Abstract
Bacteria growing in biofilms cause a wide range of human infections. Biofilm bacteria are resistant to antimicrobics at levels 500 to 5,000 times higher than those needed to kill non-biofilm bacteria. In vitro experiments have shown that electric current can enhance the activity of some antimicrobial agents against certain bacteria in biofilms; this has been termed the “bioelectric effect”. Direct electrical current has already been safely used in humans for fracture healing. Application of direct electric current with antimicrobial chemotherapy in humans could theoretically abrogate the need to remove the device in device-related infections, a procedure associated with substantial morbidity and cost. In this article, we review what has been described in the literature with regards to the bioelectric effect.
Keywords: Bioelectric effect, Biofilm
BIOFILM-RELATED INFECTIONS
The epidemic bacterial diseases that occupied human attention at the turn of the last century were generally acute in nature and caused by planktonic bacteria of highly specialized pathogenic species (e.g., Corynebacterium diphtheriae); most of these diseases are now prevented by vaccines and/or effectively controlled by currently-available antimicrobial agents (18). In their place, chronic bacterial diseases (which are poorly responsive to antimicrobics), have emerged (1). Some of the most refractory modern bacterial diseases are those associated with medical devices (e.g., joint replacements and other orthopedic instrumentation, prosthetic heart valves, pacemakers, intraventricular cardiac assist devices, automated implantable cardioverter defibrillators, urinary tract catheters and stents, peritoneal dialysis catheters, central venous catheters, neurovascular shunts, synthetic vascular grafts and stents, artificial voice prostheses, intrauterine devices) (2). When the surfaces of medical devices become the foci of device-related bacterial infections, the associated microorganisms grow in well-developed, adherent biofilms (1). It has been estimated that two thirds of human bacterial infections may involve biofilms (3).
Microorganisms growing in biofilms on medical devices are protected from killing, to a large extent, by innate host defenses and the bactericidal activity of antimicrobial agents, a type of resistance unique to biofilm-associated bacteria and distinct from conventional antimicrobial resistance (4). Bacteria in biofilms exhibit dramatically reduced (i.e., 500-5000 times) susceptibility to killing by antimicrobial agents as compared to free-floating (planktonic) cells of the same microorganism (5, 6). The resistance that bacteria exhibit when they grow in biofilms is not due to “classic” genetic mechanisms (i.e., gene mutation, genetic exchange), but is instead determined by peculiarities of biofilm growth. A variety of potential mechanisms implicated in biofilm resistance to antimicrobial agents have been proposed (2) including: restricted penetration through the biofilm matrix, antimicrobial destroying enzymes, quorum sensing signaling systems, existence of altered growth rate (persister cells) inside the biofilm, stress response to hostile environmental conditions, and overexpression of genes.
From the experience of clinical practice, it is known that device-related infections are highly refractory to antimicrobial therapy. Currently, a commonly applied therapeutic approach for implant related infections includes removal of the implanted biomaterial (1). Given the failure of conventional antimicrobics in the management of most biofilm-associated infections, novel and innovative therapeutic and preventive approaches are warranted.
Electrical current and bacteria
Bacterial cells depend on physical phenomena such as membrane potentials for their basic metabolic activity (7). It has been shown that external fields can affect the alpha-helix content and orientation of membrane proteins in eukaryotic cells, and the electrophoretic mobilities of bacterial membrane proteins (7). Moreover, electric fields and currents can influence the organization of biological membranes, metabolic and developmental processes within both prokaryotic and eukaryotic cells, and even the shape of cells, cell behavior and the dimensions of the bacterial glycocalyx (8). Directional growth in response to electric fields (galvanotropism) is well-known amongst eukaryotic cells as diverse as fibroblasts, neurons, algae, and fungal hyphae (9). This mechanism may involve differential stimulation of wall growth in both anode- and cathode-facing regions, modulating wall growth spatially (9).
The antibacterial activity of electric current has been previously demonstrated against Escherichia coli in salt solutions (10), Staphylococcus aureus in agar (11), normal flora on human skin (12), E. coli, Proteus species and Klebsiella pneumoniae in synthetic urine (13), and E. coli, S. aureus and Bacillus subtilis in water (14, 15).
The mechanism of the antibacterial activity of electric current has been variously suggested to result from toxic substances produced as a result of electrolysis (e.g., H2O2, oxidizing radicals, chlorine molecules), oxidation of enzymes and coenzymes, membrane damage leading to leakage of essential cytoplasmic constituents, and/or decreased bacterial respiratory rate (16).
According to several studies, the efficacy of biocides (17) and antibiotics (18) in killing biofilm bacteria can be radically enhanced if these agents are used within a low-intensity DC electric field; this has been termed the “bioelectric effect”. Costerton et al showed in 1994 (19) that the efficacy of certain antimicrobial agents could be increased through the application of weak electric fields. In this study, it was shown that with the combined application of direct current electric fields of about 1.5 to 20 V/cm2 (current densities of about 15 × 10−6 to 2.1 × 10−3 A/cm2) and tobramycin, the concentration of antimicrobial needed to exhibit activity against biofilm bacteria fell out 1.5 to 4.0 times compared to that needed against planktonic bacteria. Jass et al were the first to report the bioelectric effect using antimicrobials other than aminoglycosides (20). This group demonstrated that an electrical current could enhance the activity of some antimicrobials (i.e., ciprofloxacin and polymyxin B) but not of others (i.e., piperacillin) against P. aeruginosa. Wellman’s studies (21) indicate that a dose response may exist for the level of antimicrobial plus electrical field, since enhanced killing was seen at 5 mg of tobramycin per liter and 1 mA of current, but no enhanced killing was recorded at 1 mg/liter and 1 mA of current. Similar findings were presented with regards to the amount of current (21). These authors also suggested that there might be a level of current above which the bioelectric effect ceases. However, dose-response curves for both the antimicrobial agent and the current flow were not established.
Bioelectric effect mechanism of action
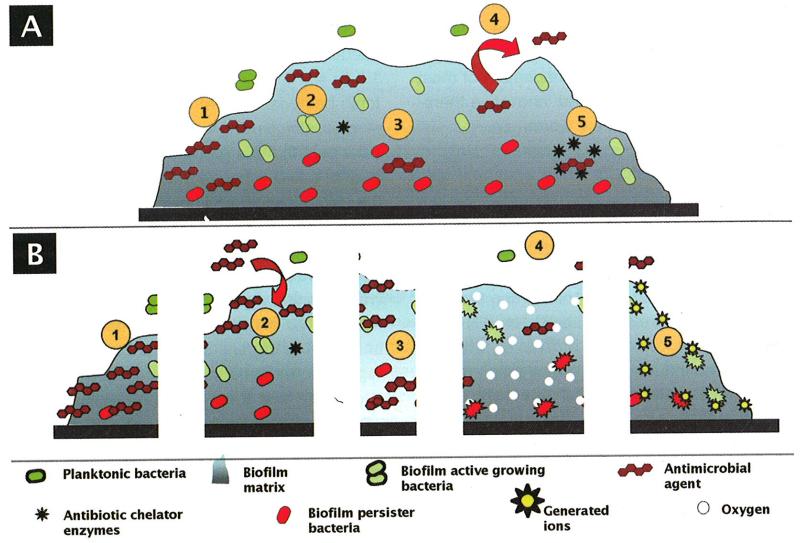
Much has been hypothesized to explain the mechanism of action of the bioelectric effect; however, a satisfactory explanation remains to be formulated. Some of the hypothetical mechanisms that have been suggested include reduction of biofilm capacity for binding the antimicrobial agent (17), increased membrane permeabilization (18), electrophoretic augmentation of antimicrobial transport (18), increased bacterial growth due to electrolytic generation of oxygen (and subsequently enhanced susceptibility to antimicrobials) (22, 23), electrochemical generation of potentiating oxidants (19), increased convective transport due to contraction and expansion of the biofilms (24), increased transport through electroosmosis, physical removal of the biofilm with electrolytically generated bubbles, and enhanced susceptibility due to a temperature increase inside the biofilm (Fig. 1).
Fig. 1.
A) Some proposed biofilm-associated resistance mechanisms: (1) antimicrobial agents may be trapped and destroyed by enzymes in the biofilm matrix; (2) antimicrobial agents may fail to penetrate beyond the surface layers of the biofilm; (3) antimicrobial agents may not be active against non-growing microorganisms; (4) expression of biofilm specific resistance genes (e.g., efflux pumps); (5) stress response to hostile environmental conditions.
B) Some proposed bioelectric effect mechanisms: (1) reduction of the biofilm capacity for binding to the antimicrobial agent; (2) electrophoretic augmentation of the antimicrobial agent transport; (3) membrane permeabilization; (4) electrolytic generation of oxygen; (5) electrochemical generation of potentiating oxidants.
a. Reduction of the biofilm capacity for binding to the antimicrobial agent
The matrix in which biofilm cells are enclosed may, in some cases, bind antimicrobial agents before they reach their target cells. Some authors have hypothesized that if an electrical current disrupts charges in the matrix, this might allow penetration of antimicrobial agents (17).
b. Membrane permeabilization and electrophoretic augmentation of antimicrobial agent transport
Electroporation-like mechanisms have been suggested as a mechanism of action of the bioelectric effect (19). Electroporation is a significant increase in the electrical conductivity and permeability of a cell plasma membrane caused by an externally applied electrical field. It is used in molecular biology as a way of introducing DNA into cells. Pores are formed when the voltage across a plasma membrane exceeds its dielectric strength. These pores formed by the electrical pulse reseal after a short period of time. In the same way, the bioelectric effect may depend largely on electrophoretic forces that allow antimicrobial agents to overcome diffusion barriers that would otherwise limit their access to their targets within bacterial cells (17, 19, 25).
c. Electrolytic generation of oxygen
The bioelectric effect could be related to electrolysis, with resultant increased delivery of oxygen to the biofilm, which might overcome biofilm biomass and cell wall barriers, as well as increase the metabolic activity and growth rate of the contained bacteria (20, 23). Reduced antimicrobial susceptibility of biofilm bacteria has been associated with localized oxygen depletion within biofilms (26, 27), and with an increased expression of the extracellular polysaccharide which mediates bacterial cell to cell adhesion (13). Production of free oxygen by electrolysis might overcome the two phenomena. However, the mechanism by which oxygen might enhance biofilm susceptibility remains to be definitively established. Stewart et al, proposed that oxygen might reach toxic levels, making the bacteria more susceptible to the antimicrobials; alternatively, increased delivery of oxygen could enhance growth of biofilm cells, overcoming reduced susceptibility associated with slow growth (23). If biofilm resistance to antimicrobials is due to oxygen deprivation (e.g., it is well know that aminoglycosides are less active under anaerobic conditions than under aerobic conditions) inside the biofilm, then an increase of the concentration of oxygen could make biofilm cells more susceptible to aminoglycosides. Stewart et al showed that when oxygen was sparged into a P. aeruginosa biofilm exposed to antimicrobial (but not electrical current), there was a significant enhancement of tobramycin efficacy (23). This enhancement was about two-thirds of that obtained when biofilm was exposed to electrical current and tobramycin.
d. Electrochemical generation of potentiating oxidants
The voltage generation of ions might be the cause of the bioelectric effect. However, Costerton et al presented experiments which indicate that this is probably not the case, on the basis of the absence of antimicrobial activity immediately downstream of an electrified chamber (19). Some studies have reported that electrical current applied alone does not result in discernible killing (17-20, 22); however, other studies have reported some effect of the electrical current alone when applied to biofilms (23, 28).
Bioelectric effect in vitro studies
A number of in vitro models have been described to test the bioelectric effect (Tab. I). Although the bioelectric effect was initially described for P. aeruginosa and biocides (isothialazone 1.5%, dimethyl ammonium chloride 50%, and glutaraldehyde 25%) (17), most subsequent studies have been done with antimicrobial agents. Khoury et al (18) used an electric modified Robbins device to demonstrate that the in vitro killing of P. aeruginosa, E. coli, and S. epidermidis biofilms by tobramycin could be enhanced by direct electric current delivered for 12 hours. Steel studs connected to a direct current electric source constituted one electrode. A platinum wire set in a groove in the bottom of the flow chamber constituted the other. The application of electric current (15-400 μA/cm2) alone showed no effect on biofilms of E. coli or S. epidermidis and a single log decrease of P. aeruginosa biofilms. When biofilm bacteria were treated with antimicrobial agents alone (0.5 to 35.0 times the minimum inhibitory concentration), no effect on biofilms was noted. When tobramycin (2.5 μg/mL) was combined with electric current, all of the S. epidermidis biofilm bacteria were killed in 8 hours (a greater than 3 log decrease compared to bacterial cells exposed to tobramycin alone). When tobramycin (8 μg/mL) was combined with electric current, all of the P. aeruginosa bacteria biofilms were killed in 12 hours (a 4-log decrease compared to bacterial cells exposed to tobramycin alone). Ciprofloxacin (1.25-5.0 μg/mL), in combination with direct electric current, also demonstrated activity against P. aeruginosa biofilms.
TABLE I.
SUMMARY OF THE BIOELECTRIC EFFECT IN VITRO STUDIES
| Method | Biofilm substrate |
Microorganism | Electrical current |
Electrode materials |
Antimicrobial agent |
Exposure time (h) |
Effect | Reference |
|---|---|---|---|---|---|---|---|---|
| Electric modified Robbins device |
Stainless steel |
Pseudomonas
aeruginosa |
2.1 mA/cm2 | Stainless steel and platinum |
Isothialazone 1.5%, Dimethyl ammonium chloride 50% and Glutaraldehyde 25% |
24 | 3-6 log reduction |
(17) |
| Electric modified Robbins device |
Stainless steel |
P. aeruginosa
P. aeruginosa Escherichia coli Staphylococcus epidermidis Candida albicans |
15-400 μA/cm2 | Stainless steel and platinum |
Tobramycin (5-100 μg/mL) Ciprofloxacin (1.25-5.0 μg/mL) Tobramycin (10-100 μg/mL) Tobramycin (2.5-100 μg/mL) Cycloheximide (100 μg/mL) |
12 | 4- 6 log reduction |
(18) |
| Two electrodes inside a Perspex flow chamber |
Stainless steel |
P. aeruginosa | 1.7 mA/cm2 | Stainless steel | Tobramycin (5 μg/mL) | 24-48 | 4-5 log reduction |
(19) |
| Two electrodes inside a Perspex flow chamber |
Dialysis membrane |
P. aeruginosa | 9 mA/cm2 | Stainless steel | Tobramycin (10 μg/mL) Ciprofloxacin (5 μg/mL) Polimixin B (20 μg/mL) Piperacillin (40 μg/mL) |
12 | 2 log reduction 0.5-1 log reduction No reduction |
(22) (20) |
| Experimental chambers |
Polycarbonate |
P. aeruginosa
and Klebsiella pneumoniae |
1 mA | Platinum | Tobramycin (5 μg/mL) | 24 | 6-8 log reduction |
(21) |
| Experimental chambers |
Polycarbonate coupons |
P. aeruginosa | 2 mA | Stainless steel | Tobramycin (5 μg/mL) | 24 | 6 log reduction |
(23) |
| Experimental chambers |
Polycarbonate coupons |
P. aeruginosa | 2 mA | Stainless steel | Tobramycin (5 μg/mL) | 24 | 5-6 log reduction |
(28) |
| Experimental chambers |
Polycarbonate |
Streptococcus
gordonii |
0.4 mA/cm2 | Stainless steel | Gentamicin (2 μg/mL) | 24 | 4-5 log reduction |
(29) |
| Microliter trays | Stainless steel |
S. epidermidis | Pulsed electromagnetic field |
No electrodes |
Gentamicin (256 times the MIC) |
12 | 1-2 log reduction |
(30) |
| Experimental chambers |
Glass | Escherichia coli | 200 mA Radio frequency current at 10 MHz |
Stainless steel No electrodes |
Gentamicin (5 μg/mL) Oxytetracycline (50 μg/mL) Gentamicin (5 μg/mL) Oxytetracycline (50 μg/mL) |
24 | 4.27 log reduction > 5.15 log reduction 3.4 log reduction 2.8 log reduction |
(31) |
| Experimental chambers |
Teflon | Methicillin resistant Staphylococcus aureus |
20, 200, 2000 mA |
Graphite | Erythromycin (2 μg/mL) Daptomycin (2 μg/mL) Moxifloxacin (4 μg/mL) Linezolid (16 μg/mL) Minocycline (4 μg/mL) Rifampin (4-32 μg/mL) |
24 | 1-2 log reduction 0-1 log reduction |
(32) |
| 2000 mA | Graphite Stainless steel |
24 | 2.1-2.4 log reduction 3.5-4.4 log reduction |
(33) |
Costerton et al (19) grew P. aeruginosa biofilms on stainless steel electrodes in a Perspex flow chamber for 24 hours. It was demonstrated that the combination of tobramycin (5 μg/mL) and direct current (1.7 mA/cm2) administered for 48 hours increased the in vitro killing of the bacteria 4-5 log orders (compared with tobramycin alone or electric current alone). Importantly, these investigators showed that the in vitro bioelectric effect applied to all areas of the active electrodes and to the surfaces of conductive elements lying within the electric field but not themselves functioning as electrodes.
Jass et al (22) used an electrical colonization cell to study the effect of tobramycin on P. aeruginosa biofilms suspended on one side of a dialysis membrane between two electrodes, thereby avoiding electrochemical and mechanical disturbances, yet remaining in the path of the electric current. Electric currents of up to 20 mA/cm2 delivered for 12 hours did not prevent biofilm formation or have any detrimental effect on an established biofilm. Tobramycin (10 μg/mL) alone did not affect the biofilm, but its antimicrobial action was enhanced nearly 2 log orders by 9 mA/cm2 electric current. In a follow-up manuscript (20), they used the same model to study the effect of ciprofloxacin (5 μg/mL) in the presence of 0 or 9 mA/cm2 current density on P. aeruginosa biofilms. Ciprofloxacin alone reduced the biofilm population; in the concomitant presence of the electrical current, the population was further reduced.
Wellman et al (21) grew mixed-culture biofilms of P. aeruginosa and K. pneumoniae for 7 days on polycarbonate coupons. Experimental chambers were built from FisherBrand five-slide, 50 gauge polypropylene slide transporter boxes modified to allow a pathway for nutrient support medium flow, and for placement of 22 gauge platinum wire electrodes at either end of the chamber. Delivery of the combination of direct electric current (1 mA) and tobramycin (5 μg/mL) for 24 hours resulted in an increase in the in vitro killing of the bacteria of 6 to 8 log orders (compared with tobramycin alone). Little killing was observed with tobramycin alone; a 1 log reduction in viable cell numbers with current alone was noted.
Stewart et al (23) placed P. aeruginosa biofilms grown for 3 days on polycarbonate slides in rectangular treatment chambers and delivered direct electric current (2 mA) through the chamber by means of a circuit containing a current controller and two stainless-steel wires at opposite ends of the long axis of the treatment chamber. When treated with tobramycin (5 μg/mL) for 24 hours, P. aeruginosa biofilms exhibited a 3 log reduction in viable cell numbers whereas a 5 log reduction was measured in a planktonic culture. When direct current was applied with tobramycin, biofilm killing increased by a further 3 log orders.
Wattanakaron et al (29) demonstrated electrical enhancement of Streptococcus gordonii biofilm killing by gentamicin in an in vitro model. The experimental methods were as described by Stewart et al (23), except that the biofilms were grown for 6 days. In this model, electric current (0.4 mA/cm2) flowed approximately parallel to the substratum to which the biofilm was attached. When treated with gentamicin (2 μg/mL) for 24 hours, S. gordonii biofilms exhibited a 1 log reduction in viable cell numbers whereas a 5 log reduction was measured in a planktonic culture. When direct current was applied during gentamicin treatment, biofilm killing increased by a further 4 log orders. Electrical current alone caused a 2 log reduction in viable cell numbers.
Pickering et al (30) investigated the in vitro effect of a pulsed electromagnetic field on the activity of gentamicin or vancomycin in the treatment of five-day-old S. epidermidis biofilms grown on the tips of stainless-steel pegs. The biofilms were exposed to varying concentrations of antimicrobic in microtiter trays at 37°C and 5% CO2 for 12 hours with or without a pulsed electromagnetic field. Exposure to a pulsed electromagnetic field increased the activity of gentamicin against the five-day biofilms of S. epidermidis. In three of five experiments there was reduction of at least 50% in the minimum biofilm inhibitory concentration. In a fourth experiment there was a 1 to 2 log reduction in colony count on exposure to 256 times the MIC of gentamicin and pulsed electromagnetic field. Analysis of variance confirmed an effect by a pulsed electromagnetic field on the activity of gentamicin (p<0.05). Importantly, however, no significant bioelectric effect was observed with vancomycin in this model.
Caubet et al (31) demonstrated electrical enhancement of E. coli biofilm killing by gentamicin or oxytetracycline in an in vitro model. They used E. coli grown for 24 hours on glass slides in rectangular treatment chambers and delivered direct electric current (200 mA) through the chamber by means of a standard constant current generator and two stainless-steel electrodes at opposite ends of the long axis of the treatment chamber. When treated with gentamicin (5 μg/mL) or oxytetracycline (50 μg/mL) for 24 hours, E. coli biofilms exhibited a 2.11 and 1.90 log reduction in viable cell numbers. When direct current was applied during gentamicin or oxytetracycline treatment, biofilms exhibited a 4.27 and >5.15 log reduction in viable cell numbers. Electrical current alone caused a 0.91 log reduction in viable cell numbers.
Our group demonstrated electrical current-mediated enhancement of the in vitro bactericidal activity of erythromycin (2 μg/mL), daptomycin (2 μg/mL) or moxifloxacin (4 μg/mL) against methicillin resistant Staphylococcus aureus (MRSA) biofilms (32). However, the activity of linezolid (16 μg/mL) or minocycline (4 μg/mL) against MRSA biofilms was not enhanced by electrical current. We designed a model that permitted us to study the interaction between the biofilm itself, the electric field, and the antimicrobial agent. An eight-channel current generator/controller and eight chambers delivering a continuous flow of fresh media with or without antimicrobial agents and/or electrical current (20, 200 or 2,000 mA) via graphite or stainless steel electrodes to biofilm-coated Teflon coupons was used. This technology was used to extensively assess whether the in vitro enhancement of killing of biofilm-associated P. aeruginosa and S. epidermidis by electric current plus aminoglycoside, quinolone, or tetracycline antimicrobics was generalizable to antimicrobial agents representing a variety of other antimicrobial classes, and to MRSA. Results of our experiments indicate that the enhanced activity of antimicrobial agents by electrical current against biofilm organisms may not be a generalizable phenomenon across microorganisms and antimicrobial agents (unpublished data).
Electrode composition may have an impact on bioelectric effect. Stainless steel electrodes have been most commonly studied (29, 31), but carbon, platinum and gold electrodes have also been used (13, 18, 32, 33). Using the previously described in vitro model, we demonstrated that electrode composition plays a role in the observed in vitro bioelectric effect. We studied the in vitro enhancement of bactericidal activity of rifampin by electrical current against MRSA biofilms using two different electrode materials. Rifampin combined with electrical current (2000 μA) delivered by stainless steel electrodes demonstrated a 3.5-4.4 log reduction of MRSA biofilms. However, a lesser effect (2.1-2.4 log reduction) was observed when electrical current was delivered by graphite electrodes (33).
Effect of electrical current alone on bacterial biofilms
Electrical current alone has been shown to have a bactericidal effect when applied as a 10 μA DC current for 16 hours to bacteria or human skin or on agar plates (11, 12). Davis et al (13, 34-36) reported that, in medium containing chloride ions, planktonic cells of E. coli, P. aeruginasa, Proteus mirabilis and Candida albicans were killed by electric fields and current densities similar to those used by Costerton et al in his first report about the bioelectrical effect (15 μA/cm2 to 2.1 μA /cm2) (19). Davis et al attributed the killing of these planktonic cells to iontophoresis, in which the accretion of metal ions on or in the bacterial cell is responsible for the effect (34, 35).
The development of biofilm-related infections begins with the adhesion of the microorganism to the biomaterial surface, mediated by the Van der Waals forces, acid base interactions and electrostatic forces. The electrostatic force between bacteria and the biomaterial is generally repulsive since almost all biomaterial surfaces are negatively charged, as are bacterial cells (37). It has been proposed that repulsive forces can be enhanced by the application of electric current, provoking surface detachment of bacterial biofilms (38, 39).
Poortinga et al (38) have demonstrated that it is possible to stimulate Streptococcus oralis detachment from conducting indium tin oxide by applying electrical currents of 10 μA/cm2. Almost total cleaning of anodic and cathodic surfaces could be achieved, even in the presence of an adsorbed conditioning film. In their study, an ionic strength-dependent transfer of electrons during an initial bacterial adhesion mechanism, that had to be reversed in order for detachment to occur, was proposed. Van der Borden et al (40) demonstrated that a variety of initially adherent Staphylococcus strains, isolated from biomaterial-related infections, could be stimulated to detach from surgical stainless steel by the application of low electrical DC currents (25-125 μA). This current-induced detachment of initially adhering bacteria from stainless steel surfaces not only involved detachment, but also the prevention of re-deposition of detached bacteria (40). It was shown that under high -flow conditions, detached bacteria were more readily transported away from the surface than under low-flow conditions, making re-deposition unlikely (40). In a follow-up manuscript (41), the effect of DC electrical currents (60 and 100 microamps) and block currents (60 and 100 μA with a 50% duty cycle, 1 Hz) against biofilms in the late stages of formation was studied. The block currents yielded higher detachment percentages than DCs due to the electro-osmotic fluid flow. Bacteria remaining on the surface after current application were less viable than they were prior to the current application, as demonstrated by confocal laser scanning microscopy (41).
Van der Borden et al designed an experimental infection model in which three percutaneous stainless steel pins were implanted in the tibia of goats and colonized with S. epidermidis (42). One pin was subjected to electric current while the other pin was used as a control (the third implanted pin was used for frame support). Infection developed after 21 days in 89% of the control pin sites, whereas only 11 % of the pin sites in the current group showed infection.
Potential applications of the bioelectric effect in the human setting
As we have shown, electrical currents may be potentially applied in the human setting either alone (the “electricidal effect”) or combined with antimicrobial agents (the bioelectric effect). Both approaches need more in vitro studies as well as studies in experimental models before they can be translated to clinical practice.
DC currents have been used clinically to drive chemotherapeutic molecules into solid tumors (43), and antibiotic molecules into the inner ear and other tissues (44). The obvious human application of the bioelectric effect could be in the management of infections associated with orthopedic hardware. In addition to systemic delivery of antimicrobics, local delivery of antimicrobics (e.g., in polymethylmethacrylate) also deserves further study as concerns the bioelectric effect. Furthermore, implantable devices could be accessed to produce effective electric fields to enhance the perioperative use of antimicrobials to kill developing bacterial biofilms, thereby preventing device-related infections.
Issues concerning electric current mediated tissue toxicity, delivery systems for electric current, and electrode geometry would need to be addressed before it could be applied in a human setting. Ideally, if the bioelectric effect is applied to human infections, the electric current should be delivered in a non-invasive (e.g., transcutaneous) or minimally invasive (e.g., subcutaneous) fashion. Attaching wires directly to the surface of foreign bodies is not ideal since the wires themselves may be a conduit for microorganisms. According to some authors, the bioelectric effect requires a current flow, not just an electrical field. Stewart et al reported that when electrodes were placed outside the treatment chamber to create essentially the same electric field, but with zero current, the electrical enhancement of killing was completely eliminated (23). However, Pickering et al (30) investigated the in vitro effect of a pulsed electromagnetic field on the efficacy of tobramycin and vancomycin against S. epidermidis biofilms on the tips of stainless-steel pegs. As it is described in their study, exposure to a pulsed electromagnetic field increased the activity of gentamicin but not vancomycin against S. epidermidis biofilms.
CONCLUSIONS
The pathogenesis of a wide variety of human infections, including device-related infections, as well as infections not associated with devices, is now recognized to relate to the presence of bacteria in biofilms. It has been estimated that two thirds of human bacterial infections involve biofilms. Existence within a biofilm represents a basic survival strategy for bacteria, within which they are protected from environmental influences and exhibit resistance to therapeutic levels of antimicrobial agents. Bacteria in biofilms exhibit dramatically reduced (i.e., by several log orders) susceptibility to killing by antimicrobics as compared to planktonic bacteria. The threat of such devastating bacterial infections is a serious problem that limits the current and future development of medical devices. In vitro experiments have demonstrated that direct electric current substantially enhances the activity of certain antimicrobial agents (i.e., aminoglycosides, quinolones, tetracycline, erythromycin, daptomycin, moxifloxacin or polymyxin B), against some biofilm-associated bacteria (P. aeruginosa, E. coli, K. pneumoniae, S. epidermidis, MRSA, S. gordonil), rendering biofilm bacteria susceptible to antimicrobial levels active against non-biofilm (planktonic) bacteria. The significance of the bioelectric effect is that it affords a potential means to overcome the reduced susceptibility of biofilm microorganisms. Although the mechanism of the bioelectric effect remains unclear, it is interesting because it may facilitate the design of technological applications to improve treatment of biofilm-related infections. Whatever the mechanism of the bioelectric effect may be, it is clear that electric currents may enhance the efficacy of certain antimicrobial agents in killing biofilm bacteria. Furthermore, it has been shown how the application of direct electric current alone can provoke the surface detachment of bacterial biofilms. Some questions about the bioelectric and electricidal effects need to be addressed. It is not clear which electric parameters are more important (e.g., electric field strength, current density, time of application). Moreover, the optimal antimicrobial concentration to achieve the maximum effect remains to be defined.
ACKNOWLEDGEMENTS
This work was supported by the National Institute of Health (R21AI061407).
Footnotes
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
REFERENCES
- 1.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–93. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.del Pozo JL, Patel R. The challenge of treating biofilm-associated bacterial infections. Clin Pharmacol Ther. 2007;82:204–9. doi: 10.1038/sj.clpt.6100247. [DOI] [PubMed] [Google Scholar]
- 3.Lewis K. Riddle of biofilm resistance. Antimicrob Agents Chemother. 2001;45:999–1007. doi: 10.1128/AAC.45.4.999-1007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderl JN, Franklin MJ, Stewart PS. Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob Agents Chemother. 2000;44:1818–24. doi: 10.1128/aac.44.7.1818-1824.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anwar H, Oasgupta MK, Costerton Jw. Testing the susceptibility of bacteria in biofilms to antibacterial agents. Antimicrob Agents Chemother. 1990;34:2043–6. doi: 10.1128/aac.34.11.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costerton JW, Cheng KJ, Geesey GG, Ladd TI, Nickel JC, Oasgupta M, et al. Bacterial biofilms in nature and disease. Annu Rev Microbiol. 1987;41:435–64. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- 7.Cevc G. Membrane electrostatics. Biochim Biophys Acta. 1990;1031:311–82. doi: 10.1016/0304-4157(90)90015-5. [DOI] [PubMed] [Google Scholar]
- 8.Sabelnikov AG, Cymbalyuk ES, Gongadze G, Borovyagin VL. Escherichia coli membranes during electrotransformation: an electron microscopy study. Biochim Biophys Acta. 1991;1066:21–8. doi: 10.1016/0005-2736(91)90245-4. [DOI] [PubMed] [Google Scholar]
- 9.Rajnicek AM. Bacterial galvanotropism: mechanisms and applications. Sci Prog. 1993;77(pt 1-2):139–51. [PubMed] [Google Scholar]
- 10.Pareilleux A, Sicard N. Lethal effects of electric current on Escherichia coli. Appl Microbiol. 1970;19:421–4. doi: 10.1128/am.19.3.421-424.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barranco SD, Spadaro JA, Berger TJ, Becker RO. In vitro effect of weak direct current on Staphylococcus aureus. Clin Orthop Relat Res. 1974;100:250–5. [PubMed] [Google Scholar]
- 12.Bolton L, Foleno B, Means B, Petrucelli S. Direct-current bactericidal effect on intact skin. Antimicrob Agents Chemother. 1980;18:137–41. doi: 10.1128/aac.18.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis CP, Wagle N, Anderson MD, Warren MM. Bacterial and fungal killing by iontophoresis with long-lived electrodes. Antimicrob Agents Chemother. 1991;35:2131–4. doi: 10.1128/aac.35.10.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsunaga T, Nakasono S, Masuda S. Electrochemical sterilization of bacteria absorbed on granular activated carbon. FEMS Microbiol Lett. 1992;72:255–9. doi: 10.1016/0378-1097(92)90471-y. [DOI] [PubMed] [Google Scholar]
- 15.Matsunaga T, Nakasono S, Takamuku T, Burgess JG, Nakamura N, Sode K. Disinfection of drinking water by using a novel electrochemical reactor employing carbon-cloth electrodes. Appl Environ Microbiol. 1992;58:686–9. doi: 10.1128/aem.58.2.686-689.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis CP, Shirtliff ME, Trieff NM, Hoskins SL, Warren MM. Quantification, qualification, and microbial killing efficiencies of antimicrobial chlorine-based substances produced by iontophoresis. Antimicrob Agents Chemother. 1994;38:2768–74. doi: 10.1128/aac.38.12.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blenkinsopp SA, Khoury AE, Costerton Jw. Electrical enhancement of biocide efficacy against Pseudomonas aeruginosa biofilms. Appl Environ Microbiol. 1992;58:3770–3. doi: 10.1128/aem.58.11.3770-3773.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khoury AE, Lam K, Ellis B, Costerton Jw. Prevention and control of bacterial infections associated with medical devices. ASAIO J. 1992;38:M174–8. doi: 10.1097/00002480-199207000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Costerton JW, Ellis B, Lam K, Johnson F, Khoury AE. Mechanism of electrical enhancement of efficacy of antibiotics in killing biofilm bacteria. Antimicrob Agents Chemother. 1994;38:2803–9. doi: 10.1128/aac.38.12.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jass J, Lappin-Scott HM. The efficacy of antibiotics enhanced by electrical currents against Pseudomonas aeruginosa biofilms. J Antimicrob Chemother. 1996;38:987–1000. doi: 10.1093/jac/38.6.987. [DOI] [PubMed] [Google Scholar]
- 21.Wellman N, Fortun SM, McLeod BR. Bacterial biofilms and the bioelectric effect. Antimicrob Agents Chemother. 1996;40:2012–4. doi: 10.1128/aac.40.9.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jass J, Costerton JW, Lappin-Scott HM. The effect of electrical currents and tobramycin on Pseudomonas aeruginosa biofilms. J Ind Microbiol. 1995;15:234–42. doi: 10.1007/BF01569830. [DOI] [PubMed] [Google Scholar]
- 23.Stewart PS, Wattanakaroon W, Goodrum L, Fortun SM, McLeod BR. Electrolytic generation of oxygen partially explains electrical enhancement of tobramycin efficacy against Pseudomonas aeruginosa biofilm. Antimicrob Agents Chemother. 1999;43:292–6. doi: 10.1128/aac.43.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoodley P, deBeer D, Lappin-Scott HM. Influence of electric fields and pH on biofilm structure as related to the bioelectric effect. Antimicrob Agents Chemother. 1997;41:1876–9. doi: 10.1128/aac.41.9.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellwood DC, Keevil CW, Marsh PD, Brown CM, Wardell JN. Surface-associated growth. Philos Trans R Soc Lond A. 1982;297:517–32. doi: 10.1098/rstb.1982.0058. [DOI] [PubMed] [Google Scholar]
- 26.Anderl JN, Zahller J, Roe F, Stewart PS. Role of nutrient limitation and stationary-phase existence in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob Agents Chemother. 2003;47:1251–6. doi: 10.1128/AAC.47.4.1251-1256.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borriello G, Werner E, Roe F, Kim AM, Ehrlich GD, Stewart PS. Oxygen limitation contributes to antibiotic tolerance of Pseudomonas aeruginosa in biofilms. Antimicrob Agents Chemother. 2004;48:2659–64. doi: 10.1128/AAC.48.7.2659-2664.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLeod BR, Fortun S, Costerton JW, Stewart PS. Enhanced bacterial biofilm control using electromagnetic fields in combination with antibiotics. Methods Enzymol. 1999;310:656–70. doi: 10.1016/s0076-6879(99)10051-x. [DOI] [PubMed] [Google Scholar]
- 29.Wattanakaroon W, Stewart PS. Electrical enhancement of Streptococcus gordonii biofilm killing by gentamicin. Arch Oral Bioi. 2000;45:167–71. doi: 10.1016/s0003-9969(99)00132-6. [DOI] [PubMed] [Google Scholar]
- 30.Pickering SA, Bayston R, Scammell BE. Electromagnetic augmentation of antibiotic efficacy in infection of orthopaedic implants. J Bone Joint Surg. 2003;85:588–93. doi: 10.1302/0301-620x.85b4.12644. [DOI] [PubMed] [Google Scholar]
- 31.Caubet R, Pedarros-Caubet F, Chu M, Freye E, de Belem Rodrigues M, Moreau JM, et al. A radio frequency electric current enhances antibiotic efficacy against bacterial biofilms. Antimicrob Agents Chemother. 2004;48:4662–4. doi: 10.1128/AAC.48.12.4662-4664.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.del Pozo JL, Rouse MS, Nguyen G, Steckelberg JM, Patel R. The effect of electrical current on antimicrobial activity against methicillin resistant Staphylococcus aureus (MRSA) biofilms; 47th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); Chicago IL, USA. September 2007. [Google Scholar]
- 33.del Pozo JL, Rouse MS, Fernandez-Sampedro M, Steckelberg JM, Patel R. Electrode composition and electrical enhancement of rifampin activity against methicillin resistant Staphylococcus aureus (MRSA) biofilms; 47th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); Chicago IL, USA. September 2007. [Google Scholar]
- 34.Davis CP, Anderson MD, Hoskins S, Warren MM. Electrode and bacterial survival with iontophoresis in syntl1etic urine. J Urol. 1992;147:1310–3. doi: 10.1016/s0022-5347(17)37551-1. [DOI] [PubMed] [Google Scholar]
- 35.Davis CP, Wagle N, Anderson MD, Warren MM. Iontophoresis generates an antimicrobial effect that remains after iontophoresis ceases. Antimicrob Agents Chemother. 1992;36:2552–5. doi: 10.1128/aac.36.11.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis CP, Weinberg S, Anderson MD, Rao GM, Warren MM. Effects of microamperage, medium, and bacterial concentration on iontophoretic killing of bacteria in fluid. Antimicrob Agents Chemother. 1989;33:442–7. doi: 10.1128/aac.33.4.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jucker BA, Harms H, Zehnder AJ. Adhesion of the positively charged bacterium Stenotrophomonas (Xanthomonas) maltophilia 70401 to glass and Teflon. J Bacteriol. 1996;178:5472–9. doi: 10.1128/jb.178.18.5472-5479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poortinga AT, Bos R, Busscher HJ. Controlled electrophoretic deposition of bacteria to surfaces for the design of biofilms. Biotechnol Bioeng. 2000;67:117–20. doi: 10.1002/(sici)1097-0290(20000105)67:1<117::aid-bit14>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 39.Ueshima M, Tanaka S, Nakamura S, Yamashita K. Manipulation of bacterial adhesion and proliferation by surface charges of electrically polarized hydroxyapatite. J Biomed Mater Res. 2002;60:578–84. doi: 10.1002/jbm.10113. [DOI] [PubMed] [Google Scholar]
- 40.van der Borden AJ, van der Mei HC, Busscher HJ. Electric-current-induced detachment of Staphylococcus epidermidis strains from surgical stainless steel. J Biomed Mater Res. 2004;68:160–4. doi: 10.1002/jbm.b.20015. [DOI] [PubMed] [Google Scholar]
- 41.van der Borden AJ, van der Werf H, van der Mei HC, Busscher HJ. Electric current-induced detachment of Staphylococcus epidermidis biofilms from surgical stainless steel. Appl Environ Microbiol. 2004;70:6871–4. doi: 10.1128/AEM.70.11.6871-6874.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van der Borden AJ, Maathuis PG, Engels E, Rakhorst G, van der Mei HC, Busscher HJ, et al. Prevention of pin tract infection in external stainless steel fixator frames using electric current in a goat model. Biomaterials. 2007;28:2122–6. doi: 10.1016/j.biomaterials.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Sersa G, Miklavcic D. Inhibition of SA-1 tumor growth in mice by human leukocyte interferon alpha combined with low-level direct current. Mol Biother. 1990;2:165–8. [PubMed] [Google Scholar]
- 44.Belehradek J, Jr, Orlowski S, Ramirez LH, Pron G, Poddevin B, Mir LM. Electropermeabilization of cells in tissues assessed by the qualitative and quantitative electroloading of bleomycin. Biochim Biophys Acta. 1994;1190:155–63. doi: 10.1016/0005-2736(94)90045-0. [DOI] [PubMed] [Google Scholar]