serum TGF-β1 concentration in rats subjected to CIP as compared with saline controls (Figure 1A).
Figure 1.
TGF-β1 concentration in rat serum and expression of TGF-β type I receptors in rat brain microvessels. (A) Rats were treated with 0.9% saline or with 3% λ-carrageenan for 3 h. Serum was collected and TGF-β1 concentration was determined by ELISA analysis. Data points are expressed as mean ± s.d. from six individual rats. (B, C) Western blot analysis of brain microvessels isolated from rats administered 0.9% saline or 3% λ-carrageenan by s.c. injection into the plantar surface of the right hind paw. Crude membrane preparations (20 µg) from rat brain microvessels were resolved on a 10% sodium dodecyl sulfate–polyacrylamide gel, transferred to a PVDF membrane, and analyzed for expression of ALK1 (A) and ALK5 (B). ALK1 was detected using the polyclonal antibody H120 (1:500 dilution) whereas ALK5 was detected using the polyclonal antibody V-22 (1:500 dilution). Relative levels of ALK1 and ALK5 protein expression were determined by densitometric analysis. Results are expressed as mean ± s.d. of three separate experiments. Asterisks represent data points that are significantly different from control.
Expression of ALK1 and ALK5 in Rat Brain Microvessels During CIP
Previous studies have reported the expression of two TGF-β type I receptors (i.e., ALK1 and ALK5) at the BBB (Ata et al, 1999; De Groot et al, 1999). Therefore, we examined the expression of both ALK1 and ALK5 in microvessels isolated from rats subjected to 3 h CIP. Western blot analysis detected single bands for ALK1 and ALK5 at 53 and 50 kDa, respectively (Figure 1B and 1C). Interestingly, 3 h CIP decreased expression of ALK1 (1.3-fold) and ALK5 (4.0-fold) as compared with saline controls. Taken together with our ELISA results, these data indicate that acute inflammatory pain may lead to reduced TGF-β-mediated signaling at the BBB.
Blood–Brain Barrier Permeability to 14C-Sucrose
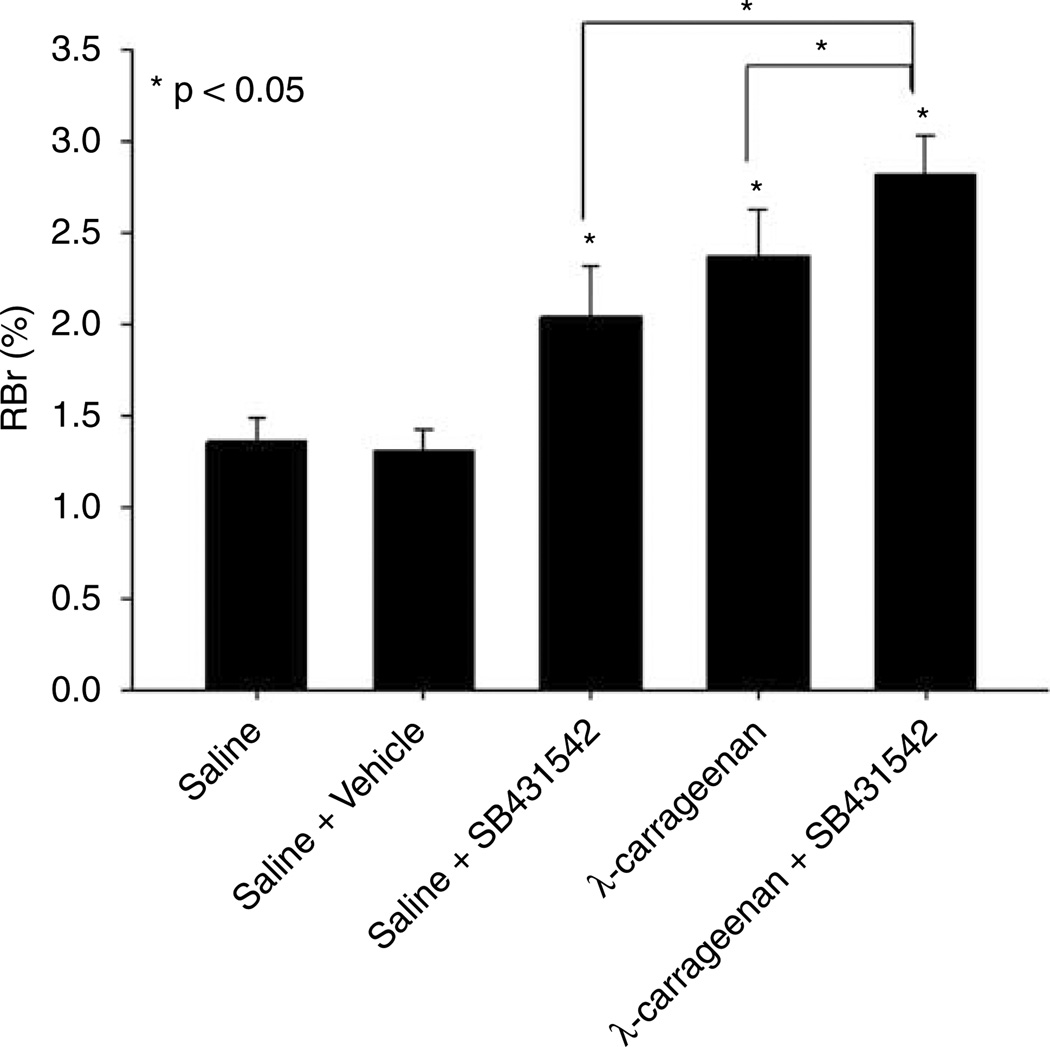
To investigate if TGF-β-mediated signaling events were involved in the regulation of BBB permeability during acute inflammatory pain, we measured the brain uptake of 14C-sucrose in the presence and absence of SB431542, a selective ALK5 inhibitor, in saline-treated and CIP animals. Because activation of ALK5-mediated signaling pathways has been reported to reduce vascular permeability (Pepper, 1997; Lebrin et al, 2005), we postulated that a decrease in TGF-β-mediated signaling through the ALK5 receptor may result in an increase in BBB permeability to sucrose. In control (i.e., saline-treated) animals, the RBr was 1.36% ± 0.13% (Figure 2). Three hours after CIP, the RBr for 14C-sucrose was significantly increased (2.37% ± 0.26%, P < 0.05), suggesting an increase in BBB permeability associated with inflammatory pain. Administration of 1.5 mg/kg SB431542 30 mins before footpad injection significantly enhanced the RBr for 14C-sucrose both in animals treated with saline (2.04% ± 0.28%, P < 0.05) and with λ-carrageenan (2.82% ± 0.22%, P < 0.05). In addition, the RBr for 14C-sucrose in rats treated with SB431542 and λ-carrageenan was significantly greater (P < 0.05) than in animals administered λ-carrageenan alone. Interestingly, the RBr for 14C-sucrose in rats treated with SB431542 and λ-carrageenan was significantly larger (P < 0.05) than in animals administered SB431542 and saline, emphasizing that TGF-β/ALK5 signaling is prominently involved in the regulation of BBB permeability. Owing to poor water solubility, we administered SB431542 in a 100% dimethyl sulfoxide vehicle. The RBr for 14C-sucrose in animals receiving 100% dimethyl sulfoxide plus saline (i.e., vehicle control) was 1.27% ± 0.11%, which was not significantly different from saline controls and implied a minimal effect on vascular permeability. A visual assessment of the brain parenchyma after in situ perfusion showed no Evans blue albumin staining, suggesting that the BBB was morphologically intact.
Figure 2.
Changes in paracellular permeability to 14C-sucrose during peripheral inflammatory pain in the presence and absence of SB431542, a selective ALK5 inhibitor. Graph shows the percent of radioactivity detected in the brain as compared with that in the perfusate (% RBr) for the five treatment groups 3 h after injection of 3% λ-carrageenan or 0.9% saline into the plantar surface of the right hind paw. SB431542 (1.5 mg/kg) or vehicle (100% dimethyl sulfoxide) were injected i.p. 30 mins before footpad injection. Results are expressed as mean ± s.d. of six animals per treatment group.
Measurement of Paw Edema and Thermal Allodynia
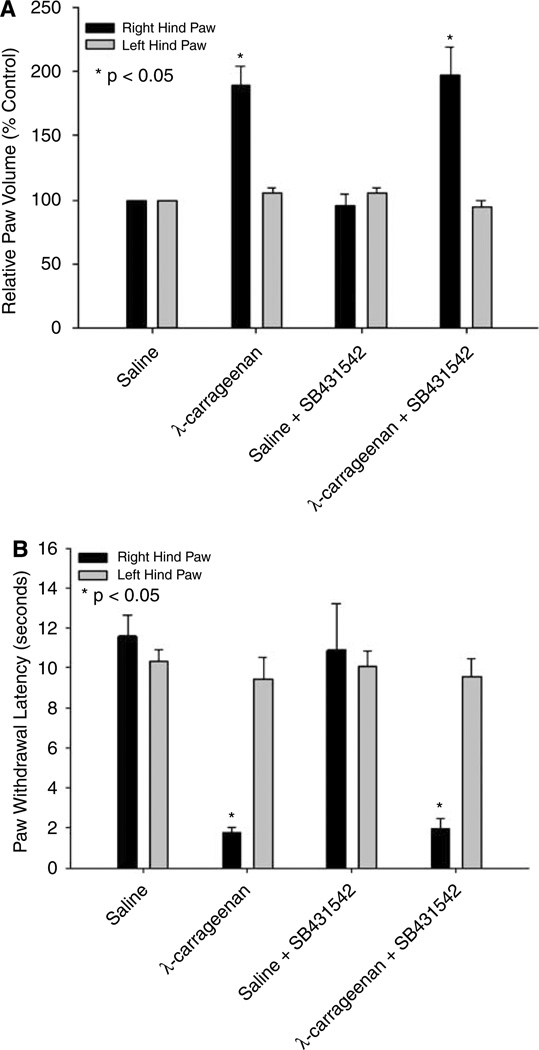
Previous studies in our laboratory have showed that CIP enhances paw swelling as well as the animal’s response to noxious thermal stimuli (Campos et al, 2008). However, it is unknown if TGF-β/ALK5 signaling has an effect on either of these parameters. Three hours after paw injection, administration of λ-carrageenan induced a significant (P < 0.05) right hind paw edema (Figure 3A). Administration of SB431542 before CIP did not alter edema formation as compared with animals administered λ-carrageenan alone. For comparison purposes, the contralateral (i.e., left) hind paw was measured and showed no volume change among any of the treatment groups studied.
Figure 3.
Effect of CIP on paw edema and thermal allodynia and the effect of SB431542 administration. (A) Edema formation in the injected paw (i.e., right) and in the contralateral paw (i.e., left) measured as the relative paw volume 3 h after injection with saline or λ-carrageenan in rats in the presence and absence of SB431542. Results are expressed as mean ± s.d. of six animals per treatment group. (B) Thermal allodynia based on paw withdrawal latency from an infrared heat source. Paw withdrawal latency was measured 3 h after injection with saline or λ-carrageenan in rats in the presence and absence of SB431542. Results are expressed as mean ± s.d. of six animals per treatment group.
Thermal allodynia during CIP was determined based on paw withdrawal latency from an infrared heat source. The response to this thermal stimulation was significantly increased (P < 0.05) in CIP animals as compared with saline controls (Figure 3B). Injection of SB431542 before footpad injection did not alter thermal allodynia in rats receiving λ-carrageenan or saline. These data imply that SB431542 administration did not affect the animals’ behavioral response to peripheral inflammatory pain.
Expression of Tight Junction Proteins
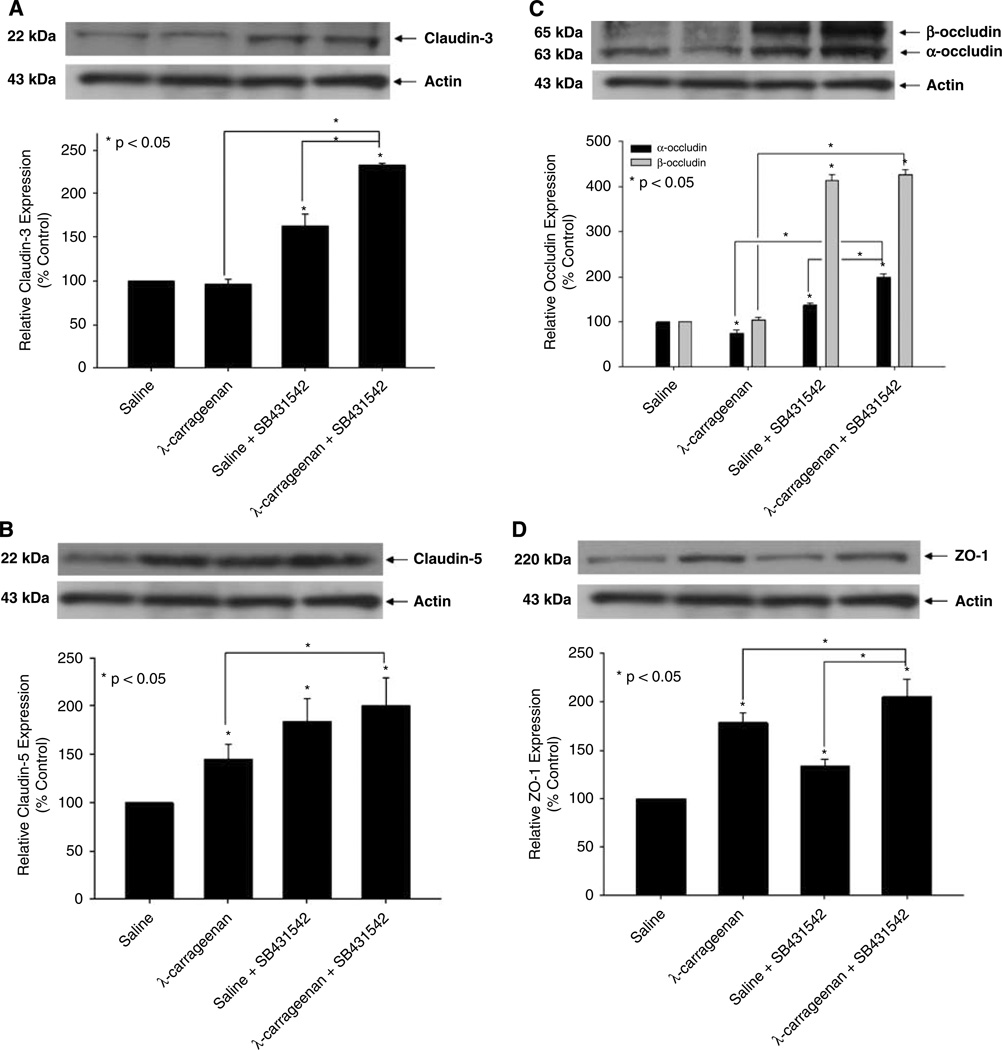
Blood–brain barrier TJs are made up of distinct proteins (i.e., claudins, occludin, ZO-1) that form intercellular and intracellular protein networks and restrict the paracellular movement of solutes. Because our results imply that TGF-β/ALK5 signaling is involved in the regulation of BBB permeability, we investigated the function of this pathway in the regulation of TJs during CIP using Western blot analysis. In microvessels obtained from CIP rats, increased expression of claudin-5 (1.5-fold) and ZO-1 (1.8-fold) was detected whereas claudin-3 expression was not altered (Figure 4). In contrast, expression of α-occludin was decreased by 1.3-fold whereas β-occludin was not altered during acute CIP. Pretreatment with SB431542 before CIP significantly enhanced (P < 0.05) protein expression of claudin-3 (2.3-fold), claudin-5 (2.0-fold), α-occludin (2.0-fold), β-occludin (4.3-fold), and ZO-1 (2.0-fold) to a greater degree than in rats administered λ-carrageenan alone. An increase in protein expression of claudin-3 (1.6-fold), claudin-5 (1.8-fold), α-occludin (1.4-fold), β-occludin (4.1-fold), and ZO-1 (1.3-fold) was also observed in animals pretreated with SB431542 before saline injection, further implicating TGF-β/ALK5-mediated signaling in TJ protein regulation. The complexity of TGF-β/ALK5 signaling at the BBB is further emphasized by increased expression of claudin-3 (1.4-fold), α-occludin (1.4-fold), and ZO-1 (1.5-fold) in rats treated with SB431542 and λ-carrageenan as compared with animals administered SB431542 alone. The presence of plasma membrane in each crude membrane sample was confirmed by expression of Glut1 (data not shown), a membrane-bound glucose transporter that is highly expressed at the plasma membrane of brain endothelial cells (Cornford et al, 1994).
Figure 4.
Expression of TJ protein in brain microvessels during CIP in the presence and absence of an ALK5 inhibitor. Western blot analysis of microvessels isolated from rats treated with saline or λ-carrageenan in the presence and absence of SB431542, a selective ALK5 inhibitor. Crude membrane preparations from rat brain microvessels (20 µg) were resolved on a 10% sodium dodecyl sulfate–polyacrylamide gel and transferred to a PVDF membrane. Samples were analyzed for expression of claudin-3 (A), claudin-5 (B), occludin (C), and ZO-1 (D). Claudin-3 was detected using the polyclonal antibody M-20 (1:500 dilution), claudin-5 was detected using the monoclonal antibody 4C3C2 (1:500 dilution), occludin was detected using the monoclonal antibody OC-3F10 (1:1000 dilution), and ZO-1 was detected using the polyclonal antibody H-300 (1:1000 dilution). Relative levels of TJ protein expression were determined by densitometric analysis. Results are expressed as mean ± s.d. of three separate experiments. Asterisks represent data points that are significantly different from control.
Expression of Phosphorylated Smad Proteins
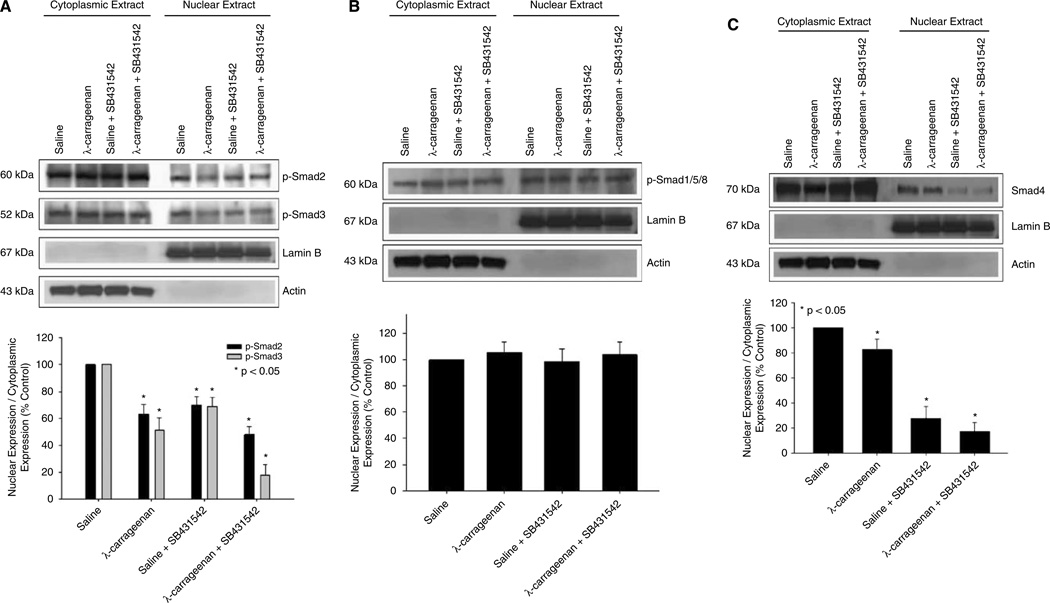
Transforming growth factor-β signaling involves phosphorylation and subsequent nuclear accumulation of (R)-Smad proteins. Therefore, we investigated the activation of (R)-Smad proteins associated with ALK5 and ALK1 in brain microvasculature during CIP. Western blot analysis of cytoplasmic and nuclear extracts from brain microvessels isolated from rats treated with saline or λ-carrageenan in the presence and absence of SB431542 was performed. Control experiments showed that total cytoplasmic expression (i.e., phosphorylated and nonphosphorylated) of all Smad isoforms studied was unchanged in response to λ-carrageenan treatment, suggesting that CIP did not alter the expression of proteins involved in the transduction of TGF-β-associated signals (data not shown). Expression of lamin B, a nuclear envelope marker, was detected in nuclear fractions but not in cytoplasmic fractions, suggesting that minimal nuclear lysis occurred during separation of these fractions. After 3 h CIP, expression of phosphorylated Smad2 (p-Smad2) and phosphorylated Smad3 (p-Smad3) was reduced in both cytoplasmic and nuclear fractions as compared with saline controls (Figure 5A). Protein expression of both p-Smad2 and p-Smad3 was also reduced in the presence of SB431542. To correlate expression levels of phosphorylated Smad proteins with the potential for signal transduction, we calculated the nucleus/cytoplasm ratio of p-Smad2 and p-Smad3 using densitometric analysis. In our study, the nucleus/cytoplasm ratio for both p-Smad2 and p-Smad3 was decreased in rats subjected to CIP for 3 h as compared with saline controls (Figure 5A). Consistent with SB431542 pharmacology, this ratio was further decreased in animals administered SB431542 during CIP. Taken together, these data provide evidence for decreased TGF-β/ALK5-mediated signaling at the BBB during CIP.
Figure 5.
Expression of Smad proteins in brain microvessels during CIP in the presence and absence of an ALK5 inhibitor. Western blot analysis of microvessels isolated from rats treated with saline or λ-carrageenan in the presence and absence of SB431542, a selective ALK5 inhibitor. Cytoplasmic and nuclear extracts from rat brain microvessels (20 µg) were resolved on a 10% sodium dodecyl sulfate–polyacrylamide gel and transferred to a PVDF membrane. Samples were analyzed for expression of p-Smad2 and p-Smad3 (A) as well as p-Smad1/5/8 (B) and Smad4 (C). P-Smad2 was detected using the polyclonal antibody Ser465/467 (1:500 dilution), p-Smad3 was detected using the polyclonal antibody Ser423/425 (1:500 dilution), pSmad1/5/8 was detected using the polyclonal antibody Ser463/465 (1:500 dilution), and Smad4 was detected using the polyclonal anti-Smad4 antibody (1:500 dilution). Relative levels of Smad proteins were determined by densitometric analysis and the nucleus/cytoplasm ratio was calculated. Results are expressed as mean ± s.d. of three separate experiments. Asterisks represent data points that are significantly different from control.
In addition to examining p-Smad2 and p-Smad3 expression, we also evaluated the activation of Smad isoforms associated with ALK1-mediated signaling (i.e., Smads 1, 5, and 8). In rats treated with λ-carrageenan alone, the expression of phosphorylated Smads 1, 5, and 8 (p-Smad1/5/8) was slightly, but not significantly, decreased (Figure 5B). In addition, p-Smad1/5/8 expression did not change in the presence of SB431542, which emphasizes the ALK5 specificity of this compound. Furthermore, the nucleus/cytoplasm ratio for p-Smad1/5/8 did not change in any of our treatment groups (Figure 5B), suggesting that ALK1-mediated signaling was not altered in brain endothelium in response to inflammatory pain.
To confirm if overall TGF-β-mediated signal transduction was altered in rat brain microvessels during CIP, we determined the protein expression of Smad4 in both cytoplasmic and nuclear fractions. Smad4 is required for the nucleocytoplasmic trafficking of phosphorylated (R)-Smad isoforms associated with both ALK1 and ALK5. Therefore, a change in the nucleus/cytoplasm ratio of Smad4 expression reflects a change in total TGF-β signaling. Densitometric analysis showed a significant (P < 0.05) decrease in the nucleus/cytoplasm ratio of Smad4 in brain microvessels isolated from rats subjected to 3 h CIP (Figure 5C). Furthermore, the nucleus/cytoplasm ratio for Smad4 was also significantly reduced (P < 0.05) in animals administered SB431542, which may reflect pharmacological inhibition of ALK5 and subsequent decrease in p-Smad2/p-Smad3 expression. Taken together, these data suggest a change in the balance between ALK1 and ALK5 signaling in rat brain microvessels during CIP and may indicate a biologic mechanism for altered BBB functional integrity during CIP.
Effect of Exogenous TGF-β1 on p-Smad2/p-Smad3 Expression and 14C-Sucrose Permeability
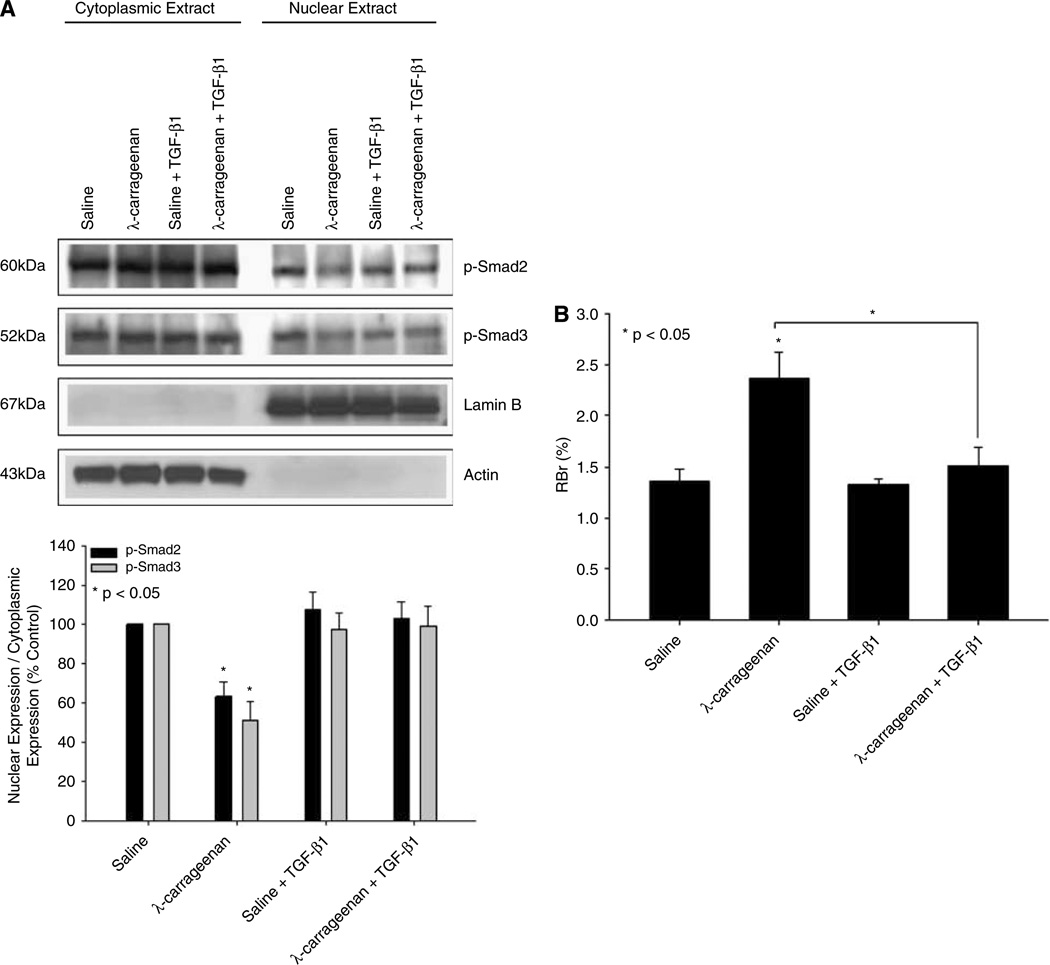
The above data imply that decreased TGF-β/ALK5 signaling may be involved in altered BBB functional integrity during inflammatory pain. Because our ELISA data showed a decrease in serum TGF-β1 during CIP, we hypothesized that exogenous administration of TGF-β1 may attenuate these changes in BBB permeability. Therefore, animals were administered TGF-β1 (12.5 ng/kg, i.p.) 30 mins before footpad injection with saline or λ-carrageenan. This TGF-β1 dose corresponds to a serum concentration of 200 pg/mL (i.e., the magnitude of TGF-β1 decrease), which was calculated based on the assumption that the total blood volume of a 250 g rat is approximately 15.77 mL (i.e., blood volume (mL) = 0.06 × body weight (g) + 0.77). Western blot analysis showed no change in the nucleus/cytoplasm ratio for either p-Smad2 or p-Smad3 in brain microvessels isolated from rats administered TGF-β1 and λ-carrageenan as compared with animals receiving λ-carrageenan alone (Figure 6A). Furthermore, the RBr for 14C-sucrose in animals dosed with TGF-β1 before CIP was significantly reduced (P < 0.05) as compared with rats administered λ-carrageenan alone (1.52 ± 0.18 for TGF-β1/λ-carrageenan versus 2.37 ± 0.26 for λ-carrageenan; Figure 6B). Taken together, these data suggest exogenous TGF-β1 can increase TGF-β/ALK5-mediated signaling during inflammatory pain and may, by extension, prevent the excess accumulation of pharmacological agents within the brain parenchyma.
Figure 6.
Effect of exogenous TGF-β1 treatment on TGF-β/ALK5 signaling and on BBB permeability. (A) Western blot analysis of microvessels isolated from rats treated with saline or λ-carrageenan in the presence and absence of SB431542, a selective ALK5 inhibitor. Cytoplasmic and nuclear extracts from rat brain microvessels (20 µg) were resolved on a 10% sodium dodecyl sulfate–polyacrylamide gel and transferred to a PVDF membrane. Samples were analyzed for expression of p-Smad2 and p-Smad3. P-Smad2 was detected using the polyclonal antibody Ser465/467 (1:500 dilution) and p-Smad3 was detected using the polyclonal antibody Ser423/425 (1:500 dilution). Relative levels of p-Smad2 and p-Smad3 were determined by densitometric analysis and the nucleus/cytoplasm ratio was calculated. Results are expressed as mean ± s.d. of three separate experiments. Asterisks represent data points that are significantly different from control. (B) Changes in paracellular permeability to 14C-sucrose during peripheral inflammatory pain in the presence and absence of TGF-β1. Graph shows the percent of radioactivity detected in the brain as compared with that in the perfusate (% RBr) for the four treatment groups 3 h after injection of 3% λ-carrageenan or 0.9% saline into the plantar surface of the right hind paw. Transforming growth factor-β1 (12.5 ng/kg) was injected i.p. 30 mins before footpad injection. Results are expressed as mean ± s.d. of six animals per treatment group.
Discussion
The BBB is a highly regulated interface between the central nervous system and the systemic circulation (Abbott, 2005; Hawkins and Davis, 2005). The BBB is characterized by the presence of TJ protein complexes between adjacent brain microvessel endothelial cells. Physiologically, the protein constituents of TJs are precisely maintained, which limits paracellular uptake of xenobiotics into the brain parenchyma. Our laboratory has previously showed reorganization of BBB TJ complexes during acute inflammatory pain (Huber et al, 2001, 2002, 2006; Campos et al, 2008). In addition, we have also reported increased brain sucrose accumulation during inflammatory pain, suggesting that these alterations in TJ protein expression may be associated with increased BBB permeability (Huber et al, 2002; Campos et al, 2008). Despite these novel observations, the intracellular signaling mechanisms involved in the regulation of TJ protein expression and BBB permeability during acute inflammatory pain have not been determined. In the present study, we examined the involvement of TGF-β signaling in the maintenance of BBB functional integrity during the acute phase of CIP. TGF-β signaling is an important regulator of endothelial cell differentiation, vascular network formation, and maintenance of vessel wall integrity (Pepper, 1997; Lebrin et al, 2005). In fact, genetic knockout of TGF-β signaling components (i.e., ALK1, ALK5) may have dramatic physiologic consequences including vascular defects and/or embryonic lethality (Dickson et al, 1995; Oh et al, 2000; Larsson et al, 2001).
Previous evidence has indicated that vascular integrity is maintained, in part, through an intricate balance between ALK1 and ALK5 signaling (Goumans et al, 2002). Functionally, ALK1-mediated signaling is involved in enhancing vascular permeability whereas ALK5-related signaling is involved in the opposite response, a decrease in vascular permeability (Lebrin et al, 2005). Both of these receptors are expressed in brain endothelium (Ata et al, 1999; De Groot et al, 1999). Because ALK5-related signaling has been reported to prevent leak across the brain microvasculature (Lebrin et al, 2005), we postulated that acute CIP may cause a decrease in TGF-β/ALK5 signaling. This may disrupt the balance between ALK1- and ALK5-mediated signaling and lead to enhanced BBB permeability. All of our experiments were conducted in female Sprague–Dawley rats to maintain consistency with previous work (Huber et al, 2001, 2002, 2006; Brooks et al, 2005; Campos et al, 2008). In addition, we performed our analyses at 3 h because it is an intermediary time point during acute CIP (i.e., between 1 and 6 h). In the present study, we showed decreased serum TGF-β1 levels and reduced protein expression of ALK5 after 3 h CIP, which is consistent with our hypothesis. Interestingly, we also observed a modest decrease in ALK1 protein expression, which may be a compensatory response to the reduction in ALK5 expression. That is, brain endothelial cells may respond to decreased TGF-β/ALK5 expression by downregulating the ALK1 pathway in an effort to maintain balance between ALK1 and ALK5 signaling. Similar compensatory responses between ALK1 and ALK5 signal transduction pathways have been reported in the literature (Lebrin et al, 2004; Fernandez et al, 2005). In light of our current data, we may have detected the initial phase of this compensation, an observation that warrants further investigation.
Based on the above results, we hypothesized that decreased TGF-β/ALK5 signaling may be a critical event leading to enhanced substrate permeability at the BBB. We tested this hypothesis by in situ brain perfusion using 14C-sucrose. The BBB is only slightly permeable to sucrose under normal conditions (Bhattacharjee et al, 2001) and, therefore, sucrose permeability is useful as a marker of BBB integrity in vivo. In the present study, λ-carrageenan treatment enhanced brain 14C-sucrose uptake within 3 h, suggesting that BBB permeability is altered in response to acute peripheral inflammatory pain. The function of TGF-β signaling on the regulation of BBB permeability during CIP was evaluated by incorporating SB431542, a selective ALK5 inhibitor (Laping et al, 2002), into our in situ perfusion studies. In vitro, SB431542 has been shown to reduce the nuclear accumulation of p-Smad2 and p-Smad3, which implies that this compound can effectively inhibit ALK5 signaling (Inman et al, 2002; Laping et al, 2002). The selectivity of SB431542 for ALK5 is 100-fold greater than for other endogenous kinases (Inman et al, 2002), suggesting minimal interaction with other serine/threonine kinase-based signaling systems. For our experiments, we administered 1.5 mg/kg SB431542, a dose selected based on the IC50 for the inhibition of Smad3 phosphorylation in a baculovirus expression system (i.e., 94 nmol/L) (Laping et al, 2002). Interestingly, pretreatment with SB431542 before CIP further increased 14C-sucrose brain uptake, suggesting that functional changes in BBB permeability are enhanced by ALK5 receptor inhibition under inflammatory pain conditions. Although studies in an immortalized mouse brain endothelial cell line (MBEC4) (Dohgu et al, 2004) and in primary cultures of bovine pulmonary artery endothelial cells (Birukova et al, 2005) have shown the involvement of ALK5-mediated signaling in the regulation of vascular permeability, we are the first to report this relationship at the BBB in vivo.
Previously, Dohgu et al (2004) demonstrated decreased permeability to sodium fluorescein and Evans blue albumin in MBEC4 cells treated with TGF-β1 for 12 h. These researchers concluded that decreased paracellular solute flux directly correlated with enhanced TJ integrity. However, this study did not incorporate Western blot analysis to determine which, if any, TJ proteins were altered in response to TGF-β-mediated signaling. In our study, we have examined in vivo the expression of various TJ proteins (i.e., claudin-3, claudin-5, occludin, ZO-1) in rat brain microvessels during the acute phase of CIP in the presence and absence of SB431542. Indeed, we observed increased claudin-5 and ZO-1 expression after 3 h CIP, which is consistent with our previous work (Huber et al, 2002; Brooks et al, 2005). Interestingly, we show that claudin-5 and ZO-1 expression is further increased in animals pretreated with SB431542 before CIP; however, SB431542 administration also increased claudin-3 expression under conditions of CIP and in saline controls. Previous reports have shown that increased expression of claudin-5 (Kojima et al, 2002; Coyne et al, 2003; Campos et al, 2008) and ZO-1 (Campos et al, 2008) is associated with a loss of barrier function. In addition, increased expression of claudin-3 was reported to correlate with increased BBB permeability in a rodent model of chronic inflammatory pain (Brooks et al, 2005). Our present data support the hypothesis that impaired TGF-β/ALK5 signaling increases BBB permeability through alterations in the protein constituents of TJ complexes; however, the profile of TJ protein expression during CIP and in animals administered SB431542 underscores the complexity of intracellular signaling processes that modulate BBB functional integrity. That is, different expression levels of ALK5 may alter TJ proteins to a different degree as evidenced by our claudin-3 data. The differential modulation of TJ proteins by varying levels of TGF-β/ALK5-mediated signaling may become more apparent at CIP time points that are greater than 3 h (i.e., chronic inflammatory pain), a possibility that requires further study.
Our occludin expression data are particularly intriguing. We observed decreased occludin expression in rat brain microvessels after 3 h CIP; however, occludin expression was dramatically increased at the BBB in animals administered SB431542. The increase in occludin expression was characterized by two single bands at 63 and 65 kDa, respectively. These bands, referred to as the α-band (i.e., lower molecular weight) and the β-band (i.e., higher molecular weight), correspond to occludin monomers that differ in their phosphorylation state (Antonetti et al, 1999). Phosphorylation of occludin is known to regulate TJ integrity by redistributing occludin from the cytoplasm to the plasma membrane (Farshori and Kachar, 1999; Andreeva et al, 2001). Furthermore, inhibition of TGF-β/ALK5 signaling has been reported to alter the posttranslational modification of some proteins by increasing phosphorylation (Kreisberg et al, 1996). The increase in immunoreactivity of occludin monomers in animals administered SB431542 may also be explained by a collapse of occludin oligomeric assemblies. Oligomerization of occludin is an essential feature of the cytoarchitecture of intact brain microvessels. Previous work by our laboratory has shown that disruption of disulfide-bonded occludin oligomers is associated with CIP (McCaffrey et al, 2008). It is possible that the increase in α- and β-band occludin monomers observed during pharmacological inhibition of ALK5 may reflect a breakdown of these occludin higher-order structures.
We further examined TGF-β signaling during CIP by determining the activation of endogenous Smad proteins. Smad proteins are activated by phosphorylation, which enables them to translocate to the nucleus and alter expression of target genes. Therefore, a change in the nucleus/cytoplasm ratio for phosphorylated Smad isoforms is indicative of altered TGF-β-mediated signal transduction. In our hands, the nucleus/cytoplasm ratio for both p-Smad2 and p-Smad3 was decreased in rat brain microvessels after 3 h CIP, suggesting a decrease in TGF-β/ALK5 signaling in response to CIP. In contrast, the nucleus/cytoplasm ratio for p-Smad1/5/8 was not altered during CIP or in the presence of SB431542, which indicates that TGF-β/ALK1 signal transduction did not change during acute inflammatory pain. Interestingly, we also observed a decrease in the Smad4 nucleus/cytoplasm ratio, which may be reflective of the decrease in signal transduction through the TGF-β/ALK5 pathway. The fact that we still observed Smad4 expression in the nucleus suggests that TGF-β is still capable of affecting target gene expression during CIP, likely through TGF-β/ALK1-mediated signaling. Because TGF-β/ALK1 activity is associated with increased vascular permeability, this may also account for altered BBB functional integrity during acute inflammatory pain.
Compromise of the BBB in response to pathophysiologic conditions may have severe consequences for the central nervous system. Reorganization of TJs leading to enhanced paracellular diffusion may render the brain more susceptible to adverse effects of pharmacological agents and toxic metabolites. Therefore, improvement of BBB function during pathophysiologic states may protect against central nervous system cellular damage. Because our data suggest that decreased TGF-β/ALK5-mediated signaling contributes to increased BBB permeability in vivo, we postulated that administration of exogenous TGF-β may reverse this effect. Indeed, pretreatment with TGF-β1 (12.5 ng/kg) increased the nucleus/cytoplasm ratio of both p-Smad2 and p-Smad3 and reduced brain uptake of 14C-sucrose during CIP. Based on these data, we propose that exogenous TGF-β1 can protect BBB integrity during CIP by maintaining activity of the TGF-β/ALK5 pathway. Furthermore, these observations emphasize the critical importance of TGF-β/ALK5-mediated signaling at the BBB functional integrity and may point to novel strategies to restore barrier functions during situations where brain endothelial integrity may be disrupted (i.e., inflammatory pain).
In summary, this study describes altered BBB functional integrity in an in vivo model of inflammatory pain. Our data provide evidence for the involvement of the TGF-β/ALK5 signaling pathway in the regulation of BBB permeability and TJ protein expression during an inflammatory pain response triggered by peripheral injection of λ-carrageenan (Figure 7). Our results also provide evidence that exogenous TGF-β1 administration may prevent permeability changes to sucrose during CIP. Overall, these observations suggest that complex signaling pathways are involved in the regulation of BBB functional integrity and may represent a novel therapeutic target for pharmacological manipulation of brain vascular permeability.
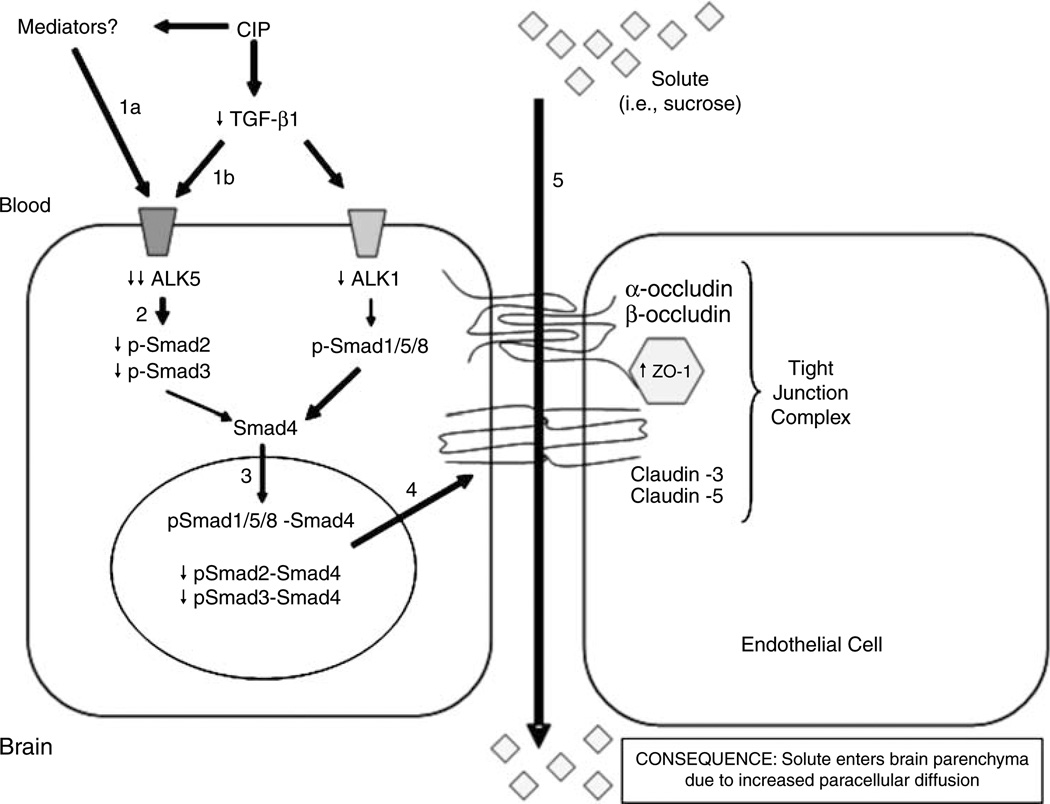
Figure 7.
Proposed mechanism of TGF-β signaling at the BBB during acute peripheral inflammatory pain. Acute CIP is characterized by an increase in production of inflammatory mediators, which leads to decreased expression of the ALK5 receptor (1a) and decreased circulating levels of TGF-β1 (1b). In turn, this leads to a decrease in signaling through the ALK5 receptor and decreased phosphorylation of Smad2 and Smad3 (2). The decreased expression of p-Smad2 and p-Smad3 results in a lower level of translocation of these Smad isoforms to the nucleus and a corresponding downregulation of TGF-β/ALK5-mediated signaling events (3). Our data also show decreased expression of the ALK1 receptor but this may be a compensatory response of the endothelial cell to maintain balance between ALK1- and ALK5-mediated signaling events. Interestingly, the expression of p-Smad1/5/8 was unchanged, suggesting that the TGF-β/ALK1 pathway may predominate at the BBB during acute CIP. Overall, this causes a dysregulation of the TJ protein complex, which is demarcated by increased expression of ZO-1 (4). Although our data also show that TGF-β/ALK5-mediated signaling can also modulate claudin-3, claudin-5, α-occludin, and β-occludin, the expression of these TJ constituents may be altered at a later time point. The result of this signaling mechanism is an increase in BBB permeability (5) that is characterized by enhanced paracellular diffusion of solutes (i.e., sucrose).
Acknowledgements
We thank Dr Robert Kuester and Dr Gwen McCaffrey for their helpful advice on this paper.
This work was supported by NIH Grants R01-NS42652 and R01-DA11271 to TPD.
Footnotes
Disclosure/conflict of interest
All the authors declare no conflict of interest.
References
- Abbott NJ. Dynamics of CNS barriers: evolution, differentiation, and modulation. Cell Mol Neurobiol. 2005;25:5–23. doi: 10.1007/s10571-004-1374-y. [DOI] [PubMed] [Google Scholar]
- Andreeva AY, Krause E, Muller EC, Blasig IE, Utepbergenov DI. Protein kinase C regulates the phosphorylation and cellular localization of occludin. J Biol Chem. 2001;276:38480–38486. doi: 10.1074/jbc.M104923200. [DOI] [PubMed] [Google Scholar]
- Antonetti DA, Barber AJ, Hollinger LA, Wolpert EB, Gardner TW. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and tumors. J Biol Chem. 1999;274:23463–23467. doi: 10.1074/jbc.274.33.23463. [DOI] [PubMed] [Google Scholar]
- Ata KA, Lennmyr F, Funa K, Olsson Y, Terent A. Expression of transforming growth factor-beta1, 2, 3 isoforms and type I and II receptors in acute focal cerebral ischemia: an immunohistochemical study in rat after transient and permanent occlusion of middle cerebral artery. Acta Neuropathol. 1999;97:447–455. doi: 10.1007/s004010051013. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee AK, Nagashima T, Kondoh T, Tamaki N. Quantification of early blood–brain barrier disruption by in situ brain perfusion technique. Brain Res Brain Res Protoc. 2001;8:126–131. doi: 10.1016/s1385-299x(01)00094-0. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Adyshev D, Gorshkov B, Birukov KG, Verin AD. ALK5 and Smad4 are involved in TGF-beta1-induced pulmonary endothelial permeability. FEBS Lett. 2005;579:4031–4037. doi: 10.1016/j.febslet.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Brooks TA, Hawkins BT, Huber JD, Egleton RD, Davis TP. Chronic inflammatory pain leads to increased blood–brain barrier permeability and tight junction protein alterations. Am J Physiol Heart Circ Physiol. 2005;289:H738–H743. doi: 10.1152/ajpheart.01288.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt AM, Jones HC, Abbott NJ. Electrical resistance across the blood–brain barrier in anaesthetized rats: a developmental study. J Physiol. 1990;429:47–62. doi: 10.1113/jphysiol.1990.sp018243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos CR, Ocheltree SM, Hom S, Egleton RD, Davis TP. Nociceptive inhibition prevents inflammatory pain induced changes in the blood–brain barrier. Brain Res. 2008;1221:6–13. doi: 10.1016/j.brainres.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornford EM, Hyman S, Swartz BE. The human brain GLUT1 glucose transporter: ultrastructural localization to the blood–brain barrier endothelia. J Cereb Blood Flow Metab. 1994;14:106–112. doi: 10.1038/jcbfm.1994.15. [DOI] [PubMed] [Google Scholar]
- Coyne CB, Gambling TM, Boucher RC, Carson JL, Johnson LG. Role of claudin interactions in airway tight junctional permeability. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1166–L1178. doi: 10.1152/ajplung.00182.2003. [DOI] [PubMed] [Google Scholar]
- De Groot CJ, Montagne L, Barten AD, Sminia P, Van DV. Expression of transforming growth factor (TGF)-beta1, -beta2, and -beta3 isoforms and TGF-beta type I and type II receptors in multiple sclerosis lesions and human adult astrocyte cultures. J Neuropathol Exp Neurol. 1999;58:174–187. doi: 10.1097/00005072-199902000-00007. [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Dickson MC, Martin JS, Cousins FM, Kulkarni AB, Karlsson S, Akhurst RJ. Defective haematopoiesis and vasculogenesis in transforming growth factor-beta 1 knock out mice. Development. 1995;121:1845–1854. doi: 10.1242/dev.121.6.1845. [DOI] [PubMed] [Google Scholar]
- Dohgu S, Yamauchi A, Takata F, Naito M, Tsuruo T, Higuchi S, Sawada Y, Kataoka Y. Transforming growth factor-beta1 upregulates the tight junction and P-glycoprotein of brain microvascular endothelial cells. Cell Mol Neurobiol. 2004;24:491–497. doi: 10.1023/b:cemn.0000022776.47302.ce. [DOI] [PubMed] [Google Scholar]
- Farshori P, Kachar B. Redistribution and phosphorylation of occludin during opening and resealing of tight junctions in cultured epithelial cells. J Membr Biol. 1999;170:147–156. doi: 10.1007/s002329900544. [DOI] [PubMed] [Google Scholar]
- Fernandez L, Sanz-Rodriguez F, Zarrabeitia R, Perez-Molino A, Hebbel RP, Nguyen J, Bernabeu C, Botella LM. Blood outgrowth endothelial cells from Hereditary Haemorrhagic Telangiectasia patients reveal abnormalities compatible with vascular lesions. Cardiovasc Res. 2005;68:235–248. doi: 10.1016/j.cardiores.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J. 2002;21:1743–1753. doi: 10.1093/emboj/21.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskins J, Gu L, Wittchen ES, Hibbard J, Stevenson BR. ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J Cell Biol. 1998;141:199–208. doi: 10.1083/jcb.141.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The blood–brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Huber JD, Campos CR, Mark KS, Davis TP. Alterations in blood–brain barrier ICAM-1 expression and brain microglial activation after lambda-carrageenan-induced inflammatory pain. Am J Physiol Heart Circ Physiol. 2006;290:H732–H740. doi: 10.1152/ajpheart.00747.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber JD, Hau VS, Borg L, Campos CR, Egleton RD, Davis TP. Blood–brain barrier tight junctions are altered during a 72-h exposure to lambda-carrageenan-induced inflammatory pain. Am J Physiol Heart Circ Physiol. 2002;283:H1531–H1537. doi: 10.1152/ajpheart.00027.2002. [DOI] [PubMed] [Google Scholar]
- Huber JD, Witt KA, Hom S, Egleton RD, Mark KS, Davis TP. Inflammatory pain alters blood–brain barrier permeability and tight junctional protein expression. Am J Physiol Heart Circ Physiol. 2001;280:H1241–H1248. doi: 10.1152/ajpheart.2001.280.3.H1241. [DOI] [PubMed] [Google Scholar]
- Inman GJ, Nicolas FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ, Hill CS. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- Ishihara H, Kubota H, Lindberg RL, Leppert D, Gloor SM, Errede M, Virgintino D, Fontana A, Yonekawa Y, Frei K. Endothelial cell barrier impairment induced by glioblastomas and transforming growth factor beta2 involves matrix metalloproteinases and tight junction proteins. J Neuropathol Exp Neurol. 2008;67:435–448. doi: 10.1097/NEN.0b013e31816fd622. [DOI] [PubMed] [Google Scholar]
- Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol. 1999;147:1351–1363. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzer RC, Raff MC. Astrocytes induce blood–brain barrier properties in endothelial cells. Nature. 1987;325:253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- Kojima S, Rahner C, Peng S, Rizzolo LJ. Claudin 5 is transiently expressed during the development of the retinal pigment epithelium. J Membr Biol. 2002;186:81–88. doi: 10.1007/s00232-001-0137-7. [DOI] [PubMed] [Google Scholar]
- Kreisberg JI, Radnik RA, Kreisberg SH. Phosphorylation of cAMP responsive element binding protein after treatment of mesangial cells with high glucose plus TGF beta or PMA. Kidney Int. 1996;50:805–810. doi: 10.1038/ki.1996.379. [DOI] [PubMed] [Google Scholar]
- Laping NJ, Grygielko E, Mathur A, Butter S, Bomberger J, Tweed C, Martin W, Fornwald J, Lehr R, Harling J, Gaster L, Callahan JF, Olson BA. Inhibition of transforming growth factor (TGF)-beta1-induced extra-cellular matrix with a novel inhibitor of the TGF-beta type I receptor kinase activity: SB-431542. Mol Pharmacol. 2002;62:58–64. doi: 10.1124/mol.62.1.58. [DOI] [PubMed] [Google Scholar]
- Larsson J, Goumans MJ, Sjostrand LJ, van Rooijen MA, Ward D, Leveen P, Xu X, ten Dijke P, Mummery CL, Karlsson S. Abnormal angiogenesis but intact hematopoietic potential in TGF-beta type I receptor-deficient mice. EMBO J. 2001;20:1663–1673. doi: 10.1093/emboj/20.7.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrin F, Deckers M, Bertolino P, ten Dijke P. TGF-beta receptor function in the endothelium. Cardiovasc Res. 2005;65:599–608. doi: 10.1016/j.cardiores.2004.10.036. [DOI] [PubMed] [Google Scholar]
- Lebrin F, Goumans MJ, Jonker L, Carvalho RL, Valdimarsdottir G, Thorikay M, Mummery C, Arthur HM, ten Dijke P. Endoglin promotes endothelial cell proliferation and TGF-beta/ALK1 signal transduction. EMBO J. 2004;23:4018–4028. doi: 10.1038/sj.emboj.7600386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey G, Seelbach MJ, Staatz WD, Nametz N, Quigley C, Campos CR, Brooks TA, Davis TP. Occludin oligomeric assembly at tight junctions of the blood–brain barrier is disrupted by peripheral inflammatory hyperalgesia. J Neurochem. 2008;106:2395–2409. doi: 10.1111/j.1471-4159.2008.05582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne-Clavel J, Oliveras JL. The ‘plantar test’ apparatus (Ugo Basile Biological Apparatus), a controlled infrared noxious radiant heat stimulus for precise withdrawal latency measurement in the rat, as a tool for humans? Somatosens Mot Res. 1996;13:215–223. doi: 10.3109/08990229609052577. [DOI] [PubMed] [Google Scholar]
- Oh SP, Seki T, Goss KA, Imamura T, Yi Y, Donahoe PK, Li L, Miyazono K, ten Dijke P, Kim S, Li E. Activin receptor-like kinase 1 modulates transforming growth factor-beta 1 signaling in the regulation of angiogenesis. Proc Natl Acad Sci USA. 2000;97:2626–2631. doi: 10.1073/pnas.97.6.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper MS. Transforming growth factor-beta: vasculogenesis, angiogenesis, and vessel wall integrity. Cytokine Growth Factor Rev. 1997;8:21–43. doi: 10.1016/s1359-6101(96)00048-2. [DOI] [PubMed] [Google Scholar]
- Seki T, Yun J, Oh SP. Arterial endothelium-specific activin receptor-like kinase 1 expression suggests its role in arterialization and vascular remodeling. Circ Res. 2003;93:682–689. doi: 10.1161/01.RES.0000095246.40391.3B. [DOI] [PubMed] [Google Scholar]
- Watabe T, Nishihara A, Mishima K, Yamashita J, Shimizu K, Miyazawa K, Nishikawa S, Miyazono K. TGF-beta receptor kinase inhibitor enhances growth and integrity of embryonic stem cell-derived endothelial cells. J Cell Biol. 2003;163:1303–1311. doi: 10.1083/jcb.200305147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Ma J, Han JD, Wang N, Chen YG. Distinct regulation of gene expression in human endothelial cells by TGF-beta and its receptors. Microvasc Res. 2006;71:12–19. doi: 10.1016/j.mvr.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Zastre JA, Chan GN, Ronaldson PT, Ramaswamy M, Couraud PO, Romero IA, Weksler B, Bendayan M, Bendayan R. Up-regulation of P-glycoprotein by HIV protease inhibitors in a human brain microvessel endothelial cell line. J Neurosci Res. 2009;87:1023–1036. doi: 10.1002/jnr.21898. [DOI] [PubMed] [Google Scholar]