Abstract
Granulicella mallensis MP5ACTX8T is a novel species of the genus Granulicella in subdivision 1of Acidobacteria. G. mallensis is of ecological interest being a member of the dominant soil bacterial community active at low temperatures and nutrient limiting conditions in Arctic alpine tundra. G. mallensis is a cold-adapted acidophile and a versatile heterotroph that hydrolyzes a suite of sugars and complex polysaccharides. Genome analysis revealed metabolic versatility with genes involved in metabolism and transport of carbohydrates. These include gene modules encoding the carbohydrate-active enzyme (CAZyme) family involved in breakdown, utilization and biosynthesis of diverse structural and storage polysaccharides including plant based carbon polymers. The genome of Granulicella mallensis MP5ACTX8T consists of a single replicon of 6,237,577 base pairs (bp) with 4,907 protein-coding genes and 53 RNA genes.
Keywords: cold adapted, acidophile, tundra soil, Acidobacteria
Introduction
Strain MP5ACTX8T (= ATCC BAA-1857T = DSM 23137T), is the type strain of the species Granulicella mallensis [1]. The genus Granulicella, in subdivision 1 of Acidobacteria, was first described by Pankratov et al. in 2010 [2]. Granulicella mallensis (mal.len' sis. N. L. fem. adj. mallensis; pertaining to its isolation from soil of Malla Nature Reserve, Kilpisjärvi, Finland; 69°01’N, 20°50’E) was described along with other species of the genus Granulicella isolated from tundra soil [1] and is one of the two with sequenced genomes, out of eight validly described Granulicella species.
Acidobacteria is one of the most ubiquitous bacterial phyla found in diverse habitats and is abundant in most soil environments [3,4] including Arctic tundra soils [5,6]. Acidobacteria are phylogenetically and physiologically diverse [7] represented by 26 phylogenetic subdivisions [8] of which only subdivisions 1, 3, 4, 8, and 10 are defined by taxonomically characterized representatives. To date, subdivision 1 is comprised of eight genera: Acidobacterium [9], Terriglobus [10,11], Edaphobacter [12], Granulicella [1,2], Acidipila [13], Telmatobacter [14], Acidicapsa [15] and Bryocella [16]. Subdivision 3, 4 and 10 include only one genus each, namely Bryobacter [17], Blastocatella [18] and Thermotomaculum [19], respectively, while subdivision 8 includes three genera; Holophaga [20], Geothrix [21] and Acanthopleuribacter [22]. Three species, ‘Candidatus Koribacter versatilis’ [23], ‘Candidatus Solibacter usitatus’ [23] and ‘Candidatus Chloracidobacterium thermophilum’ [24] have been described as ‘Candidatus’ taxa. Acidobacteria are relatively difficult to cultivate with slow growth rates and typically require up to several weeks to develop visible colonies on solid media. Nevertheless, the phylogenetic diversity, ubiquity and abundance of this group suggest that they play important ecological roles in soils. The abundance of Acidobacteria has been found to correlate with soil pH [25,26] and carbon [27,28], with subdivision 1 Acidobacteria being most abundant in slightly acidic soils. Our previous studies have shown that Acidobacteria dominate in the acidic tundra heaths of northern Finland [25,29-31]. Using selective isolation techniques we have been able to isolate several slow growing and fastidious strains of Acidobacteria [1,11]. On the basis of phylogenetic, phenotypic and chemotaxonomic data, including 16S rRNA, rpoB gene sequence similarity and DNA–DNA hybridization, strain MP5ACTX8T was classified as a novel species of the genus Granulicella [1]. Here, we summarize the physiological features together with the complete genome sequence, annotation and data analysis of Granulicella mallensis MP5ACTX8T (Table 1).
Table 1. Classification and general features of G. mallensis strain MP5ACTX8T according to the MIGS recommendations [32].
| MIGS ID | Property | Term | Evidence codea |
|---|---|---|---|
| Classification | Domain Bacteria | TAS [33] | |
| Phylum Acidobacteria | TAS [34,35] | ||
| Class Acidobacteria | TAS [36,37] | ||
| Order Acidobacteriales | TAS [36,38] | ||
| Family Acidobacteriaceae | TAS [34,39] | ||
| Genus Granulicella | TAS [1,2] | ||
| Species Granulicella mallensis | TAS [1] | ||
| Type strain: MP5ACTX8T (= ATCC BAA-1857T = DSM 23137T) | |||
| Gram stain | negative | TAS [1] | |
| Cell shape | rod | TAS [1] | |
| Motility | non-motile | TAS [1] | |
| Sporulation | not reported | NAS | |
| Temperature range | 4–28 °C | TAS [1] | |
| Optimum temperature | 24–27 °C | TAS [1] | |
| pH range | 3.5–6.5 | TAS [1] | |
| Optimum pH | 5 | TAS [1] | |
| Carbon source | D-glucose, maltose, D-fructose, D-galactose, lactose, lactulose, D-mannose, D-ribose, raffinose, sucrose, trehalose, cellobiose, D-xylose, glucuronate |
TAS [1] | |
| MIGS-6 | Habitat | terrestrial | TAS [1] |
| MIGS-6.3 | Salinity | Growth with up to 1.5% NaCl | TAS [1] |
| MIGS-22 | Oxygen requirement | aerobic | TAS [1] |
| MIGS-15 | Biotic relationship | free-living | TAS [1] |
| MIGS-14 | Pathogenicity | non-pathogenic | NAS |
| MIGS-4 | Geographic location | Arctic-alpine tundra, Finland | TAS [1] |
| MIGS-5 | Sample collection | 2006 | TAS [1] |
| MIGS-4.1 | Latitude | 69°01’N, | TAS [1] |
| MIGS-4.2 | Longitude | 20°50’E | |
| MIGS-4.4 | Altitude | 700 m | TAS [1] |
aEvidence codes - IDA: Inferred from Direct Assay; TAS: Traceable Author Statement (i.e., a direct report exists in the literature); NAS: Non-traceable Author Statement (i.e., not directly observed for the living, isolated sample, but based on a generally accepted property for the species, or anecdotal evidence). These evidence codes are from the Gene Ontology project [40].
Classification and features
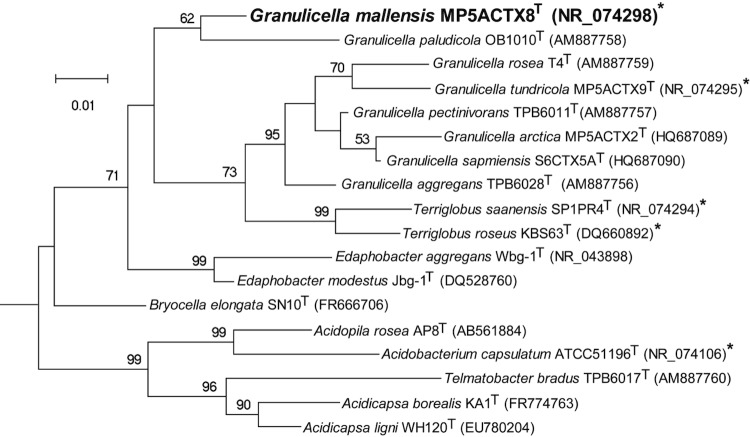
Within the genus Granulicella, eight species are described with validly published names: G. mallensis MP5ACTX8T, G. tundricola MP5ACTX9T, G. arctica MP5ACTX2T and G. sapmiensis S6CTX5AT isolated from Arctic tundra soil [1] and G. paludicola OB1010T, G. pectinivorans TPB6011T, G. rosea TPO1014T and G. aggregans TPB6028T isolated from sphagnum peat bogs [3]. Strain MP5ACTX8T showed 95.5 -96.1% 16S rRNA gene sequence identity to tundra soil strains, G. tundricola MP5ACTX9T (95.5%), G. sapmiensis S6CTX5AT (96.2%) and G. arctica MP5ACTX2T (96.1%) and 94.6 – 97.4% to G. rosea TPO1014T (94.6%), G. aggregans TPB6028T (96.0%), G. pectinivorans TPB6011T (96.1%), G. paludicola OB1010T (96.5%) and G. paludicola LCBR1 (97.4%). Phylogenetic analysis based on the 16S rRNA gene of taxonomically classified strains of family Acidobacteriaceae placed G. paludicola type strain OB1010 T as the closest taxonomically classified relative of G. mallensis MP5ACTX8T (Figure 1).
Figure 1.
Phylogenetic tree highlighting the position of G. mallensis MP5ACTX8T (shown in bold) relative to the other type strains within SD1 Acidobacteria. The maximum likelihood tree was inferred from 1,361 aligned positions of the 16S rRNA gene sequences and derived based on the Tamura-Nei model using MEGA 5 [41]. Bootstrap values >50 (expressed as percentages of 1,000 replicates) are shown at branch points. Bar: 0.02 substitutions per nucleotide position. The corresponding GenBank accession numbers are displayed in parentheses. Strains whose genomes have been sequenced, are marked with an asterisk; G. mallensis MP5ACTX8T (CP003130), G. tundricola MP5ACTX9T (CP002480), T. saanensis SP1PR4T (CP002467), T. roseus KBS63T (CP003379) and A. capsulatum ATCC 51196T (CP001472). Bryobacter aggregatus MPL3 (AM162405) in SD3 Acidobacteria was used as an outgroup.
Morphology and physiology
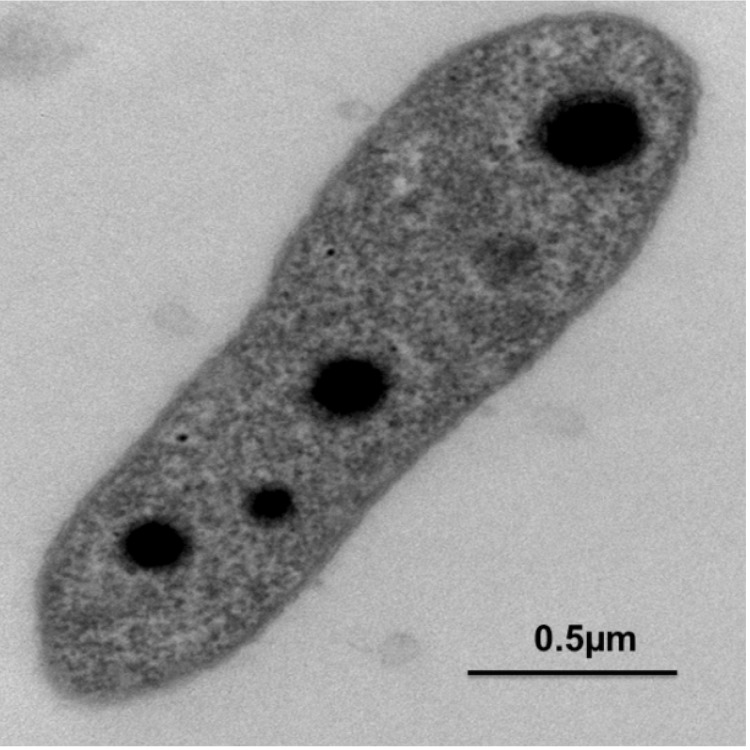
G. mallensis grows on R2 medium (Difco) at pH 3.5–6.5 (optimum pH 5) and at +4 to +28 °C (optimum 24–27 °C) [1]. On R2 agar, strain MP5ACTX8T forms opaque white mucoid colonies with a diameter of approximately 1 mm. Cells are Gram-negative, non-motile, aerobic rods, approximately 0.5–0.7 mm wide and 0.6–1.3 mm long. Growth observed with up to 1.5% NaCl (w/v) (Table 1). The cell-wall structure in ultrathin sections of electron micrographs of cells of MP5ACTX8T is shown in Figure 2.
Figure 2.
Electron micrograph of G. mallensis MP5ACTX8T.
G. mallensis utilizes D-glucose, maltose, cellobiose, D-fructose, D-galactose, lactose, lactulose, D-mannose, D-ribose, raffinose, sucrose, trehalose, D-xylose, N-acetyl-D-glucosamine, glucuronate, glutamate, melezitose and salicin, but does not utilize D-arabinose, acetate, formate, pyruvate, malate, mannitol, D- or L-alanine, D-glycine, L-leucine, L-ornithine, gluconic acid, aspartate, dulcitol, butyrate, caproate, valerate, lactate, oxalate, propionate, fumarate, adonitol, methanol, ethanol, succinate, D-sorbitol or myoinositol, when grown using VL55 mineral medium with 100 mg yeast extract l-1. G. mallensis hydrolyzes aesculin, starch, pectin, laminarin and lichenan, but not gelatin, cellulose, xylan, sodium alginate, pullulan, chitosan or chitin on R2 medium. Strains show positive reaction for acid and alkaline phosphatases, leucine arylamidase, a-chymotrypsin, naphthol-AS-BI-phosphohydrolase, α- and β-galactosidases, α- and β-glucosidases, N-acetyl- β-glucosaminidase, β-glucuronidase, trypsin and valine arylamidase, but negative for α-fucosidase, α-mannosidase, esterase (C4 and C8), lipase (C14) and cystine arylamidase. Strain MP5ACTX8T reduces nitrate to nitrite. Strain MP5ACTX8T is resistant to the antibiotics erythromycin, chloramphenicol, neomycin, rifampicin, streptomycin, gentamicin, polymyxin B and penicillin, but susceptible to ampicillin, kanamycin, tetracycline, lincomycin, novobiocin and bacitracin [1].
Chemotaxonomy
The major cellular fatty acids in G. mallensis are iso-C15:0 (45.3%), C16:1ω7c (28.7%), iso-C13:0 (8.3%) and C16:0 (8.9%). The cellular fatty acid compositions of strain MP5ACTX8T were relatively similar to that of other Granulicella strains with fatty acids iso-C15:0 and C16:1ω7c being most abundant in all strains. Strain MP5ACTX8T contains MK-8 as the major quinone.
Genome sequencing and annotation
Genome project history
G. mallensis strain MP5ACTX8T was selected for sequencing in 2009 by the DOE Joint Genome Institute (JGI) community sequencing program. The Quality Draft (QD) assembly and annotation were completed on December 26, 2010. The complete genome was made available on Dec. 1, 2011. The genome project is deposited in the Genomes On-Line Database (GOLD) [42] and the complete genome sequence of strain MP5ACTX8T is deposited in GenBank (CP003130). Table 2 presents the project information and its association with MIGS version 2.0 [32].
Table 2. Project information.
| MIGS ID | Property | Term |
|---|---|---|
| MIGS 31 | Finishing quality | Finished |
| MIGS-28 | Libraries used | Three libraries, an Illumina GAii shotgun library (GSGY), a 454 Titanium standard library (GSXT, GWTA) and a paired end 454 (GSFP) library |
| MIGS 29 | Sequencing platforms | 454 Titanium standard, 454 Paired End, Illumina |
| MIGS 31.2 | Fold coverage | 18.5× (454), 213× (Illumina) |
| MIGS 30 | Assemblers | Newbler, VELVET, PHRAP |
| MIGS 32 | Gene calling method | ProdigaL, GenePRIMP |
| Locus Tag | AciX8 | |
| Genbank ID | CP003130.1 | |
| GenBank Date of Release | December 1, 2011 | |
| GOLD ID | Gc02349 | |
| BIOPROJECT | PRJNA49957, PRJNA47903 | |
| Project relevance | Environmental, Biogeochemical cycling of Carbon, Biotechnological, GEBA |
Growth conditions and genomic DNA extraction
G. mallensis MP5ACTX8T was cultivated on R2 medium as previously described [1]. Genomic DNA (gDNA) of high sequencing quality was isolated using a modified CTAB method and evaluated according to the Quality Control (QC) guidelines provided by the DOE Joint Genome Institute [43].
Genome sequencing and assembly
The finished genome of G. mallensis MP5ACTX8T (JGI ID 4088692) was generated at the DOE Joint genome Institute (JGI) using a combination of Illumina [44] and 454 technologies [45]. For this genome, an Illumina GAii shotgun library which generated 59,701,420 reads totaling 4537.3 Mb, a 454 Titanium standard library which generated 136,708 reads and a paired end 454 library with an average insert size of 10.3 kb which generated 157,336 reads totaling 172.0 Mb of 454 data, were constructed and sequenced. All general aspects of library construction and sequencing performed at the JGI can be found at the JGI website [43]. The 454 Titanium standard data and the 454 paired end data were assembled with Newbler, version 2.3. Illumina sequencing data was assembled with Velvet, version 0.7.63 [46]. The 454 Newbler consensus shreds, the Illumina Velvet consensus shreds and the read pairs in the 454 paired end library were integrated using parallel phrap, version SPS - 4.24 (High Performance Software, LLC) [47]. The software Consed [48] was used in the finishing process. The Phred/Phrap/Consed software package [49] was used for sequence assembly and quality assessment in the subsequent finishing process. Illumina data was used to correct potential base errors and increase consensus quality using the software Polisher developed at JGI (Alla Lapidus, unpublished). Possible misassemblies were corrected using gapResolution (Cliff Han, un-published), Dupfinisher [50] or sequencing cloned bridging PCR fragments with sub-cloning. Gaps between contigs were closed by editing in Consed, by PCR and by Bubble PCR (J-F Cheng, unpublished) primer walks. The final assembly is based on 74.2 Mb of 454 data which provides an average 18.5× coverage and 1318.5 Mb of Illumina data which provides an average 213× coverage of the genome.
Genome annotation
Genes were identified using Prodigal [51] as part of the Oak Ridge National Laboratory genome annotation pipeline, followed by a round of manual curation using the JGI GenePRIMP pipeline [52]. The predicted CDSs were translated and used to search the National Center for Biotechnology Information (NCBI) non-redundant database, UniProt, TIGRFam, Pfam, PRIAM, KEGG, COGs [53,54], and InterPro. These data sources were combined to assert a product description for each predicted protein. Non-coding genes and miscellaneous features were predicted using tRNAscan-SE [55], RNAMMer [56], Rfam [57], TMHMM [58], and signalP [59]. Additional gene prediction analysis and functional annotation were performed within the Integrated Microbial Genomes Expert Review (IMG-ER) platform [60].
Genome properties
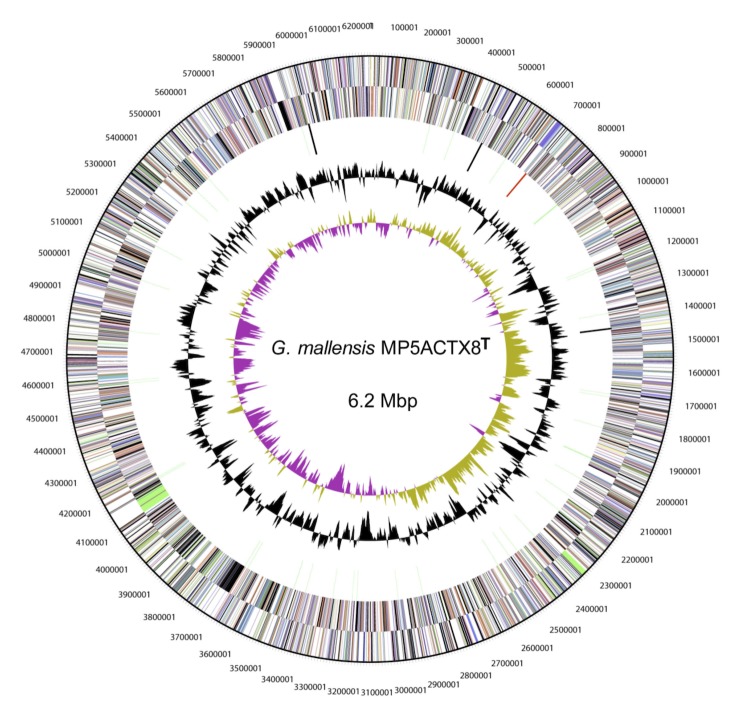
The genome consists of one circular chromosome of 6,211,694 bp in size with a GC content of 57.8 mol% and consists of 53 RNA genes (Figure 3 and Table 3). Of the 4,960 predicted genes, 4,907 are protein-coding genes (CDSs) and 90 are pseudogenes. Of the total CDSs, 70.5% represent COG functional categories and 16% consist of signal peptides. The distribution of genes into COG functional categories is presented in Figure 3 and Table 4.
Figure 3.
Circular representation of the chromosome of G. mallensis MP5ACTX8T displaying relevant genome features. From outside to center; Genes on forward strand (color by COG categories), genes on reverse strand (color by COG categories), RNA genes (tRNAs green, rRNAs red, other RNAs black), GC content and GC skew.
Table 3. Genome statistics.
| Attribute | Value | % of Total |
|---|---|---|
| Genome size (bp) | 6,237,577 | 100 |
| DNA coding region(bp) | 5,499,388 | 88.2 |
| DNA G+C content (bp) | 3612173 | 57.9 |
| DNA scaffolds | 1 | 100 |
| Total genes | 4,960 | 100 |
| Protein coding genes | 4,907 | 98.9 |
| RNA genes | 53 | 1.3 |
| Pseudo genes | 90 | 1.8 |
| Genes in internal clusters | 2,679 | 54 |
| Genes with function prediction | 3,511 | 70.8 |
| Genes assigned to COGs | 3,496 | 70.5 |
| Genes with Pfam domains | 3,754 | 75.7 |
| Genes with signal peptides | 797 | 16.1 |
| Genes with transmembrane helices | 1,291 | 26.0 |
| CRISPR repeats | 0 | - |
The total is based on either the size of the genome in base pairs or the protein coding genes in the annotated genome.
Table 4. Number of genes associated with general COG functional categories.
| Code | Value | %age | Description |
|---|---|---|---|
| J | 167 | 4.32 | Translation, ribosomal structure and biogenesis |
| A | 2 | 0.05 | RNA processing and modification |
| K | 332 | 8.58 | Transcription |
| L | 156 | 4.03 | Replication, recombination and repair |
| B | 1 | 0.03 | Chromatin structure and dynamics |
| D | 27 | 0.7 | Cell cycle control, Cell division, chromosome partitioning |
| Y | 0.0 | 0.0 | Nuclear structure |
| V | 76 | 1.96 | Defense mechanisms |
| T | 139 | 3.59 | Signal transduction mechanisms |
| M | 322 | 8.32 | Cell wall/membrane biogenesis |
| N | 17 | 0.44 | Cell motility |
| Z | 0.0 | 0.0 | Cytoskeleton |
| W | 0.0 | 0.0 | Extracellular structures |
| U | 79 | 2.04 | Intracellular trafficking and secretion |
| O | 123 | 3.18 | Posttranslational modification, protein turnover, chaperones |
| C | 193 | 4.99 | Energy production and conversion |
| G | 355 | 9.18 | Carbohydrate transport and metabolism |
| E | 258 | 6.67 | Amino acid transport and metabolism |
| F | 76 | 1.96 | Nucleotide transport and metabolism |
| H | 155 | 4.01 | Coenzyme transport and metabolism |
| I | 164 | 4.24 | Lipid transport and metabolism |
| P | 157 | 4.06 | Inorganic ion transport and metabolism |
| Q | 125 | 3.23 | Secondary metabolites biosynthesis, transport and catabolism |
| R | 527 | 13.62 | General function prediction only |
| S | 418 | 10.8 | Function unknown |
| - | 1,464 | 29.52 | Not in COGs |
The total is based on the total number of protein coding genes in the genome.
Discussion
Granulicella mallensis type strain MP5ACTX8T has the largest genome size of 6.2 Mbp. among the three tundra soil strains of subdivision 1 Acidobacteria [28]. Genome analysis of Granulicella mallensis identified a high abundance of genes assigned to COG functional categories for transport and metabolism of carbohydrates (9.1%) and amino acids (6.7%) and involved in cell envelope biogenesis (8.3%) and transcription (8.6%). Further genome analysis revealed an abundance of gene modules encoding for functional activities within the carbohydrate-active enzymes (CAZy) family [61] involved in breakdown, utilization and biosynthesis of carbohydrates. G. mallensis hydrolyzed complex carbon polymers, including CMC, pectin, lichenin, laminarin and starch, and utilized sugars such as cellobiose, D-mannose, D-xylose, D-trehalose. This parallels genome predictions for CDSs encoding for enzymes such as cellulases, pectinases, alginate lyases, trehalase and amylases. In addition, the G. mallensis genome contained a cluster of genes in the neighborhood of the cellulose synthase gene (bcsAB) which included cellulase (bscZ) (endoglucanase Y) of family GH8, cellulose synthase operon protein (bcsC) and a cellulose synthase operon protein (yhjQ) involved in cellulose biosynthesis. Detailed comparative genome analysis of G. mallensis MP5ACTX8T with other Acidobacteria strains for which finished genomes were available is reported in Rawat et al. [28]. The data thus suggests that G. mallensis is involved in hydrolysis, the utilization of stored carbohydrates, and in the biosynthesis of exopolysaccharides from organic matter and plant based polymers in the soil. Therefore, we infer that strain G. mallensis may be central to carbon cycling processes in arctic and boreal soil ecosystems.
Acknowledgements
The work conducted by the US Department of Energy Joint Genome Institute is supported by the Office of Science of the US Department of Energy Under Contract No. DE-AC02-05CH11231. This work was funded in part by the Academy of Finland and the New Jersey Agricultural Experiment Station.
References
- 1.Männistö MK, Rawat S, Starovoytov V, Häggblom MM. Granulicella arctica sp. nov., Granulicella mallensis sp. nov., Granulicella sapmiensis sp. nov. and Granulicella tundricola sp. nov., novel Acidobacteria from tundra soil of Northern Finland. Int J Syst Evol Microbiol 2012; 62:2097-2106 10.1099/ijs.0.031864-0 [DOI] [PubMed] [Google Scholar]
- 2.Pankratov TA, Dedysh SN. Granulicella paludicola gen. nov., sp. nov., Granulicella pectinivorans sp. nov., Granulicella aggregans sp. nov. and Granulicella rosea sp. nov., acidophilic, polymer degrading acidobacteria from Sphagnum peat bogs. Int J Syst Evol Microbiol 2010; 60:2951-2959 10.1099/ijs.0.021824-0 [DOI] [PubMed] [Google Scholar]
- 3.Janssen PH. Identifying the dominant soil bacterial taxa in libraries of 16S rRNA and 16S rRNA genes. Appl Environ Microbiol 2006; 72:1719-1728 10.1128/AEM.72.3.1719-1728.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fierer N, Bradford MA, Jackson RB. Toward an ecological classification of soil bacteria. Ecology 2007; 88:1354-1364 10.1890/05-1839 [DOI] [PubMed] [Google Scholar]
- 5.Campbell BJ, Polson SW, Hanson TE, Mack MC, Schuur EA. The effect of nutrient deposition on bacterial communities in Arctic tundra soil. Environ Microbiol 2010; 12:1842-1854 10.1111/j.1462-2920.2010.02189.x [DOI] [PubMed] [Google Scholar]
- 6.Chu H, Fierer N, Lauber CL, Caporaso JG, Knight R, Grogan P. Soil bacterial diversity in the Arctic is not fundamentally different from that found in other biomes. Environ Microbiol 2010; 12:2998-3006 10.1111/j.1462-2920.2010.02277.x [DOI] [PubMed] [Google Scholar]
- 7.Jones RT, Robeson MS, Lauber CL, Hamady M, Knight R, Fierer N. A comprehensive survey of soil acidobacterial diversity using pyrosequencing and clone library analyses. ISME J 2009; 3:442-453 10.1038/ismej.2008.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barns SM, Cain EC, Sommerville L, Kuske CR. Acidobacteria phylum sequences in uranium-contaminated subsurface sediments greatly expand the known diversity within the phylum. Appl Environ Microbiol 2007; 73:3113-3116 10.1128/AEM.02012-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kishimoto N, Kosako Y, Tano T. Acidobacterium capsulatum gen. nov., sp. nov.: an acidophilic chemoorganotrophic bacterium containing menaquinone from acidic mineral environment. Curr Microbiol 1991; 22:1-7 10.1007/BF02106205 [DOI] [PubMed] [Google Scholar]
- 10.Eichorst SA, Breznak JA, Schmidt TM. Isolation and characterization of soil bacteria that define Terriglobus gen. nov., in the phylum Acidobacteria. Appl Environ Microbiol 2007; 73:2708-2717 10.1128/AEM.02140-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Männistö MK, Rawat SR, Starovoytov V, Häggblom MM. Terriglobus saanensis sp. nov., an acidobacterium isolated from tundra soil. Int J Syst Evol Microbiol 2011; 61:1823-1828 10.1099/ijs.0.026005-0 [DOI] [PubMed] [Google Scholar]
- 12.Koch IH, Gich F, Dunfield PF, Overmann J. Edaphobacter modestus gen. nov., sp. nov., and Edaphobacter aggregans sp. nov., acidobacteria isolated from alpine and forest soils. Int J Syst Evol Microbiol 2008; 58:1114-1122 10.1099/ijs.0.65303-0 [DOI] [PubMed] [Google Scholar]
- 13.Okamura K, Kawai A, Yamada T, Hiraishi A. Acidipila rosea gen. nov.,sp nov., an acidophilic chemoorganotrophic bacterium belonging to the phylum Acidobacteria. FEMS Microbiol Lett 2011; 317:138-142 10.1111/j.1574-6968.2011.02224.x [DOI] [PubMed] [Google Scholar]
- 14.Pankratov TA, Kirsanova LA, Kaparullina EN, Kevbrin VV, Dedysh SN. Telmatobacter bradus gen. nov., sp. nov., a cellulolytic facultative anaerobe from subdivision 1 of the Acidobacteria and emended description of Acidobacterium capsulatum Kishimoto et al. Int J Syst Evol Microbiol 2012; 62:430-437 10.1099/ijs.0.029629-0 [DOI] [PubMed] [Google Scholar]
- 15.Kulichevskaya IS, Kostina LA, Valásková V, Rijpstra IC, Sinninghe Damsté JS, de Boer W, Dedysh SN. Acidicapsa borealis gen. nov., sp. nov. and A. ligni sp. nov., two novel subdivision 1 Acidobacteria from sphagnum peat and decaying wood. Int J Syst Evol Microbiol 2012; 62:1512-1520 10.1099/ijs.0.034819-0 [DOI] [PubMed] [Google Scholar]
- 16.Dedysh SN, Kulichevskaya IS, Serkebaeva YM, Mityaeva MA, Sorokin VV, Suzina NE, Rijpstra WI, Damste JS. Bryocella elongata gen. nov., sp. nov., a novel member of Subdivision 1 of the Acidobacteria isolated from a methanotrophic enrichment culture, and emended description of Edaphobacter aggregans Koch et al. 2008. Int J Syst Evol Microbiol 2012; 62:654-664 10.1099/ijs.0.031898-0 [DOI] [PubMed] [Google Scholar]
- 17.Kulichevskaya IS, Suzina NE, Liesack W, Dedysh SN. Bryobacter aggregatus gen. nov., sp. nov., a peat-inhabiting, aerobic chemoorganotroph from subdivision 3 of the Acidobacteria. Int J Syst Evol Microbiol 2010; 60:301-306 10.1099/ijs.0.013250-0 [DOI] [PubMed] [Google Scholar]
- 18.Foesel BU, Rohde M, Overmann J. Blastocatella fastidiosa gen. nov., sp. nov., isolated from semiarid savanna soil – The first described species of Acidobacteria subdivision 4. Syst Appl Microbiol 2013; 36:82-89 10.1016/j.syapm.2012.11.002 [DOI] [PubMed] [Google Scholar]
- 19.Izumi H, Nunoura T, Miyazaki M, Mino S, Toki T, Takai K, Sako Y, Sawabe T, Nakagawa S. Thermotomaculum hydrothermale gen. nov., sp. nov., a novel heterotrophic thermophile within the phylum Acidobacteria from a deep-sea hydrothermal vent chimney in the Southern Okinawa Trough. Extremophiles 2012; 16:245-253 10.1007/s00792-011-0425-9 [DOI] [PubMed] [Google Scholar]
- 20.Liesack W, Bak F, Kreft JU, Stackebrandt E. Holophaga foetida gen.nov., sp. nov., a new homoacetogenic bacterium degrading methoxylated aromatic compounds. Arch Microbiol 1994; 162:85-90 10.1007/BF00264378 [DOI] [PubMed] [Google Scholar]
- 21.Coates JD, Ellis DJ, Gaw CV, Lovley DR. Geothrix fermentans gen. nov., sp. nov., a novel Fe(III)-reducing bacterium from a hydrocarbon contaminated aquifer. Int J Syst Bacteriol 1999; 49:1615-1622 10.1099/00207713-49-4-1615 [DOI] [PubMed] [Google Scholar]
- 22.Fukunaga Y, Kurahashi M, Yanagi K, Yokota A, Harayama S. Acanthopleuribacter pedis gen. nov., sp. nov., a marine bacterium isolated from a chiton, and description of Acanthopleuribacteraceae fam. nov., Acanthopleuribacterales ord. nov., Holophagales ord. nov. and Holophagae classis nov. in the phylum ‘Acidobacteria’. Int J Syst Evol Microbiol 2008; 58:2597-2601 10.1099/ijs.0.65589-0 [DOI] [PubMed] [Google Scholar]
- 23.Ward NL, Challacombe JF, Janssen PH, Henrissat B, Coutinho PM, Wu M, Xie G, Haft DH, Sait M, Badger J, et al. Three genomes from the phylum Acidobacteria provide insight into the lifestyles of these microorganisms in soils. Appl Environ Microbiol 2009; 75:2046-2056 10.1128/AEM.02294-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bryant DA, Amaya M. Garcia Costas AMG, Maresca JA, Chew AGM, Klatt CG, Bateson MM, Tallon LJ, Hostetler J, Nelson WC, Heidelberg JF, Ward DM. Candidatus Chloracidobacterium thermophilum: an aerobic phototrophic acidobacterium. Science 2007; 317:523-526 10.1126/science.1143236 [DOI] [PubMed] [Google Scholar]
- 25.Männistö MK, Tiirola M, Häggblom MM. Microbial communities in Arctic fjelds of Finnish Lapland are stable but highly pH dependent. FEMS Microbiol Ecol 2007; 59:452-465 10.1111/j.1574-6941.2006.00232.x [DOI] [PubMed] [Google Scholar]
- 26.Sait M, Davis KE, Janssen PH. Effect of pH on isolation and distribution of members of subdivision 1 of the phylum Acidobacteria occurring in soil. Appl Environ Microbiol 2006; 72:1852-1857 10.1128/AEM.72.3.1852-1857.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eichorst SA, Kuske CR, Schmidt TM. Influence of plant polymers on the distribution and cultivation of bacteria in the phylum Acidobacteria. Appl Environ Microbiol 2011; 77:586-596 10.1128/AEM.01080-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rawat SR, Männistö MK, Bromberg Y, Häggblom MM. Comparative genomic and physiological analysis provides insights into the role of Acidobacteria in organic carbon utilization in Arctic tundra soils. FEMS Microbiol Ecol 2012; 82:341-355 10.1111/j.1574-6941.2012.01381.x [DOI] [PubMed] [Google Scholar]
- 29.Rawat S, Männistö MK, Starovoytov V, Goodwin L, Nolan M, Hauser L, Land M, Davenport KW, Woyke T, Häggblom MM. Complete genome sequence of Terriglobus saanensis strain SP1PR4T, an Acidobacteria from tundra soil. Stand Genomic Sci 2012; 7:59-69 10.4056/sigs.3036810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Männistö MK, Tiirola M, Häggblom MM. Effect of freeze-thaw cycles on bacterial communities of Arctic tundra soil. Microb Ecol 2009; 58:621-631 10.1007/s00248-009-9516-x [DOI] [PubMed] [Google Scholar]
- 31.Männistö MK, Kurhela E, Tiirola M, Häggblom MM. Acidobacteria dominate the active bacterial communities of sub-Arctic tundra with widely divergent winter-time snow accumulation and soil temperatures. FEMS Microbiol Ecol 2013; 84:47-59 10.1111/1574-6941.12035 [DOI] [PubMed] [Google Scholar]
- 32.Field D, Garrity G, Gray T, Morrison N, Selengut J, Sterk P, Tatusova T, Thomson N, Allen MJ, Angiuoli SV, et al. The minimum information about a genome sequence (MIGS) specification. Nat Biotechnol 2008; 26:541-547 10.1038/nbt1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woese CR, Kandler O, Wheelis ML. Towards a natural system of organisms: proposal for the do-mains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci USA 1990; 87:4576-4579 10.1073/pnas.87.12.4576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Validation List No 143. Int J Syst Evol Microbiol 2012; 62:1-4 10.1099/ijs.0.039487-0 [DOI] [Google Scholar]
- 35.Thrash JC, Coates JD. Phylum XVII. Acidobacteria phyl. nov. In: Krieg NR, Staley JT, Brown DR, Hedlund BP, Paster BJ, Ward NL, Ludwig W, Whitman WB (eds), Bergey's Manual of Systematic Bacteriology, Second Edition, Volume 4, Springer, New York, 2011, p. 725. [Google Scholar]
- 36.Judicial Commission of the International Committee on Systematics of Prokaryotes The nomenclatural types of the orders Acholeplasmatales, Halanaerobiales, Halobacteriales, Methanobacteriales, Methanococcales, Methanomicrobiales, Planctomycetales, Prochlorales, Sulfolobales, Thermococcales, Thermoproteales and Verrucomicrobiales are the genera Acholeplasma, Halanaerobium, Halobacterium, Methanobacterium, Methanococcus, Methanomicrobium, Planctomyces, Prochloron, Sulfolobus, Thermococcus, Thermoproteus and Verrucomicrobium, respectively. Opinion 79. Int J Syst Evol Microbiol 2005; 55:517-518 10.1099/ijs.0.63548-0 [DOI] [PubMed] [Google Scholar]
- 37.Cavalier-Smith T. The neomuran origin of archaebacteria, the negibacterial root of the universal tree and bacterial megaclassification. Int J Syst Evol Microbiol 2002; 52:7-76 [DOI] [PubMed] [Google Scholar]
- 38.Ludwig W, Euzeby J, Whitman WG. Draft taxonomic outline of the Bacteroidetes, Planctomycetes, Chlamydiae, Spirochaetes, Fibrobacteres, Fusobacteria, Acidobacteria, Verrucomicrobia, Dictyoglomi, and Gemmatimonadetes http://www.bergeys.org/outlines/Bergeys_Vol_4_Outline.pdf Taxonomic Outline 2008.
- 39.Thrash JC, Coates JD. Family I. Acidobacteriaceae fam. nov. In: Krieg NR, Staley JT, Brown DR, Hedlund BP, Paster BJ, Ward NL, Ludwig W, Whitman WB (eds), Bergey's Manual of Systematic Bacteriology, Second Edition, Volume 4, Springer, New York, 2011, p. 728. [Google Scholar]
- 40.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 2000; 25:25-29 10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 2011; 28:2731-2739 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liolios K, Mavromatis K, Tavernarakis N, Kyrpides NC. The Genomes On Line Database (GOLD) in 2007: status of genomic and metagenomic projects and their associated metadata. Nucleic Acids Res 2007; 36:D475-D479 10.1093/nar/gkm884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DOE Joint Genome Institute http://www.jgi.doe.gov
- 44.Bennett S. Solexa Ltd. Pharmacogenomics 2004; 5:433-438 10.1517/14622416.5.4.433 [DOI] [PubMed] [Google Scholar]
- 45.Margulies M, Egholm M, Altman WE. Genome sequencing in microfabricated high-density picolitre reactors. Nature 2005; 437:376-380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zerbino DR, Birney E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res 2008; 18:821-829 10.1101/gr.074492.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ewing B, Hillier L, Wendl MC, Green P. Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res 1998; 8:175-185 10.1101/gr.8.3.175 [DOI] [PubMed] [Google Scholar]
- 48.Gordon D, Abajian C, Green P. Consed: a graphical tool for sequence finishing. Genome Res 1998; 8:195-202 10.1101/gr.8.3.195 [DOI] [PubMed] [Google Scholar]
- 49.The Phred/Phrap/Consed software package. http://www.phrap.com
- 50.Han CS, Chain P. Finishing repeat regions automatically with Dupfinisher CSREA Press. In: Arabnia AR, Valafar H, editors. Proceedings of the 2006 international conference on bioinformatics & computational biology; 2006; June 26-29. CSREA Press. p 141-146. [Google Scholar]
- 51.Hyatt D, Chen GL, Locascio PF, Land ML, Larimer FW, Hauser LJ. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics 2010; 11:119 10.1186/1471-2105-11-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pati A, Ivanova NN, Mikhailova N, Ovchinnikova G, Hooper SD, Lykidis A, Kyrpides NC. GenePRIMP: a gene prediction improvement pipeline for prokaryotic genomes. Nat Methods 2010; 7:455-457 10.1038/nmeth.1457 [DOI] [PubMed] [Google Scholar]
- 53.Tatusov RL, Koonin EV, Lipman DJ. A genomic perspective on protein families. Science 1997; 278:631-637 10.1126/science.278.5338.631 [DOI] [PubMed] [Google Scholar]
- 54.Clusters of Orthologous Groups http://www.ncbi.nlm.nih.gov/COG
- 55.Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res 1997; 25:955-964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lagesen K, Hallin P, Rodland EA, Staerfeldt HH, Rognes T, Ussery DW. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res 2007; 35:3100-3108 10.1093/nar/gkm160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Griffiths-Jones S, Bateman A, Marshall M, Khanna A, Eddy SR. Rfam: an RNA family database. Nucleic Acids Res 2003; 31:439-441 10.1093/nar/gkg006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 2001; 305:567-580 10.1006/jmbi.2000.4315 [DOI] [PubMed] [Google Scholar]
- 59.Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 2004; 340:783-795 10.1016/j.jmb.2004.05.028 [DOI] [PubMed] [Google Scholar]
- 60.Markowitz VM, Mavromatis K, Ivanova N, Chen IM, Chu K, Kyrpides N. Expert Review of Functional Annotations for Microbial Genomes. Bioinformatics 2009; 25:2271-2278 10.1093/bioinformatics/btp393 [DOI] [PubMed] [Google Scholar]
- 61.Carbohydrate-active enzymes. http://www.cazy.org