Abstract
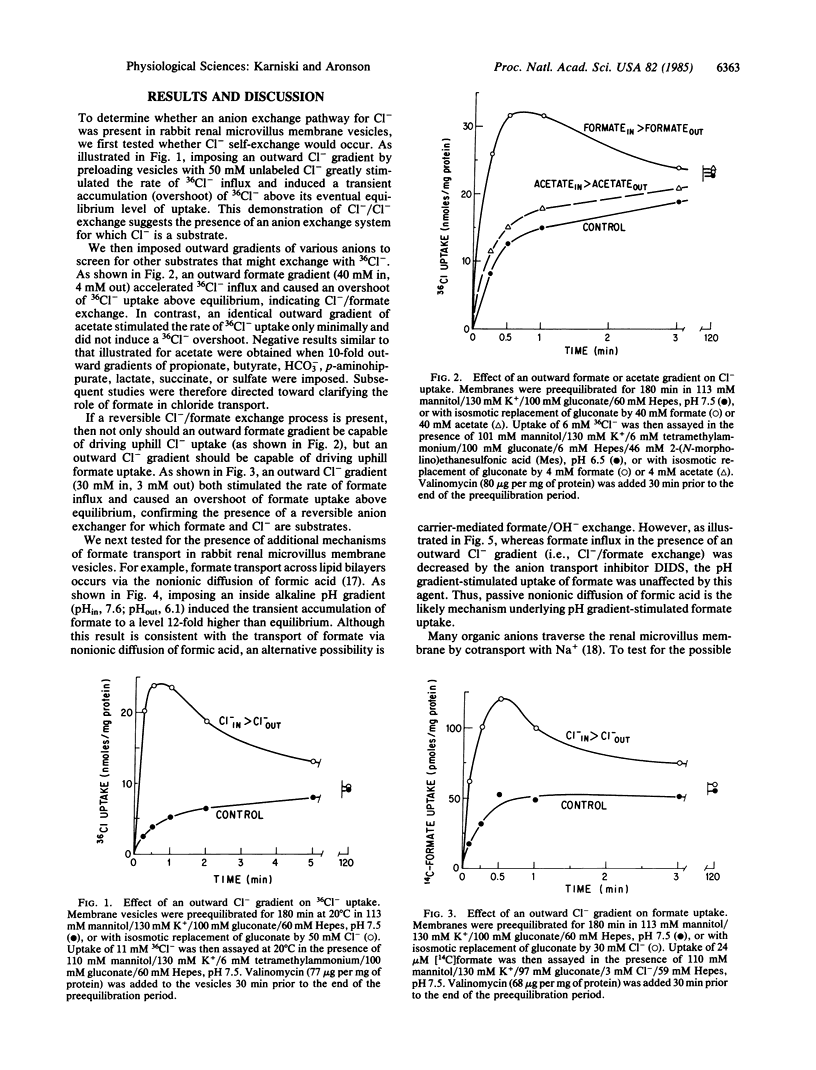
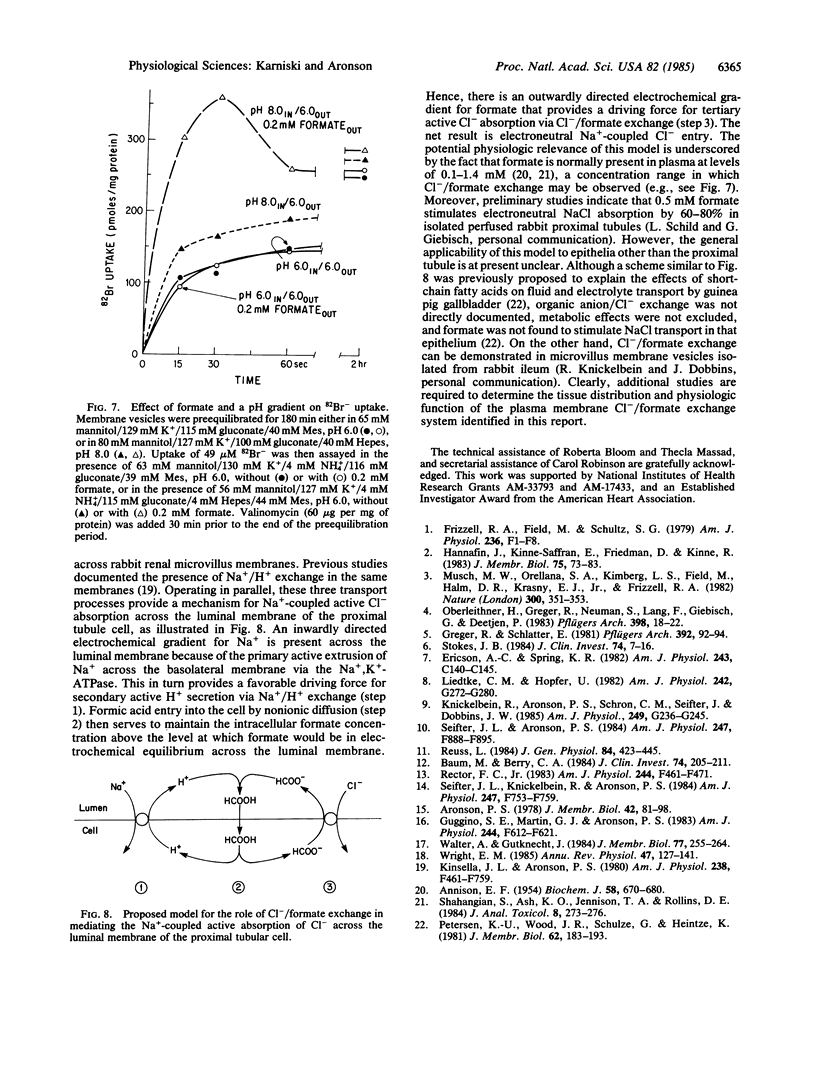
The pathways for transport of Cl- and formate in microvillus membrane vesicles isolated from rabbit renal cortex were evaluated. An outward formate gradient stimulated the uptake of Cl-, and an outward Cl- gradient stimulated the uptake of formate, indicating Cl-/formate exchange. In addition, an inside alkaline pH gradient induced the accumulation of formate, consistent with nonionic diffusion of formic acid. Although an inward Na+ gradient also stimulated uphill formate accumulation, suggesting Na+/formate cotransport, this effect was abolished when ionophores were used to prevent the generation of a transmembrane pH gradient, indicating an indirect coupling of formic acid transport to Na+/H+ exchange. An inside alkaline pH gradient only minimally stimulated the uptake of 82Br-, used as tracer for Cl-, confirming the absence of appreciable Cl-/OH- exchange. However, the same pH gradient in the presence of a physiologic formate concentration (0.2 mM) markedly stimulated 82Br- influx. These data suggest that Cl-/formate exchange with recycling of formic acid by nonionic diffusion is a potential mechanism for active Cl- absorption across the luminal membrane in the proximal tubule and perhaps in other epithelia.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANNISON E. F. Studies on the volatile fatty acids of sheep blood with special reference to formic acid. Biochem J. 1954 Dec;58(4):670–680. doi: 10.1042/bj0580670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson P. S. Energy-dependence of phlorizin binding to isolated renal microvillus membranes. Evidence concerning the mechanism of coupling between the electrochemical Na+ gradient the sugar transport. J Membr Biol. 1978 Jul 21;42(1):81–98. doi: 10.1007/BF01870395. [DOI] [PubMed] [Google Scholar]
- Baum M., Berry C. A. Evidence for neutral transcellular NaCl transport and neutral basolateral chloride exit in the rabbit proximal convoluted tubule. J Clin Invest. 1984 Jul;74(1):205–211. doi: 10.1172/JCI111403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson A. C., Spring K. R. Coupled NaCl entry into Necturus gallbladder epithelial cells. Am J Physiol. 1982 Sep;243(3):C140–C145. doi: 10.1152/ajpcell.1982.243.3.C140. [DOI] [PubMed] [Google Scholar]
- Frizzell R. A., Field M., Schultz S. G. Sodium-coupled chloride transport by epithelial tissues. Am J Physiol. 1979 Jan;236(1):F1–F8. doi: 10.1152/ajprenal.1979.236.1.F1. [DOI] [PubMed] [Google Scholar]
- Greger R., Schlatter E. Presence of luminal K+, a prerequisite for active NaCl transport in the cortical thick ascending limb of Henle's loop of rabbit kidney. Pflugers Arch. 1981 Nov;392(1):92–94. doi: 10.1007/BF00584588. [DOI] [PubMed] [Google Scholar]
- Guggino S. E., Martin G. J., Aronson P. S. Specificity and modes of the anion exchanger in dog renal microvillus membranes. Am J Physiol. 1983 Jun;244(6):F612–F621. doi: 10.1152/ajprenal.1983.244.6.F612. [DOI] [PubMed] [Google Scholar]
- Hannafin J., Kinne-Saffran E., Friedman D., Kinne R. Presence of a sodium-potassium chloride cotransport system in the rectal gland of Squalus acanthias. J Membr Biol. 1983;75(1):73–83. doi: 10.1007/BF01870801. [DOI] [PubMed] [Google Scholar]
- Kinsella J. L., Aronson P. S. Properties of the Na+-H+ exchanger in renal microvillus membrane vesicles. Am J Physiol. 1980 Jun;238(6):F461–F469. doi: 10.1152/ajprenal.1980.238.6.F461. [DOI] [PubMed] [Google Scholar]
- Knickelbein R., Aronson P. S., Schron C. M., Seifter J., Dobbins J. W. Sodium and chloride transport across rabbit ileal brush border. II. Evidence for Cl-HCO3 exchange and mechanism of coupling. Am J Physiol. 1985 Aug;249(2 Pt 1):G236–G245. doi: 10.1152/ajpgi.1985.249.2.G236. [DOI] [PubMed] [Google Scholar]
- Liedtke C. M., Hopfer U. Mechanism of Cl- translocation across small intestinal brush-border membrane. II. Demonstration of Cl--OH- exchange and Cl- conductance. Am J Physiol. 1982 Mar;242(3):G272–G280. doi: 10.1152/ajpgi.1982.242.3.G272. [DOI] [PubMed] [Google Scholar]
- Musch M. W., Orellana S. A., Kimberg L. S., Field M., Halm D. R., Krasny E. J., Jr, Frizzell R. A. Na+-K+-Cl- co-transport in the intestine of a marine teleost. Nature. 1982 Nov 25;300(5890):351–353. doi: 10.1038/300351a0. [DOI] [PubMed] [Google Scholar]
- Oberleithner H., Greger R., Neuman S., Lang F., Giebisch G., Deetjen P. Omission of luminal potassium reduces cellular chloride in early distal tubule of amphibian kidney. Pflugers Arch. 1983 Jun;398(1):18–22. doi: 10.1007/BF00584707. [DOI] [PubMed] [Google Scholar]
- Petersen K. U., Wood J. R., Schulze G., Heintze K. Stimulation of gallbladder fluid and electrolyte absorption by butyrate. J Membr Biol. 1981;62(3):183–193. doi: 10.1007/BF01998164. [DOI] [PubMed] [Google Scholar]
- Rector F. C., Jr Sodium, bicarbonate, and chloride absorption by the proximal tubule. Am J Physiol. 1983 May;244(5):F461–F471. doi: 10.1152/ajprenal.1983.244.5.F461. [DOI] [PubMed] [Google Scholar]
- Reuss L. Independence of apical membrane Na+ and Cl- entry in Necturus gallbladder epithelium. J Gen Physiol. 1984 Sep;84(3):423–445. doi: 10.1085/jgp.84.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifter J. L., Aronson P. S. Cl- transport via anion exchange in Necturus renal microvillus membranes. Am J Physiol. 1984 Dec;247(6 Pt 2):F888–F895. doi: 10.1152/ajprenal.1984.247.6.F888. [DOI] [PubMed] [Google Scholar]
- Seifter J. L., Knickelbein R., Aronson P. S. Absence of Cl-OH exchange and NaCl cotransport in rabbit renal microvillus membrane vesicles. Am J Physiol. 1984 Nov;247(5 Pt 2):F753–F759. doi: 10.1152/ajprenal.1984.247.5.F753. [DOI] [PubMed] [Google Scholar]
- Shahangian S., Ash K. O., Rollins D. E. An enzymatic method for the analysis of formate in human plasma. J Anal Toxicol. 1984 Nov-Dec;8(6):273–276. doi: 10.1093/jat/8.6.273. [DOI] [PubMed] [Google Scholar]
- Stokes J. B. Sodium chloride absorption by the urinary bladder of the winter flounder. A thiazide-sensitive, electrically neutral transport system. J Clin Invest. 1984 Jul;74(1):7–16. doi: 10.1172/JCI111420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter A., Gutknecht J. Monocarboxylic acid permeation through lipid bilayer membranes. J Membr Biol. 1984;77(3):255–264. doi: 10.1007/BF01870573. [DOI] [PubMed] [Google Scholar]
- Wright E. M. Transport of carboxylic acids by renal membrane vesicles. Annu Rev Physiol. 1985;47:127–141. doi: 10.1146/annurev.ph.47.030185.001015. [DOI] [PubMed] [Google Scholar]


