Abstract
The purpose of this study was to analyze the effects of lubricin on tendon stiffness and viscoelasticity.
A total of 36 mice were tested with 12 mice in each of the following groups: lubricin knock-out (−/−), heterozygous (+/−) and wild-type (+/+). A ramp test was used to determine the elastic modulus by pulling the fascicles to 2.5% strain amplitude at a rate of 0.05 mm/s. Then, followed by a relaxation test that pulled the fascicles to 5% strain amplitude at a rate of 2 mm/s. The fascicles were allowed to relax for 2 min at the maximum strain and a single-cycle relaxation ratio was used to characterize viscoelastic properties.
There was no significant difference in the Young’s modulus between the three groups (p > 0.05), but the knockout mice had a significantly (p < 0.05) lower relaxation ratio than the wild type mice.
Based on these data, we concluded that lubricin expression has an effect on the viscoelastic properties of tendon fascicles. The clinical significance of this finding, if any, remains to be demonstrated.
Keywords: Lubricin, Tendon, Fascicle, Viscoelasticity, Mechanical
1. Introduction
Lubricin, also known as proteoglycan 4 or superficial zone protein, has many biological functions. These include cytoprotection, lubrication and anti-adhesion (Rhee et al., 2005; Sun et al., 2006). Lubricin improves tendon gliding (Taguchi et al., 2008), and is present between tendon fascicles as well (Sun et al., 2006). Recent evidence (Kohrs et al., 2011) suggests a role for lubricin in interfascicular gliding, but the effect of lubricin on tendon stiffness and viscoelasticity is unknown.
The function of the tendon is to efficiently transfer forces from the muscle to the skeleton, minimizing energy loss during the load transfer as well as allowing enough extension to avoid injury (Gupta et al., 2010; Ker, 2007; Screen, 2008; Yin and Elliott, 2004). Tendons consist of bundles of collagen fibers, or fascicles, which play an important role in their tensile properties (Bensamoun et al., 2006). Collagen makes up 70–80% of a tendon or ligament’s dry weight (Yamamoto et al., 1999). Tendons also consist of proteoglycans, which play a dominant role in the viscoelastic behavior and strain transfer within the tendon (Yin and Elliott, 2004). Proteoglycans are the major crosslinking elements between collagens. Loss of proteoglycans in tendon leads to altered assembly of collagen fibrils and changes the tendon mechanical properties (Danielson et al., 1997; Kuc and Scott, 1997; Pins et al., 1997; Robinson et al., 2005). It is also clear that age affects the material properties of tendons and tendon fascicles (Bensamoun et al., 2006).
The purpose of this study was to analyze the effects of lubricin on tendon mechanical properties by comparing tail fascicles from wild, heterozygous and lubricin knockout (Kohrs et al., 2011) mice. The hypothesis was that KO mice fascicles would have a higher stiffness and a lower relaxation ratio.
2. Materials and methods
2.1. Fascicle isolation and preparation
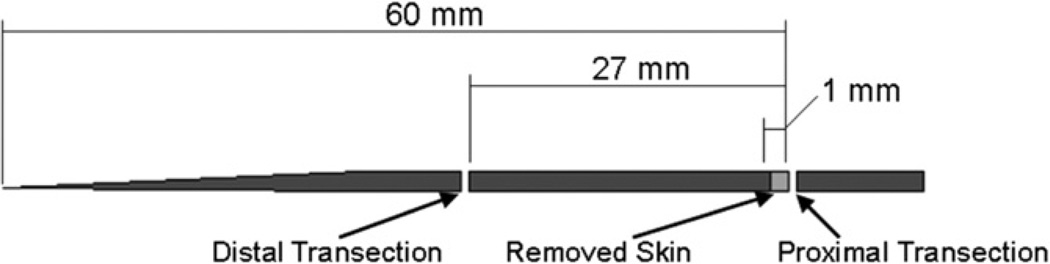
Animals were obtained after use in other studies approved by the Institutional Animal Care and Use Committee (IACUC). A total of 36 mice were used with 12 mice in each of the following groups: Lubricin knock-out (−/−), heterozygous (+/−) and wild-type (+/+). After sacrifice, the mouse tails were resected and stored frozen at −80 °C. This process has been shown not to affect tendon mechanical properties (Graf et al., 1992; Lee et al., 2009). The tails were then thawed, dissected and tested at room temperature. The age range for all mice was between 10 and 13 weeks. The gender was random; the specific male–female distribution was 7–5, 4–8 and 6–6 for the wild type, heterozygous and lubricin knock-out groups, respectively. The mouse tails were transected 60 mm from the distal end of the tail. The skin was removed 1 mm from the proximal end to provide space to find an appropriate fascicle. The tail was transected again at a level 33 mm from the distal end, leaving a 27 mm tail piece for fascicle isolation (Fig. 1). Fascicles were carefully removed from the proximal end of this section using forceps. The only fascicles used in these tests were those that slid out smoothly with minimal resistance. We considered the experimental unit to be the animal, not the individual fascicle, and we also considered that there might be some variability in the diameter of individual fascicles within the same animal. Four fascicles – two fascicles from each of the left and right dorsal tendons (Fig. 2) – were used for mechanical evaluation, and the data was averaged to yield a single data point (Bensamoun et al., 2006; Kohrs et al., 2011). During dissection and testing, the fascicles were kept moist with saline.
Fig. 1.
A mouse tail diagram showing the transection cuts.
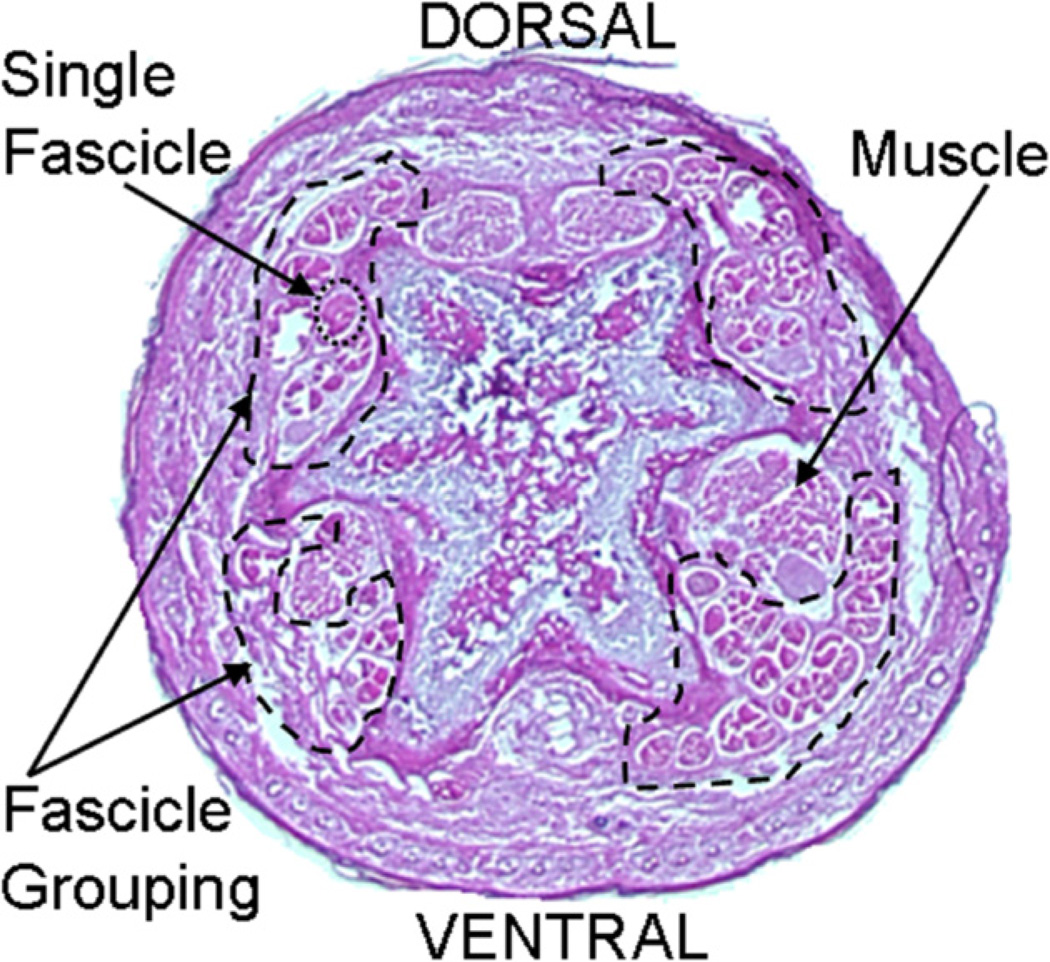
Fig. 2.
A stained cross-section of a mouse tail showing the four tendons and the fascicle grouping within the tendons.
2.2. Fascicle cross-sectional area
Once removed, each fascicle was secured with Loctite 401 cyanoacrylate adhesive to thin sheets of Nitrile rubber on both ends, exposing 15 mm of fascicle between sheets (Fig. 3). Care was taken to ensure there was no adhesive on the exposed 15 mm section. Each fascicle was mounted onto a custom fixture which allowed it to be rotated 90° about the long axis while being submerged in saline. Eight scaled measurements of the diameter were taken under 200× magnification at 0° and 90° and rotated around the long axis for a total of 16 measurements. The cross-sectional area was calculated using the average diameter and assuming a round cross-section. Before being mounted on the testing apparatus, the initial length (L0) corresponding to 0% strain (under a 2 g preload) was measured with a digital caliper (Interapid, Brown & Sharpe, North Kingstown, RI). Strain for each specimen was calculated based on this measured initial length.
Fig. 3.
A photograph showing the process of attaching a fascicle to the medium material (Nitrile rubber) with glue (Loctite 401 cyanoacrylate adhesive). The fascicle is kept moist in saline throughout the process.
2.3. Mechanical testing
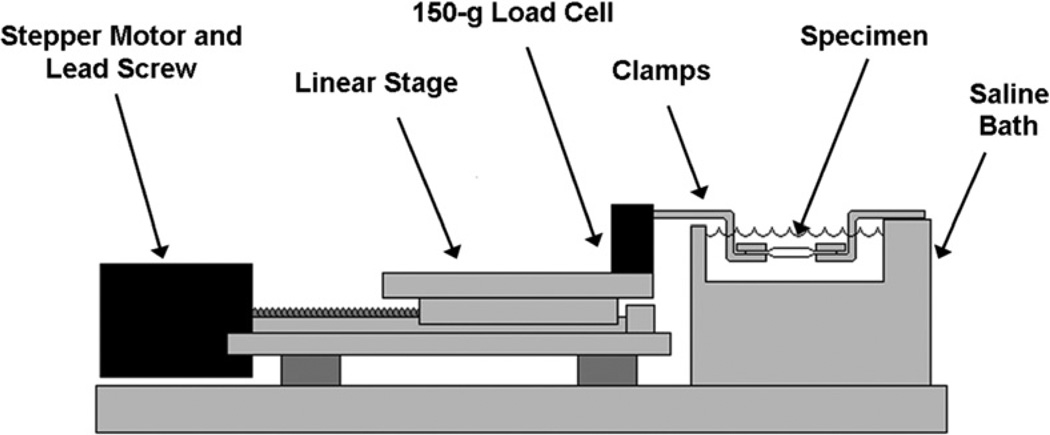
The fascicles were mounted onto a custom-designed mechanical test system (Fig. 4), which includes two clamps, a 150-g transducer (Transducer Techniques, Temecula, CA), and a stepper-motor-driven linear actuator (Servo Systems, Montville, NJ). Data was collected at a sampling rate of 100 Hz. Fascicles were kept submerged in a saline bath at room temperature throughout testing.
Fig. 4.
The mechanical testing set-up consisting of two clamps submerged in a saline bath, a 150-g load cell, and a stepper-motor-driven actuator.
2.4. Ramp test
There was no cyclic preconditioning of the fascicles. Each fascicle was subjected to a preload of approximately 2 g at the start of the ramp test. The fascicle was then pulled to 2.5% strain amplitude at a rate of 0.05 mm/s and returned to the initial position at the same speed. The fascicle was then maintained at L0 for 60 s prior to the relaxation test. The elastic modulus was calculated from the slope of the linear region of the stress–strain curve.
2.5. Relaxation test
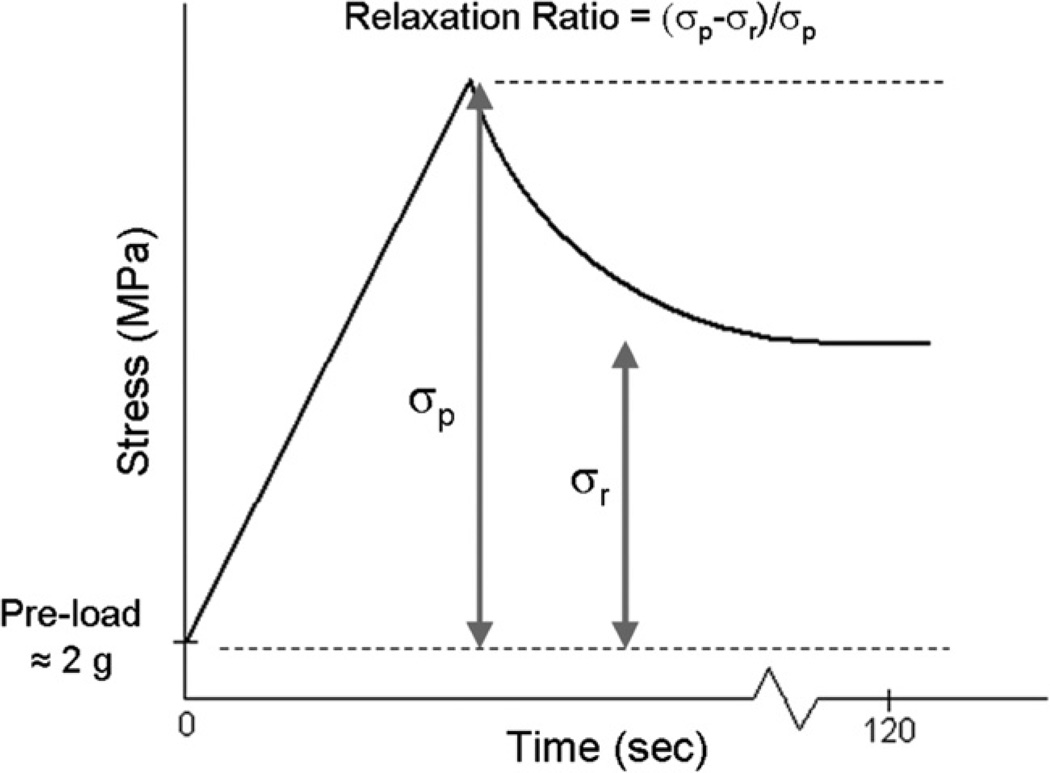
Following the ramp test, each fascicle was once again preloaded to approximately 2 g and then pulled to 5% strain amplitude at a speed of 2 mm/s. The fascicles were allowed to relax at this maximum strain amplitude for 2 min. Based on pilot data, 2 min was determined to be a suitable relaxation period to achieve a static stress. The elastic modulus was again calculated from the slope of the linear region of the stress–strain curve. The viscoelastic properties of the fascicles were characterized by defining a stress relaxation ratio (Eq. (1)), a ratio of the stress at 5% (maximum) strain, σp, to the static stress after 2 min of relaxation, σr (Fig. 5). The preload was subtracted from the maximum and static stresses before calculating this ratio.
| (1) |
Fig. 5.
Expected stress relaxation test results and relevant parameters.
2.6. Data analysis
Young’s modulus, stress relaxation ratio and fascicle diameter values from the three groups were compared in the analysis. Young’s modulus and stress relaxation ratio calculation was performed using MATLAB (Mathworks, Natick, MA) program. Each individual data point was an average of mechanical properties of four fascicles from a single tail, two from each of the left and right dorsal tendons. Young’s modulus data were analyzed using repeated measures ANOVA to determine if the animal type caused differences between loading rates or if the loading rate caused an overall difference. Univariate repeated measures ANOVA was used to determine if the gene type caused a difference at either load rate. Relaxation ratio and fascicle diameter between animal type was compared using one way ANOVA followed by a Tukey–Kramer post hoc test. All statistical tests were one-sided, and p-values less than 0.05 were considered significant. JMP (SAS, Cary, NC) statistical analysis software was used to perform ANOVA.
3. Results
Fascicle diameter of the wild type, heterozygous and lubricin knock-out groups were 0.1098 (±0.0087), 0.1094 (±0.0063) and 0.1076 (±0.0110) mm, respectively. There were no significant differences across these values (p = 0.7843).
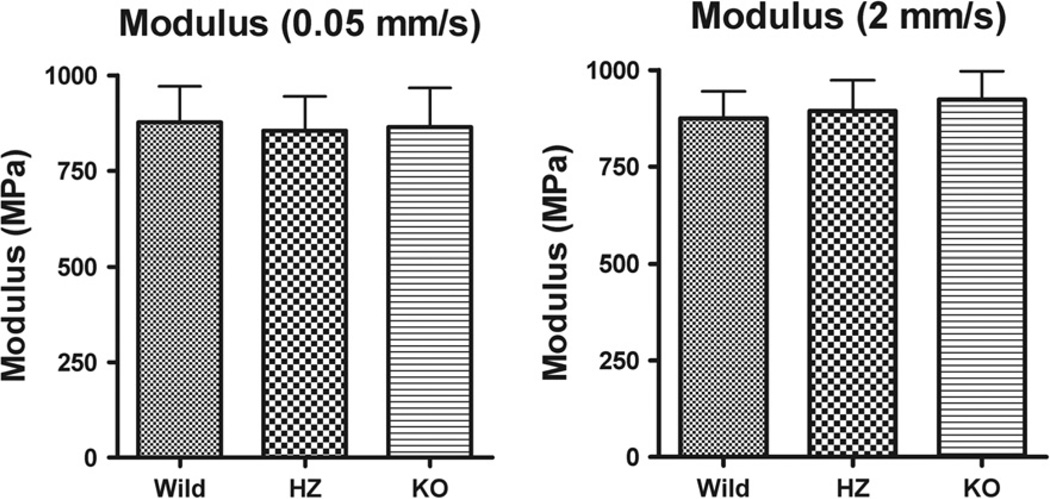
Typical stress–strain results of the fascicle mechanical testing at the two different load rates are shown in Fig. 6. At the slower loading rate, the means (±SD) for Young’s modulus of the wild type, heterozygous and lubricin knock-out groups were 877.80 (±93.79), 855.45 (±89.68) and 865.74 (±102.036) MPa, respectively, and at the higher loading rate values of 874.83 (±70.14), 893.80 (±80.03) and 923.20 (±73.40) MPa (Fig. 7). Animal type neither caused significant difference in Young’s modulus between loading rates (p = 0.1575), nor was there an interaction effect. Loading rate did cause a significant overall difference in Young’s modulus (p = 0.0212). Animal type did not cause a significant difference in Young’s modulus at either loading rate (p = 0.7907).
Fig. 6.
Representative mechanical test data for a fascicle including ramped loading modulus test (2.5% strain at 0.05 mm/s) and ramped loading for the stress relaxation test (5% strain at 2 mm/s).
Fig. 7.
Mean Young’s modulus for different strain rates in WT, HZ and KO mice. Whiskers represent standard deviation. There were no significant differences.
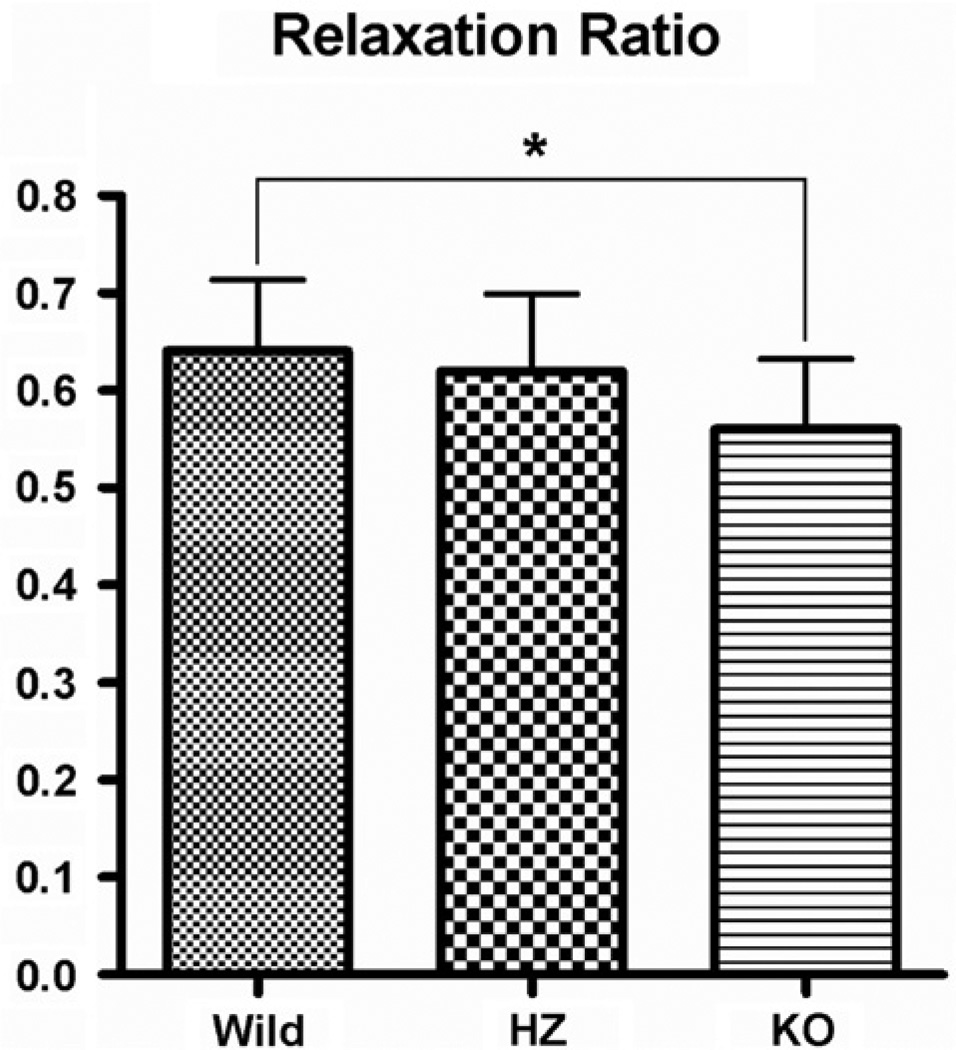
The means (±SD) for the relaxation ratio of the wild type, heterozygous and lubricin knock-out groups were 0.6402 (±0.07293), 0.6194 (±0.07963) and 0.5582 (±0.07191), respectively (Fig. 8). ANOVA testing showed a significant difference between groups (p = 0.0305). A Tukey–Kramer post hoc test showed that the difference in Young’s modulus between wild type and heterozygous and heterozygous and KO types was not significant (p = 0.7760 and p = 0.1278, respectively); but the relaxation ratio of KO mice was significantly lower than wild types (p = 0.0297).
Fig. 8.
Mean relaxation ratio in WT, HZ and KO mice. Whiskers represent standard deviation. Significant differences (p < 0.05) are noted (*).
4. Discussion
Immunohistochemical staining has consistently demonstrated in the past that lubricin is predominantly located at the surface of fibrocartilaginous regions of tendon and that lubricin concentration is higher in areas of tendon that experience higher compressive stresses than tensile stresses (Rees et al., 2002). Immunohistochemical staining was not used to identify lubricin expression because it had been done previously. More recently, the staining of mouse fascicles also showed small amounts of lubricin within fascicles, but it was most prominent on the surface; the staining appeared to be greater in wild type than heterozygous fascicles, with no staining in the KO mice (Kohrs et al., 2011). Such observation lead us to hypothesize the role of lubricin in the mechanical function of the fascicles in the tendon.
Strain between fibers in fascicles changes with respect to time, and is the largest contributor to stress relaxation, while intra-fibrillar strain does not significantly change with time at the micro and nano scale (Gupta et al., 2010; Yin and Elliott, 2004). This supports our data, which shows lubricin as a factor in viscoelastic properties of the fascicle but not tensile strength. Viscoelastic properties are important for optimizing tissue stiffness under various loading conditions and providing damping for load response (Gupta et al., 2010; Paxton and Baar, 2007). Thus, our results suggest that a lack of lubricin in the tendon may result in more stress on the muscle and bone from load transfers.
It is important to note that decorin, the main proteoglycan in tendons, also plays a role in viscoelastic properties of the tendon; however, decorin knockout mice show reduced stress relaxation rates (Elliott et al., 2003; Yin and Elliott, 2004). Young mice have greater decorin content and slower relaxation rates (Ker, 2007; Yin and Elliott, 2004). All mice in this experiment were adults 10–13 weeks old, so age was not a factor affecting the results. It is possible that other proteins have effects on these mechanical properties. It is also possible that the data from this experiment does not truly represent the in vivo state for fascicle.
Previous testing of fascicle stiffness have resulted in Young’s modulus values that are smaller, but on the same order of magnitude as those obtained in this study. A study comparing fascicle properties of TGF-b inducible early gene-1 (TIEG) knock-out and wild type mice revealed a mean Young’s modulus for similar-aged wild type mice approximately half of that reported in this study (Bensamoun et al., 2006). One possible explanation for this difference could be that the specimen ends were prepared and gripped differently, the method described here resulting in a very rigid construct.
This study has several limitations. A disadvantage of using mice fascicles is that they are very small and delicate. Measurement of the diameter was difficult because of the small size and non-uniform cross-sections along the length of the fascicle. A more accurate or direct measurement of the cross sectional area may have helped to improve the variability within groups for the modulus data. The cross-sectional area is not a factor for the relaxation ratio calculation, and thus the variability of this parameter was acceptable. Because the fascicles are so small and delicate, excessive handling for measurements may cause damage. One method that may mitigate both of these concerns would be to measure the weight of a fascicle immediately after removal and use the known density and length to determine a cross sectional area. Even though the size of fascicles was challenging, great care was taken methodologically. We did not test the compressive mechanical properties of the mouse fascicles in this study, but it would be worthwhile to analyze this in a future study in light of the relationship between lubricin and compressive loading. The effects of lubricin on mechanical properties should also be analyzed on tendons experiencing higher compressive stresses.
Prior data indicates that lubricin exists on the tendon fascicle surfaces (Sun et al., 2006). Previous studies also demonstrated that relative motion among the tendon fascicles occurs during tendon strain (Haraldsson et al., 2008). Recently, several studies further revealed that lubricin has an important effect on tendon and fascicle lubrication (Kohrs et al., 2011; Sun et al., 2008; Taguchi et al., 2008, 2009). Based on these findings, we believe that lubricin may also affect tendon viscoelastic properties. There is only a small amount of lubricin within the mouse fascicle. However, it is possible that the structural make-up of the tendon fascicles may be affected by a lack of lubricin, due to a direct effect caused by a secondary impact on synthesis of matrix macromolecules. Future studies could further analyze the structure of fascicles, including nanostructure assessment through transmission electron microscopy or assessing bulk properties, such as correlating fibril size to lubricin concentration. The effect of the absence of lubricin on tendon matrix composition and the synthesis of other glycoproteins, such as decorin, could also be investigated.
5. Conclusions
Lubricin has been generally accepted as an important molecule for cartilage function. Depletion of lubricin function has been associated with camptodactyly-arthropathy-coxa vara-pericarditis (CACP) syndrome, an arthritis-like autosomal recessive disorder, which disrupts normal joint and tendon function (Marcelino et al., 1999; Rhee et al., 2005). This study was the first to investigate the mechanical properties of lubricin knockout mouse tendon. Although data from the current study indicated no significant effect on elastic properties or fascicle size, the lower relaxation ratio in lubricin knockout mice indicated that the viscoelastic properties of the tendon fascicle might be altered with lubricin depletion.
Acknowledgment
This study was supported by a grant from Mayo Foundation.
Abbreviations
- L0
initial length
- TIEG
TGF-β inducible early gene-1
- KO
knockout
Contributor Information
John Reuvers, Email: jreuvers@iastate.edu.
Andrew R. Thoreson, Email: thoreson.andrew@mayo.edu.
Chunfeng Zhao, Email: zhao.chunfeng@mayo.edu.
Ling Zhang, Email: LZhang1@Lifespan.org.
Gregory D. Jay, Email: Gjay@Lifespan.org.
Kai-Nan An, Email: an@mayo.edu.
Matthew L. Warman, Email: matthew.warman@childrens.harvard.edu.
Peter C. Amadio, Email: pamadio@mayo.edu.
References
- Bensamoun SF, Tsubone T, Subramaniam M, Hawse JR, Boumediene E, et al. Age-dependent changes in the mechanical properties of tail tendons in TGF-beta inducible early gene-1 knockout mice. J. Appl. Physiol. 2006;101:1419–1424. doi: 10.1152/japplphysiol.00800.2005. [DOI] [PubMed] [Google Scholar]
- Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, et al. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J. Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott DM, Robinson PS, Gimbel JA, Sarver JJ, Abboud JA, et al. Effect of altered matrix proteins on quasilinear viscoelastic properties in transgenic mouse tail tendons. Ann. Biomed. Eng. 2003;31:599–605. doi: 10.1114/1.1567282. [DOI] [PubMed] [Google Scholar]
- Graf BK, Fujisaki K, Vanderby R, Jr, Vailas AC. The effect of in situ freezing on rabbit patellar tendon. A histologic, biochemical, and biomechanical analysis. Am. J. Sports Med. 1992;20:401–405. doi: 10.1177/036354659202000406. [DOI] [PubMed] [Google Scholar]
- Gupta HS, Seto J, Krauss S, Boesecke P, Screen HR. In situ multi-level analysis of viscoelastic deformation mechanisms in tendon collagen. J. Struct. Biol. 2010;169:183–191. doi: 10.1016/j.jsb.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Haraldsson BT, Aagaard P, Qvortrup K, Bojsen-Moller J, Krogsgaard M, et al. Lateral force transmission between human tendon fascicles. Matrix Biol. 2008;27:86–95. doi: 10.1016/j.matbio.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Ker RF. Mechanics of tendon, from an engineering perspective. Int. J. Fatigue. 2007;29:1001–1009. [Google Scholar]
- Kohrs RT, Zhao C, Sun YL, Jay GD, Zhang L, et al. Tendon fascicle gliding in wild type, heterozygous, and lubricin knockout mice. J. Orthop. Res. 2011;29:384–389. doi: 10.1002/jor.21247. [DOI] [PubMed] [Google Scholar]
- Kuc IM, Scott PG. Increased diameters of collagen fibrils precipitated in vitro in the presence of decorin from various connective tissues. Connect. Tissue Res. 1997;36:287–296. doi: 10.3109/03008209709160228. [DOI] [PubMed] [Google Scholar]
- Lee GH, Kumar A, Berkson E, Verma N, Bach BR, Jr, et al. A biomechanical analysis of bone-patellar tendon-bone grafts after repeat freeze-thaw cycles in a cyclic loading model. J. Knee Surg. 2009;22:111–113. doi: 10.1055/s-0030-1247734. [DOI] [PubMed] [Google Scholar]
- Marcelino J, Carpten JD, Suwairi WM, Gutierrez OM, Schwartz S, et al. CACP, encoding a secreted proteoglycan, is mutated in camptodactylyarthropathy-coxa vara-pericarditis syndrome. Nat. Genet. 1999;23:319–322. doi: 10.1038/15496. [DOI] [PubMed] [Google Scholar]
- Paxton JZ, Baar K. Tendon mechanics: the argument heats up. J. Appl. Physiol. 2007;103:423–424. doi: 10.1152/japplphysiol.00426.2007. [DOI] [PubMed] [Google Scholar]
- Pins GD, Christiansen DL, Patel R, Silver FH. Self-assembly of collagen fibers. Influence of fibrillar alignment and decorin on mechanical properties. Biophys. J. 1997;73:2164–2172. doi: 10.1016/S0006-3495(97)78247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees SG, Davies JR, Tudor D, Flannery CR, Hughes CE, et al. Immunolocalisation and expression of proteoglycan 4 (cartilage superficial zone proteoglycan) in tendon. Matrix Biol. 2002;21:593–602. doi: 10.1016/s0945-053x(02)00056-2. [DOI] [PubMed] [Google Scholar]
- Rhee DK, Marcelino J, Baker M, Gong Y, Smits P, et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J. Clin. Invest. 2005;115:622–631. doi: 10.1172/JCI200522263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson PS, Huang T-F, Kazam E, Iozzo RV, Birk DE, et al. Influence of decorin and biglycan on mechanical properties of multiple tendons in knockout mice. J. Biomech. Eng. 2005;127:181–185. doi: 10.1115/1.1835363. [DOI] [PubMed] [Google Scholar]
- Screen HR. Investigating load relaxation mechanics in tendon. J. Mech. Behav. Biomed. Mater. 2008;1:51–58. doi: 10.1016/j.jmbbm.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Sun Y, Berger EJ, Zhao C, Jay GD, An K, et al. Expression and mapping of lubricin in canine flexor tendon. J. Orthop. Res. 2006;24:1861–1868. doi: 10.1002/jor.20239. [DOI] [PubMed] [Google Scholar]
- Sun Y, Chen M-Y, Zhao C, An K, Amadio PC. The effect of hyaluronidase, phospholipase, lipid solvent and trypsin on the lubrication of canine flexor digitorum profundus tendon. J. Orthop. Res. 2008;26:1225–1229. doi: 10.1002/jor.20624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi M, Sun Y-L, Zhao C, Zobitz ME, Cha C-J, et al. Lubricin surface modification improves extrasynovial tendon gliding in a canine model in vitro. J. Bone Joint Surg. Am. 2008;90:129–135. doi: 10.2106/JBJS.G.00045. [DOI] [PubMed] [Google Scholar]
- Taguchi M, Sun Y, Zhao C, Zobitz M, Cha C, et al. Lubricin surface modification improves tendon gliding after tendon repair in a canine model in vitro. J. Orthop. Res. 2009;27:257–263. doi: 10.1002/jor.20731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto E, Hayashi K, Yamamoto N. Mechanical properties of collagen fascicles from stress-shielded patellar tendons in the rabbit. Clin. Biomech. 1999;14:418–425. doi: 10.1016/s0268-0033(99)00006-6. [DOI] [PubMed] [Google Scholar]
- Yin L, Elliott DM. A biphasic and transversely isotropic mechanical model for tendon: application to mouse tail fascicles in uniaxial tension. J. Biomech. 2004;37:907–916. doi: 10.1016/j.jbiomech.2003.10.007. [DOI] [PubMed] [Google Scholar]