Abstract
Clostridium dakarense strain FF1T, is the type strain of Clostridium dakarense sp. nov., a new species within the genus Clostridium. This strain, whose genome is described here, was isolated from the fecal flora of a 4-month-old Senegalese child suffering from gastroenteritis. C. dakarense sp. nov. strain FF1T is an obligate anaerobic Gram-positive bacillus. Here we describe the features of this organism, together with the complete genome sequence and annotation. The 3,735,762 bp long genome (1 chromosome but no plasmid) exhibits a G+C content of 27.98% and contains 3,843 protein-coding and 73 RNA genes, including 8 rRNA genes.
Keywords: Clostridium dakarense, genome, culturomics, taxono-genomics
Introduction
Clostridium dakarense strain FF1T (= CSUR P243 = DSM 27086), is the type strain of Clostridium dakarense sp. nov. This bacterium is a Gram-positive, anaerobic, spore-forming, indole negative bacillus that was isolated from the stool of a 4-month-old Senegalese child suffering from gastroenteritis as part of a “culturomics” study aiming at cultivating individually all species within human feces.
The elevated cost and lack of intra- and inter-laboratory reproducibility of the “gold standard” of taxonomic tools. i.e. DNA-DNA hybridization and G+C content determination [1], put bacterial taxonomic classification in a precarious state. In addition, the internationally-validated cutoff values of 16S rRNA sequence comparison [2] do not apply to all validly published genera and species. Recently, high throughput genome sequencing and mass spectrometric analyses of bacteria have allowed unprecedented access to a wealth of genetic and proteomic information [3]. As a consequence, we proposed to use a polyphasic approach [4] to describe new bacterial taxa, including genome sequence, MALDI-TOF spectrum and main phenotypic characteristics [5-11].
The genus Clostridium (Prazmowski, 1880), classified among the Firmicutes, was created in 1880 [12] and consists of obligate anaerobic rod-shaped bacilli capable of producing endospores [12]. More than 180 Clostridium species have been described to date [13]. Members of the genus Clostridium are mostly environmental bacteria or associated with the commensal digestive flora of mammals, but several are major human pathogens, including C. botulinum, C. difficile, C. tetani and C. perfringens [14,15]. A few species, such as C. butyricum and C. pasteurianum, fix nitrogen and have gained importance in agricultural and industrial applications [16,17].
Here we present a summary classification and a set of features for C. dakarense sp. nov. strain FF1T (= CSUR P243 = DSM 27086) together with the description of the complete genomic sequencing and annotation. These characteristics support the circumscription of the species C. dakarense sp. nov.
Classification and features
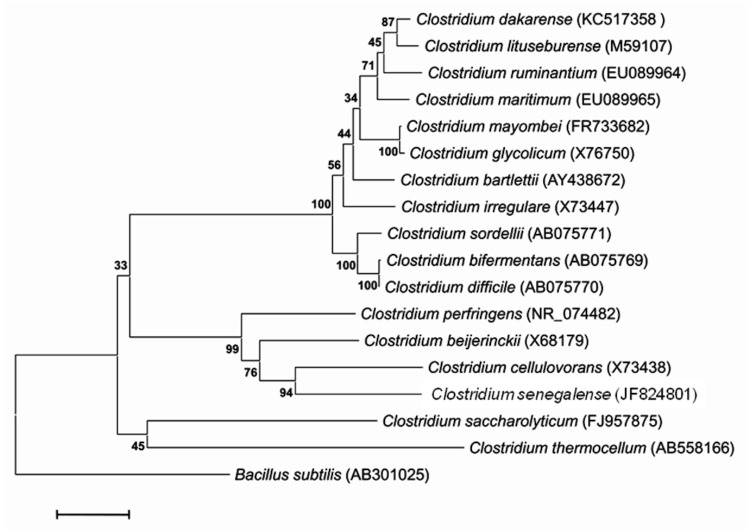
A stool specimen was collected from a 4-month-old Senegalese child suffering from gastroenteritis. Informed consent was obtained from the child’s parents and approval from the ethics committee from the Institut Federatif de Recherche 48 (Faculté de Médecine, Marseille, France). The fecal specimen was preserved at -20°C after collection and sent to Marseille. Strain FF1T (Table 1) was isolated in July 2011 by anaerobic cultivation on 5% sheep blood-enriched Columbia agar (BioMerieux, Marcy l’Etoile, France). This strain exhibited a 96.90% 16S rRNA nucleotide sequence similarity with C. lituseburense, the phylogenetically closest validated Clostridium species (Figure 1). Although sequence similarity of the 16S rRNA is not uniform across taxa, this value was lower than the 98.7% threshold recommended by Stackebrandt and Ebers to delineate a new species without carrying out DNA-DNA hybridization [30]. In addition, it was consistent with 16S rRNA identity values observed among validated species within the Clostridium genus that range from 78.4 to 99.8%.
Table 1. Classification and general features of Clostridium dakarense strain FF1T according to the MIGS recommendations [18].
| MIGS ID | Property | Term | Evidence codea |
|---|---|---|---|
| Domain Bacteria | TAS [19] | ||
| Phylum Firmicutes | TAS [20-22] | ||
| Class Clostridia | TAS [23,24] | ||
| Current classification | Order Clostridiales | TAS [25,26] | |
| Family Clostridiaceae | TAS [25,27] | ||
| Genus Clostridium | TAS [12,25,28] | ||
| Species Clostridium dakarense | IDA | ||
| Type strain FF1 | IDA | ||
| Gram stain | Positive | IDA | |
| Cell shape | Rod-shaped | IDA | |
| Motility | Motile | IDA | |
| Sporulation | Sporulating | IDA | |
| Temperature range | Mesophile | IDA | |
| Optimum temperature | 37°C | IDA | |
| MIGS-6.3 | Salinity | Growth in BHI medium + 5% NaCl | IDA |
| MIGS-22 | Oxygen requirement | Anaerobic | IDA |
| Carbon source | Unknown | NAS | |
| Energy source | Unknown | NAS | |
| MIGS-6 | Habitat | Human gut | IDA |
| MIGS-15 | Biotic relationship | Free living | IDA |
| MIGS-14 | Pathogenicity Biosafety level Isolation |
Unknown 2 Human feces |
NAS |
| MIGS-4 | Geographic location | Senegal | IDA |
| MIGS-5 | Sample collection time | June 2011 | IDA |
| MIGS-4.1 | Latitude | 13.7167 | IDA |
| MIGS-4.1 | Longitude | - 16.4167 | IDA |
| MIGS-4.3 | Depth | Surface | IDA |
| MIGS-4.4 | Altitude | 51 m above sea level | IDA |
aEvidence codes - IDA: Inferred from Direct Assay; TAS: Traceable Author Statement (i.e., a direct report exists in the literature); NAS: Non-traceable Author Statement (i.e., not directly observed for the living, isolated sample, but based on a generally accepted property for the species, or anecdotal evidence). These evidence codes are from the Gene Ontology project [29]. If the evidence is IDA, then the property was directly observed for a live isolate by one of the authors or an expert mentioned in the acknowledgements.
Figure 1.
Phylogenetic tree highlighting the position of C. dakarense sp. nov. strain FF1T relative to other type strains within the Clostridium genus. GenBank accession numbers are indicated in parentheses. Sequences were aligned using CLUSTALW, and phylogenetic inferences obtained using the maximum-likelihood method within the MEGA software. Numbers at the nodes are bootstrap values obtained by repeating 500 times the analysis to generate a majority consensus tree. Bacillus subtilis was used as an outgroup. The scale bar represents a 2% nucleotide sequence divergence.
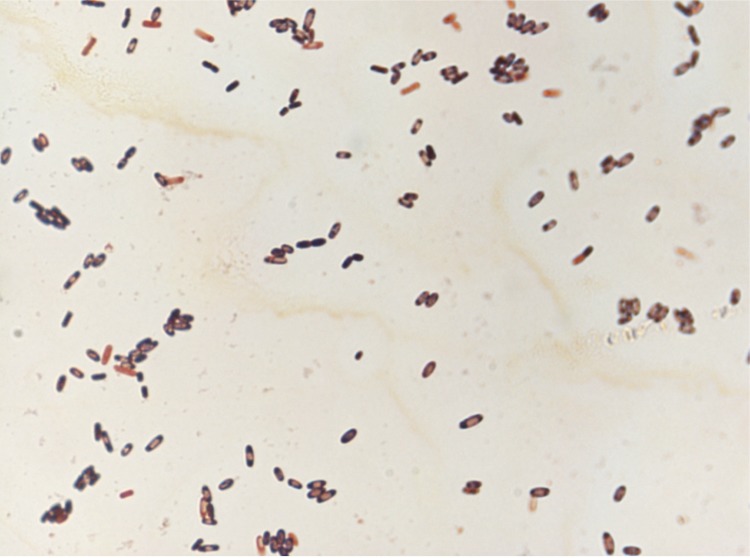
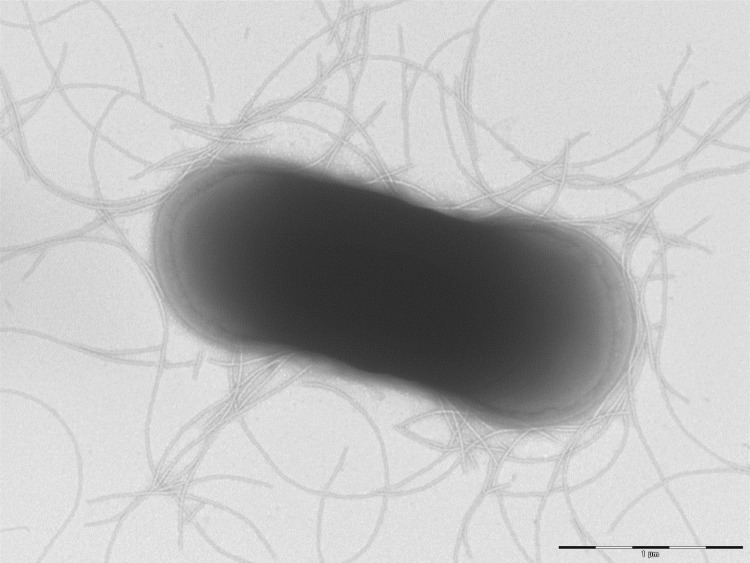
Different growth temperatures (25, 30, 37, 45 and 56°C) were tested. Growth was observed between 25 and 37°C, with optimal growth at 37°C after 24 hours of inoculation in anaerobic conditions. Colonies were 1.5 mm in diameter and opaque and smooth appearance on blood-enriched Columbia agar. Growth of the strain was tested under anaerobic and microaerophilic conditions using GENbag anaer and GENbag microaer systems, respectively (BioMerieux), and under aerobic conditions, with or without 5% CO2. The strain growth was obtained only in anaerobic conditions. Gram staining showed rod-shaped Gram-positive bacilli able to form spores (Figure 2). The motility test was positive. Cells grown on agar have a mean diameter of 1.2 µm (Figure 3).
Figure 2.
Gram staining of C. dakarense sp. nov. strain FF1T.
Figure 3.
Transmission electron microscopy of C. dakarense sp. nov. strain FF1T, using a Morgani 268D (Philips) at an operating voltage of 60kV. The scale bar represents 1 µm.
Strain FF1T exhibited neither catalase nor oxidase activities. Using API Rapid ID 32A (BioMerieux, Marcy l’Etoile), a positive reaction were observed for arginine dihydrolase, N-acetyl-β-glucosaminidase and pyroglutamic acid arylamidase. Negative reactions were observed for urease, indole and nitrate reduction. Using API 50 CH (BioMerieux, Marcy l’Etoile), positive reactions were observed for galactose, glucose, maltose and saccharose fermentation and negative reaction were observed for ribose, lactose and fructose. C. dakarense is susceptible to amoxicillin, metronidazole, vancomycin, imipenem and rifampicin and resistant to trimethoprim/ sulfamethoxazole. When compared with representative species from the genus Clostridium, C. dakarense strain FF1T exhibited the phenotypic differences detailed in Table 2.
Table 2. Differential characteristics of C. dakarense sp. nov. strain FF1T (Cda).
| Properties | CDa | CBa | CBe | CC | CDi | CG | CP | CSa | CSe | CT |
|---|---|---|---|---|---|---|---|---|---|---|
| Cell diameter (µm) | 1.2 | 1.5 | 1.7 | 2.5 | 3.0 | 0.4-1.0 | 1.3 | 3.0 | 1.1 | 2.5 |
| Oxygen requirement | - | - | - | - | - | - | - | - | - | na |
| Pigment production | - | - | - | - | + | + | + | na | - | + |
| Gram stain | + | + | V | - | + | + | - | + | - | |
| Salt requirement | - | na | na | na | na | - | - | na | - | na |
| Motility | + | - | + | - | + | + | - | - | + | - |
| Endospore formation | + | + | + | + | + | + | w | + | + | + |
| Production of | ||||||||||
| Acid phosphatase | + | + | na | na | na | na | + | na | na | na |
| Catalase | - | - | - | - | na | na | na | na | - | na |
| Oxidase | - | na | na | na | na | na | na | na | - | na |
| Nitrate reductase | - | - | - | na | - | - | + | + | - | - |
| Urease | - | - | - | na | na | na | na | na | - | na |
| β-galactosidase | - | + | na | na | na | - | + | na | - | na |
| Acid from | ||||||||||
| L-Arabinose | - | na | + | - | - | - | - | + | na | na |
| Ribose | - | + | - | - | - | + | w | na | na | |
| Mannose | - | - | + | + | - | + | na | na | na | |
| Mannitol | - | + | + | + | + | - | - | w | na | na |
| Sucrose | - | + | + | + | + | - | + | w | na | na |
| D-glucose | + | + | + | + | na | + | + | na | na | |
| D-fructose | - | + | + | + | + | + | + | + | na | na |
| D-maltose | + | + | + | + | - | + | + | w | na | na |
| D-lactose | - | na | + | + | - | - | + | w | na | na |
| Hydrolysis of | ||||||||||
| Gelatin | na | - | + | - | na | - | na | na | na | + |
| Starch | na | na | + | - | - | - | + | - | na | |
| G+C content (mol%) | 27.98 | 29.8 | 28 | 27 | 28 | 29 | 27 | 28 | 26.8 | 39 |
| Habitat | Human gut | Human gut | Human gut | Poplar wood | Human gut | Mud, wastewater | Colonic flora | Sewage sludge | Human gut | Sewage sludge |
C. bartlettii (CBa), C. beijerinckii (CBe), C. cellulovorans (CC), C. difficile (CDi), C. glycolicum (CG), C. perfringens (CP), C. saccharolyticum (CSa), C. senegalense (CSe) and C. thermocellum (CT).
na = data not available; w = weak, v = variable reaction
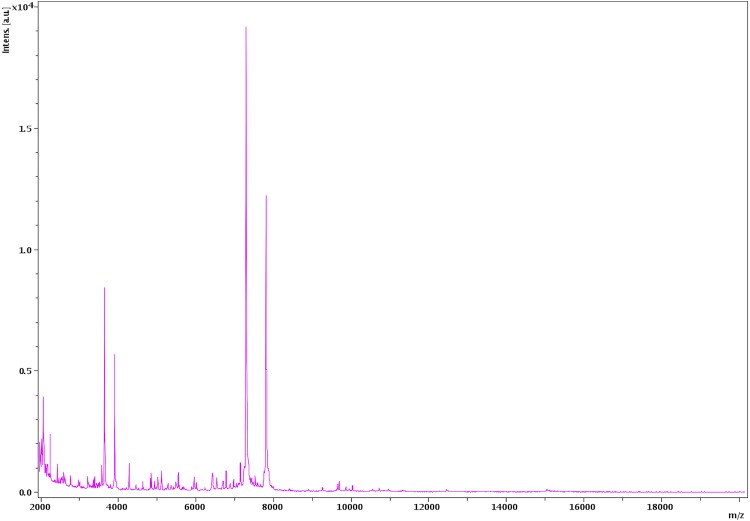
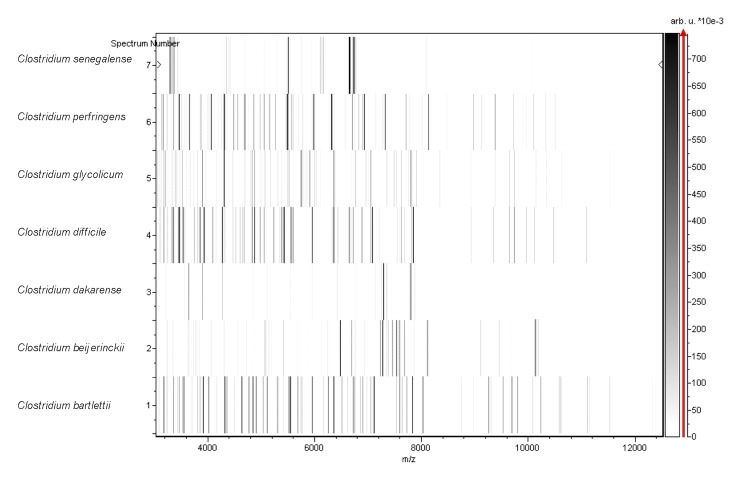
Matrix-assisted laser-desorption/ionization time-of-flight (MALDI-TOF) MS protein analysis was carried out as previously described [31]. Briefly, a pipette tip was used to pick one isolated bacterial colony from a culture agar plate, and to spread it as a thin film on a MTP 384 MALDI-TOF target plate (Bruker Daltonics, Leipzig, Germany). Eighteen distinct deposits were made for strain FF1T from eighteen isolated colonies. Each smear was overlaid with 2 µL of matrix solution (saturated solution of alpha-cyano-4-hydroxycinnamic acid) in 50% acetonitrile, 2.5% tri-fluoracetic-acid, and allowed to dry for five minutes. Measurements were performed with a Microflex spectrometer (Bruker). Spectra were recorded in the positive linear mode for the mass range of 2,000 to 20,000 Da (parameter settings: ion source 1 (IS1), 20 kV; IS2, 18.5 kV; lens, 7 kV). A spectrum was obtained after 675 shots at a variable laser power. The time of acquisition was between 30 seconds and 1 minute per spot. The eighteen spectra were imported into the MALDI BioTyper software (version 2.0, Bruker) and analyzed by standard pattern matching (with default parameter settings) against the main spectra of 4,706 bacteria including 216 spectra from validly published species of Clostridium, that are part of the reference data contained in the BioTyper database. The method of identification included the m/z from 2,000 to 20,000 Da. For every spectrum, 100 peaks at most were taken into account and compared with spectra in the database. A score enabled the identification, or not, from the tested species: a score > 2 with a validly published species enabled the identification at the species level, and a score < 1.7 did not enable any identification at the genus level. For strain FF1T, the maximal obtained score was lower than 1.9, thus suggesting that our isolate was not a member of a known species. We added the spectrum from strain FF1T to our database for future reference (Figure 4). Finally, the gel view allows us to highlight the spectrum differences with other members of the genus Clostridium (Figure 5).
Figure 4.
Reference mass spectrum from C. dakarense strain FF1T. Spectra from 18 individual colonies were compared and a reference spectrum was generated.
Figure 5.
Gel view comparing C. dakarense sp. nov. strain FF1T spectra with other members of the Clostridium genus (C. bartlettii, C. beijerinckii, C. difficile, C. glycolicum, C. perfringens, C. senegalense). The Gel View displays the raw spectra of all loaded spectrum files arranged in a pseudo-gel like look. The x-axis records the m/z value. The left y-axis displays the running spectrum number originating from subsequent spectra loading. The peak intensity is expressed by a Gray scale scheme code. The color bar and the right y-axis indicate the relation between the color a peak is displayed with and the peak intensity in arbitrary units.
Genome sequencing information
Genome project history
The organism was selected for sequencing on the basis of its phylogenetic position and 16S rRNA similarity to other members of the genus Clostridium, and is part of a “culturomics” study of the human digestive flora aiming at isolating all bacterial species within human feces. It was the 94th genome of a Clostridium species and the first genome of Clostridium dakarense sp. nov. The Genbank accession number is CBTZ00000000 and consists of 257 contigs. Table 3 shows the project information and its association with MIGS version 2.0 compliance [32].
Table 3. Project information.
| MIGS ID | Property | Term |
|---|---|---|
| MIGS-31 | Finishing quality | High-quality draft |
| MIGS-28 | Libraries used | One 454 paired end 3-kb library |
| MIGS-29 | Sequencing platforms | 454 GS FLX Titanium |
| MIGS-31.2 | Fold coverage | 35 |
| MIGS-30 | Assemblers | Newbler version 2.5.3 |
| MIGS-32 | Gene calling method | Prodigal |
| Genbank ID | CBTZ00000000 | |
| Genbank Date of Release | ||
| MIGS-13 | Project relevance | Study of the human gut microbiome |
Growth conditions and DNA isolation
C. dakarense sp. nov. strain FF1T (= CSUR P243 = DSM 27086), was grown anaerobically on sheep blood-enriched Columbia agar medium at 37°C. Eight petri dishes were spread and resuspended in 4x100µl of G2 buffer (EZ1 DNA Tissue kit, Qiagen). A first mechanical lysis was performed by glass powder on the Fastprep-24 device (Sample Preparation system) from MP Biomedicals, USA) using 2x20 seconds cycles. DNA was then treated with 2.5 µg/µL lysozyme (30 minutes at 37°C) and extracted through the BioRobot EZ 1 Advanced XL (Qiagen). The DNA was then concentrated and purified on a Qiamp kit (Qiagen). DNA concentration was 70.7ng/µl as determined by the Genios Tecan fluorometer, using the Quant-it Picogreen kit (Invitrogen).
Genome sequencing and assembly
This project was loaded twice on a 1/4 region for the paired-end application and once on a 1/8 region for the shotgun on PTP Picotiterplates. The shotgun library was constructed with 500 ng of DNA as described by the manufacturer (Roche) with the GS Rapid library Prep kit. For the paired-end sequencing, 5 µg of DNA was mechanically fragmented on the Hydroshear device (Digilab, Holliston, MA, USA) with an enrichment size of 3-4kb. The DNA fragmentation was visualized using an Agilent 2100 BioAnalyzer on a DNA labchip 7500, which yield an optimal size of 3.6 kb. The library was constructed according to the 454_Titanium paired-end protocol and manufacturer. Circularization and nebulization were performed and generated a pattern with an optimum at 561 bp. After PCR amplification through 15 cycles followed by double size selection, the single stranded paired end library was then quantified with Quant-it Ribogreen kit (Invitrogen) on the Genios_Tecan fluorometer at 52 pg/µL. The library concentration equivalence was calculated as 1.7E+08 molecules/µL. The library was stored at -20°C until use.
The shotgun library was clonally amplified with 3cpb in 3 emPCR reactions and the paired end library was amplified with lower cpb (1cpb) in 4 emPCR reactions with the GS Titanium SV emPCR Kit (Lib-L) v2. The yield of the emPCR was 5.37% for the shotgun reads and 19.27% for the paired-end reads, according to the quality expected by the range of 5 to 20% from the Roche procedure. A total of 340,000 beads from the 1/8 region of the shotgun reads and 790,000 beads from the 1/4 region of the paired-end reads were loaded on the GS Titanium PicoTiterPlates (PTP Kit 70×75) and sequenced with the GS Titanium Sequencing Kit XLR70.
The runs were performed overnight and then analyzed on the cluster through the gsRunBrowser and gsAssembler_Roche. The global 383,079 passed filter sequences generated 96.50 Mb with a length average of 277 bp. These sequences were assembled using the Newbler software from Roche with 90% identity and 40 bp as overlap. Fourteen scaffolds and 257 large contigs (>1500bp) were obtained, for a genome size of 3,735,762 bp.
Genome annotation
Open Reading Frames (ORFs) were predicted using Prodigal [33] with default parameters but the predicted ORFs were excluded if they spanned a sequencing gap region. The predicted bacterial protein sequences were searched against the GenBank database [34] and the Clusters of Orthologous Groups (COG) databases using BLASTP. The tRNAScanSE tool [35] was used to find tRNA genes, whereas ribosomal RNAs were found by using RNAmmer [36] and BLASTn against the GenBank database. Lipoprotein signal peptides and numbers of transmembrane helices were predicted using SignalP [37] and TMHMM [38] respectively. ORFans were identified if their BLASTP E-value was lower than 1e-03 for alignment length greater than 80 amino acids. If alignment lengths were smaller than 80 amino acids, we used an E-value of 1e-05. Such parameter thresholds have already been used in previous works to define ORFans. Artemis [39] was used for data management and DNA Plotter [40] was used for visualization of genomic features. Mauve alignment tool was used for multiple genomic sequence alignment and visualization [41].
To estimate the mean level of nucleotide sequence similarity at the genome level between C. dakarense and nine other members of the genus Clostridium (Table 6), we use the Average Genomic Identity of gene Sequences (AGIOS) home-made software. Briefly, this software combines the Proteinortho software [42] for detecting orthologous proteins between genomes compared two by two, then retrieves the corresponding genes and determines the mean percentage of nucleotide sequence identity among orthologous ORFs using the Needleman-Wunsch global alignment algorithm. Clostridium dakarense strain FF1T, was compared to C. bartlettii strain DSM 16795 (GenBank accession number NZ_DS499569), C. beijerinckii strain NCIMB 8052 (NC_009617), C. cellulovorans strain 743B (NC_014393), C. difficile strain 630 (NC8009089), C. glycolicum strain ATCC 14880 (ARES01000000), C. perfringens strain ATCC 13124 (BA000016), C. saccharolyticum strain WM1 (NC_014376), C. senegalense strain JC122T (CAEV00000000), and C. thermocellum strain ATCC 27405 (CP000568).
Table 6. Numbers of orthologous proteins shared between genomes (upper right) .
| CDa | CC | CBe | CP | CSe | CSa | CT | CBa | CG | CDi | |
|---|---|---|---|---|---|---|---|---|---|---|
| CDa | 3,808 | 1,045 | 1,230 | 1,089 | 1,131 | 1,013 | 806 | 1,324 | 1,690 | 1,203 |
| CC | 68.22 | 4,254 | 1,490 | 1,163 | 1,181 | 1,057 | 967 | 871 | 1,038 | 1,021 |
| CBe | 68.84 | 70.36 | 5,020 | 1,300 | 1,289 | 1,207 | 968 | 989 | 1,204 | 1,129 |
| CP | 70.02 | 70.43 | 72.15 | 2,660 | 1,168 | 920 | 777 | 845 | 1,005 | 1,147 |
| CSe | 69.91 | 70.37 | 70.82 | 70.13 | 3,704 | 930 | 821 | 856 | 1,134 | 1,008 |
| CSa | 61.94 | 62.50 | 62.44 | 62.22 | 62.05 | 4,154 | 854 | 833 | 1,004 | 998 |
| CT | 64.49 | 64.84 | 64.56 | 64.78 | 64.53 | 63.83 | 3,173 | 713 | 840 | 952 |
| CBa | 74.98 | 68.22 | 68.84 | 69.46 | 69.52 | 62.15 | 64.73 | 2,787 | 1,517 | 1,303 |
| CG | 75.70 | 68.28 | 68.83 | 69.49 | 69.57 | 62.26 | 64.59 | 76.04 | 3,840 | 1,568 |
| CDi | 71.34 | 69.57 | 68.52 | 71.52 | 65.49 | 66.37 | 64.32 | 74.45 | 74.50 | 3,798 |
average percentage similarity of nucleotides corresponding to orthologous protein shared between genomes (lower left) and numbers of proteins per genome (bold). CDa: C. dakarense; CC: C. cellulovorans; CBe: C. beijerinckii; CP: C. perfringens; CSe: C. senegalense; CSa: C. saccharolyticum; CT: C. thermocellum; CBa: C. bartlettii; CG: C. glycolicum; CDi: C. difficile.
Genome properties
The genome of C. dakarense sp. nov. strain FF1T is 3,735,762 bp long (1 chromosome, but no plasmid) with a 27,98% G + C content of (Figure 6 and Table 4). Of the 3,916 predicted genes, 3,843 protein-coding genes, and 73 were RNAs. Eight rRNA genes (one 16S rRNA, one 23S rRNA and six 5S rRNA) and 65 predicted tRNA genes were identified in the genome. A total of 2,769 genes (72.05%) were assigned a putative function (by COG or NR blast). Two hundred ninety-eight genes were identified as ORFans (7.75%). The remaining 515 genes were annotated as hypothetical proteins (13, 40%). The distribution of genes into COGs functional categories is presented in Table 4. The properties and the statistics of the genome are summarized in Tables 4 and 5.
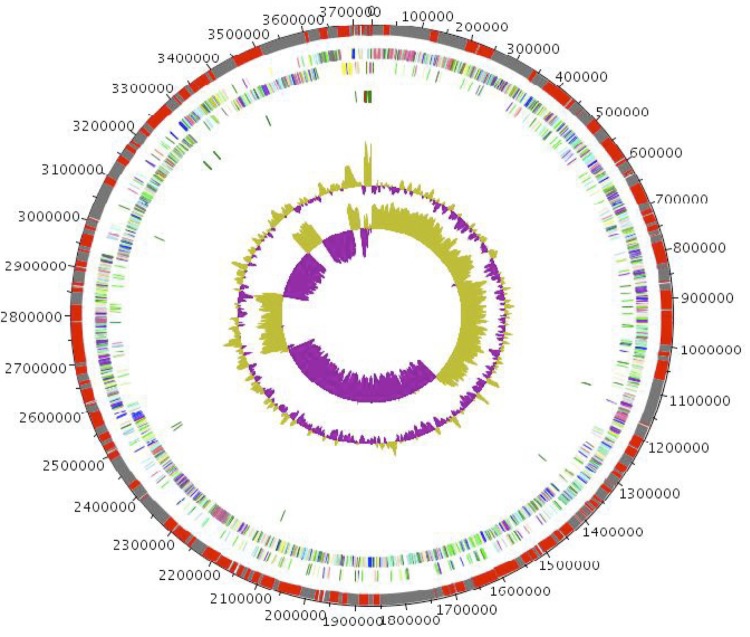
Figure 6.
Graphical circular map of the chromosome. From the outside in, the outer two circles show open reading frames oriented in the forward and reverse directions (colored by COG categories), respectively. The third circle marks the rRNA gene operon (red) and tRNA genes (green). The fourth circle shows the G+C% content plot. The inner-most circle shows the GC skew, purple and olive indicating negative and positive values, respectively.
Table 4. Nucleotide content and gene count levels of the genome.
| Attribute | Value | % of totala |
|---|---|---|
| Genome size (bp) | 3,735,762 | 100 |
| DNA coding region (bp) | 3,239,020 | 86.70 |
| DNA G+C content (bp) | 1,045,424 | 27.98 |
| Total genes | 3,916 | 100 |
| RNA genes | 73 | 1.86 |
| Protein-coding genes | 3,843 | 98.14 |
| Genes with function prediction | 2,769 | 72.05 |
| Genes assigned to COGs | 2,849 | 74.13 |
| Genes with peptide signals | 410 | 10.67 |
| Genes with transmembrane helices | 1,016 | 26.44 |
a The total is based on either the size of the genome in base pairs or the total number of protein coding genes in the annotated genome
Table 5. Number of genes associated with the 25 general COG functional categories.
| Code | Value | %agea | Description |
|---|---|---|---|
| J | 171 | 4.45 | Translation |
| A | 0 | 0 | RNA processing and modification |
| K | 325 | 8.46 | Transcription |
| L | 158 | 4.11 | Replication, recombination and repair |
| B | 1 | 0.03 | Chromatin structure and dynamics |
| D | 34 | 0.88 | Cell cycle control, mitosis and meiosis |
| Y | 0 | 0 | Nuclear structure |
| V | 111 | 2.89 | Defense mechanisms |
| T | 225 | 5.85 | Signal transduction mechanisms |
| M | 165 | 4.29 | Cell wall/membrane biogenesis |
| N | 58 | 1.51 | Cell motility |
| Z | 0 | 0 | Cytoskeleton |
| W | 0 | 0 | Extracellular structures |
| U | 45 | 1.17 | Intracellular trafficking and secretion |
| O | 95 | 2.47 | Posttranslational modification, protein turnover, chaperones |
| C | 194 | 5.04 | Energy production and conversion |
| G | 248 | 6.45 | Carbohydrate transport and metabolism |
| E | 248 | 6.45 | Amino acid transport and metabolism |
| F | 88 | 2.29 | Nucleotide transport and metabolism |
| H | 117 | 3.04 | Coenzyme transport and metabolism |
| I | 72 | 1.87 | Lipid transport and metabolism |
| P | 181 | 4.71 | Inorganic ion transport and metabolism |
| Q | 52 | 1.35 | Secondary metabolites biosynthesis, transport and catabolism |
| R | 386 | 10.04 | General function prediction only |
| S | 261 | 6.79 | Function unknown |
| - | 994 | 25.87 | Not in COGs |
a The total is based on the total number of protein coding genes in the annotated genome.
Comparison with the genomes from other Clostridium species
The genome sequence of Clostridium sp. is currently available for more than seventy-five Clostridium species. Here we compared the genome sequence of C. dakarense strain FF1T with than those of C. bartlettii, C. beijerinckii, C. cellulovorans, C. difficile, C. glycolicum, C. perfringens, C. saccharolyticum, C. senegalense, and C. thermocellum.
The draft genome sequence of C. dakarense strain FF1T is smaller than those of C. cellulovorans, C. beijerinckii, C. senegalense, C. saccharolyticum, C. thermocellum, C. difficile, C. glycolicum (3.73, 5.26, 6.0, 3.89, 4.66, 3.84, 4.3 and 3.99 Mb, respectively) but larger than those of C. perfringens and C. bartletii (3.26 and 2.97 Mb, respectively). The G+C content of C. dakarense is lower than those of C. cellulovorans, C. beijerinckii, C. perfringens, C. saccharolyticum, C. thermocellum, C. difficile (31.2, 29.9, 28.4, 45, 39 and 29.1%, respectively) but higher than those of C. bartlettii, C. glycolicum and C. senegalense (28.8, 28 and 26.8%, respectively). The gene content of C. dakarense is larger than those of C. thermocellum, C. senegalense, C. perfringens, C. glycolicum, C. bartlettii (3,916, 3,173, 3,761, 2,876, 3,840 and 2,787, respectively) and smaller than those of C. cellulovorans, C. beijerinckii, C. saccharolyticum and C. difficile, (4,501, 5,243, 4,154 and 4,019, respectively). The ratio of genes per Mb of C. dakarense is larger to those of C. cellulovorans, C. beijerinckii, C. senegalense, C. saccharolyticum, C. thermocellum, C. difficile, C. bartlettii, C. glycolicum and C. perfringens (1,049, 856, 874, 966, 891, 826, 934, 938, 962 and 882, respectively).
The number of orthologous genes shared between C. dakarense and other compared Clostridium species has been summarized in Table 6. The average percentage of nucleotide sequence identity ranged from 62.05 to 74.5% among previously published Clostridium species, and from 61.94 to 75.7% between C. dakarense and other studied Clostridium species, thus confirming its new species status.
Conclusion
On the basis of phenotypic, phylogenetic and genomic analyses, we formally propose the creation of Clostridium dakarense sp. nov. which contains strain FF1T. This bacterium strain has been isolated from the fecal flora of a 4-months-old Senegalese child suffering from gastroenteritis.
Description of Clostridium senegalense sp. nov.
Clostridium dakarense (da.kar.e′n.se. L. gen. neutr. n. dakarense, pertaining to, or originating from Dakar, the capital of Senegal, where the type strain was isolated).
Colonies were 1.5 mm in diameter on blood-enriched Columbia agar and Chocolate agar + PolyViteX. Cells are rod-shaped with a mean diameter of 1.2 μm. Optimal growth is achieved anaerobically. No growth is observed in aerobic conditions. Growth occurs between 25-37°C, with optimal growth observed at 37°C, in medium 5% sheep blood-enriched Columbia agar. Cells stain Gram-positive, are endospore-forming, and motile. Catalase, oxidase, urease, indole and nitrate reduction activity are absent. Arginine dihydrolase, N-acetyl-β-glucosanimidase and pyroglutamic acid arylamidase activity are present. Cells are susceptible to amoxicillin, metronidazole, vancomycin, imipenem and rifampicin but resistant to trimethoprim/sulfamethoxazole.
The G+C content of the genome is 27.98%. The 16S rRNA gene sequence and whole-genome shotgun sequence of C. dakarense strain FF1T (= CSUR P243 = DSM 27086) are deposited in GenBank under accession numbers KC517358 and CBTZ00000000, respectively. The type strain FF1T (= CSUR P243 = DSM 27086) was isolated from the fecal flora of a 4-months-old child in Dakar, Senegal.
Acknowledgements
The authors thank the Xegen Company (www.xegen.fr) for automating the genomic annotation process. This study was funded by the Mediterranee-Infection Foundation.
References
- 1.Rossello-Mora R. DNA-DNA Reassociation Methods Applied to Microbial Taxonomy and Their Critical Evaluation. In: Stackebrandt E (ed), Molecular Identification, Systematics, and population Structure of Prokaryotes. Springer, Berlin, 2006, p. 23-50. [Google Scholar]
- 2.Stackebrandt E, Ebers J. Taxonomic parameters revisited: tarnished gold standards. Microbiol Today 2006; 33:152-155 [Google Scholar]
- 3.Welker M, Moore ER. Applications of whole-cell matrix-assisted laser-desorption/ionization time-of-flight mass spectrometry in systematic microbiology. Syst Appl Microbiol 2011; 34:2-11 10.1016/j.syapm.2010.11.013 [DOI] [PubMed] [Google Scholar]
- 4.Tindall BJ, Rosselló-Móra R, Busse HJ, Ludwig W, Kämpfer P. Notes on the characterization of prokaryote strains for taxonomic purposes. Int J Syst Evol Microbiol 2010; 60:249-266 10.1099/ijs.0.016949-0 [DOI] [PubMed] [Google Scholar]
- 5.Kokcha S, Mishra AK, Lagier JC, Million M, Leroy Q, Raoult D, Fournier PE. Non contiguous-finished genome sequence and description of Bacillus timonensis sp. nov. Stand Genomic Sci 2012; 6:346-355 10.4056/sigs.2776064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lagier JC, El Karkouri K, Nguyen TT, Armougom F, Raoult D, Fournier PE. Non-contiguous finished genome sequence and description of Anaerococcus senegalensis sp. nov. Stand Genomic Sci 2012; 6:116-125 10.4056/sigs.2415480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mishra AK, Gimenez G, Lagier JC, Robert C, Raoult D, Fournier PE. Non-contiguous finished genome sequence and description of Alistipes senegalensis sp. nov. Stand Genomic Sci 2012; 6:304-314 10.4056/sigs.2625821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lagier JC, Armougom F, Mishra AK, Ngyuen TT, Raoult D, Fournier PE. Non-contiguous finished genome sequence and description of Alistipes timonensis sp. nov. Stand Genomic Sci 2012; 6:315-324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mishra AK, Lagier JC, Robert C, Raoult D, Fournier PE. Non-contiguous finished genome sequence and description of Clostridium senegalense sp. nov. Stand Genomic Sci 2012; 6:386-395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mishra AK, Lagier JC, Robert C, Raoult D, Fournier PE. Non-contiguous finished genome sequence and description of Peptoniphilus timonensis sp. nov. Stand Genomic Sci 2012; 7:1-11 10.4056/sigs.2956294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mishra AK, Lagier JC, Rivet R, Raoult D, Fournier PE. Non-contiguous finished genome sequence and description of Paenibacillus senegalensis sp. nov. Stand Genomic Sci 2012; 7:70-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prazmowski A. "Untersuchung über die Entwickelungsgeschichte und Fermentwirking einiger Bakterien-Arten." Ph.D. Dissertation, University of Leipzig, Germany, 1880, p. 366-371. [Google Scholar]
- 13.List of prokaryotic names with standing with nomenclature. http://www.bacterio.net [DOI] [PMC free article] [PubMed]
- 14.Evaluations and Standards Laboratory. “Identification of Clostridium species”, 2008 pp. 14. [Google Scholar]
- 15.Wells CL, Wilkins TD. (1996). Clostridia: Spore forming Anaerobic Bacilli In: Baron's Medical Microbiology (Baron S et al., eds.) (4th ed.). University of Texas Medical Branch. [Google Scholar]
- 16.Keis S, Shaheen R, David T. Jones Emended descriptions of Clostridium acetobutylicum and Clostridium beijerinckii, and descriptions of Clostridium saccharoperbutylacetonicum sp. nov. and Clostridium saccharobutylicum sp. nov. Int J Syst Evol Microbiol 2001; 51:2095-2103 10.1099/00207713-51-6-2095 [DOI] [PubMed] [Google Scholar]
- 17.Carnahan JE, Castle JE. Nitrogen Fixation. Annu Rev Plant Physiol 1963; 14:125-136 10.1146/annurev.pp.14.060163.001013 [DOI] [Google Scholar]
- 18.Field D, Garrity G, Gray T, Morrison N, Selengut J, Sterk P, Tatusova T, Thomson N, Allen MJ, Angiuoli SV, et al. The minimum information about a genome sequence(MIGS) specification. Nat Biotechnol 2008; 26:541-547 10.1038/nbt1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woese CR, Kandler O, Wheelis ML. Towards a natural system of organisms: proposal for the domains Archae, Bacteria, and Eukarya. Proc Natl Acad Sci USA 1990; 87:4576-4579 10.1073/pnas.87.12.4576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibbons NE, Murray RGE. Proposals Concerning the Higher Taxa of Bacteria. Int J Syst Bacteriol 1978; 28:1-6 10.1099/00207713-28-1-1 [DOI] [Google Scholar]
- 21.Garrity GM, Holt JG. The Road Map to the Manual. In: Garrity GM, Boone DR, Castenholz RW (eds), Bergey's Manual of Systematic Bacteriology, Second Edition, Volume 1, Springer, New York, 2001, p. 119-169. [Google Scholar]
- 22.Murray RGE. The Higher Taxa, or, a Place for Everything...? In: Holt JG (ed), Bergey's Manual of Systematic Bacteriology, First Edition, Volume 1, The Williams and Wilkins Co., Baltimore, 1984, p. 31-34. [Google Scholar]
- 23.List of new names and new combinations previously effectively, but not validly, published. List no. 132. Int J Syst Evol Microbiol 2010; 60:469-472 10.1099/ijs.0.022855-0 [DOI] [PubMed] [Google Scholar]
- 24.Rainey FA. Class II. Clostridia class nov. In: De Vos P, Garrity G, Jones D, Krieg NR, Ludwig W, Rainey FA, Schleifer KH, Whitman WB (eds), Bergey's Manual of Systematic Bacteriology, Second Edition, Volume 3, Springer-Verlag, New York, 2009, p. 736. [Google Scholar]
- 25.Skerman VBD, Sneath PHA. Approved list of bacterial names. Int J Syst Bacteriol 1980; 30:225-420 10.1099/00207713-30-1-225 [DOI] [Google Scholar]
- 26.Prévot AR. In: Hauderoy P, Ehringer G, Guillot G, Magrou. J., Prévot AR, Rosset D, Urbain A (eds), Dictionnaire des Bactéries Pathogènes, Second Edition, Masson et Cie, Paris, 1953, p. 1-692. [Google Scholar]
- 27.Pribram E. Klassification der Schizomyceten. Klassifikation der Schizomyceten (Bakterien), Franz Deuticke, Leipzig, 1933, p. 1-143. [Google Scholar]
- 28.Smith LDS, Hobbs G. Genus III. Clostridium Prazmowski 1880, 23. In: Buchanan RE, Gibbons NE (eds), Bergey's Manual of Determinative Bacteriology, Eighth Edition, The Williams and Wilkins Co., Baltimore, 1974, p. 551-572. [Google Scholar]
- 29.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 2000; 25:25-29 10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stackebrandt E, Ebers J. Taxonomic parameters revisited: tarnished gold standards. Microbiol Today 2006; 33:152-155 [Google Scholar]
- 31.Seng P, Drancourt M, Gouriet F, La Scola B, Fournier PE, Rolain JM, Raoult D. Ongoing revolution in bacteriology: routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin Infect Dis 2009; 49:543-551 10.1086/600885 [DOI] [PubMed] [Google Scholar]
- 32.Field D, Garrity G, Gray T, Morrison N, Selengut J, Sterk P, Tatusova T, Thomson N, Allen MJ, Angiuoli SV, et al. The minimum information about a genome sequence (MIGS) specification. Nat Biotechnol 2008; 26:541-547 10.1038/nbt1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prodigal. http://prodigal.ornl.gov
- 34.GenBank database. http://www.ncbi.nlm.nih.gov/genbank
- 35.Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res 1997; 25:955-964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lagesen K, Hallin P, Rodland EA, Staerfeldt HH, Rognes T, Ussery DW. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res 2007; 35:3100-3108 10.1093/nar/gkm160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 2004; 340:783-795 10.1016/j.jmb.2004.05.028 [DOI] [PubMed] [Google Scholar]
- 38.Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 2001; 305:567-580 10.1006/jmbi.2000.4315 [DOI] [PubMed] [Google Scholar]
- 39.Rutherford K, Parkhill J, Crook J, Horsnell T, Rice P, Rajandream MA, Barrell B. Artemis: sequence visualization and annotation. Bioinformatics 2000; 16:944-945 10.1093/bioinformatics/16.10.944 [DOI] [PubMed] [Google Scholar]
- 40.Carver T, Thomson N, Bleasby A, Berriman M, Parkhill J. DNAPlotter: circular and linear interactive genome visualization. Bioinformatics 2009; 25:119-120 10.1093/bioinformatics/btn578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Darling AC, Mau B, Blattner FR, Perna NT. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res 2004; 14:1394-1403 10.1101/gr.2289704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lechner M, Findeib S, Steiner L, Marz M, Stadler PF, Prohaska SJ. Proteinortho: Detection of (Co-)orthologs in large-scale analysis. BMC Bioinformatics 2011; 12:124 10.1186/1471-2105-12-124 [DOI] [PMC free article] [PubMed] [Google Scholar]