Abstract
Purpose: To evaluate the relative diagnostic value of nonmydriatic fundus photography (nFP) among patients screened for diabetic retinopathy in remote rural medical clinics and an urban academic medical center for nonadherence to recommended annual dilated eye examination. Subjects and Methods: A retrospective cross-sectional study was performed among diabetic patients seen in primary outpatient clinics between 2006 and 2011 who were screened for diabetic retinopathy with nFP for history of nonadherence to recommended annual dilated eye examination. A single nonstereoscopic, 45°, 10-megapixel digital image of the disc and macula of both eyes was obtained locally and transmitted electronically to a retinal specialist for remote review. The results from remote rural Native American Indian reservations were compared with those from an urban academic family practice clinic. The proportion of subjects diagnosed with diabetic retinopathy and the quality of fundus images were compared. Results: Among 872 patients (1,744 eyes) screened from rural sites and 517 subjects (1,034 eyes) screened from an urban site, images were of good quality for evaluation in 82.4% and 85.7% of subjects, respectively. Diabetic retinopathy was noted in 12.6% of rural subjects and 29.6% of urban subjects (p<0.001). Conclusions: nFP can be a useful tool in both rural and urban settings to screen for diabetic retinopathy in patients who are nonadherent to the recommended dilated annual eye exam. In our study population, a surprisingly higher percentage of diabetic subjects screened from the urban clinic had retinopathy compared with subjects screened in rural clinics.
Key words: : teleophthalmology, telemedicine, diabetic retinopathy, rural, urban, screening, nonmydriatic fundus photography
Introduction
Diabetes mellitus is a chronic illness that affects 25.8 million people, or 8.3% of the U.S. population.1 The most common ocular presentation is diabetic retinopathy, which is the leading cause of blindness among middle-aged adults in the United States.2 Approximately 40–45% of Americans who are diagnosed with diabetes mellitus already have some degree of diabetic retinopathy at the time of diagnosis.3
Vision loss associated with diabetic retinopathy can be prevented or minimized with tighter control of blood glucose, blood pressure, and cholesterol and appropriate timely ophthalmologic care.4 Large randomized prospective studies have demonstrated the benefit of laser photocoagulation and intravitreal injections of corticosteroids and inhibitors of vascular endothelial growth factors in minimizing vision loss associated with complications of diabetic retinopathy.5–8 These treatments have become standard of care. However, because the patients with vision-threatening diabetic retinopathy are often asymptomatic during the period in which treatments such as laser photocoagulation or intravitreal injections can be applied, regular screening of asymptomatic patients by an eye care specialist is needed to minimize the risk of irreversible vision loss.9 An annual dilated eye exam is recommended for diabetic patients1; however, ophthalmology services are not always readily or widely accessible in remote rural areas.10 In urban areas where ophthalmology services are available, many patients (perhaps as many as 40–50%11) are not screened annually because of nonadherence to recommended guidelines.12–14 Teleophthalmology is therefore a cost-effective means for screening diabetic retinopathy15 and in fact less expensive than conventional retinal examination.16 This technology may reduce traveling and time for the patient and provider.17 It may also provide an alternative method with greater convenience and access for the remote and indigent populations.
The purpose of this study is to evaluate the diagnostic value of diabetic retinopathy screening using nonmydriatic fundus photography (nFP) in remote rural sites and an urban academic medical center among diabetic patients seen in primary care clinics who reported no recent history of an annual dilated fundoscopic examination as routinely recommended. Previously, we showed that this method of screening is a useful tool to access and triage these diabetic patients for follow-up comprehensive eye examination.18
Subjects and Methods
This is a retrospective review of all patients screened for diabetic retinopathy using nFP between July 2006 and May 2011 and whose electronic images were reviewed by a retinal specialist (S.S.P.) remotely. The patients were seen either at rural medical clinics in one of nine remote California Native American Indian Reservations (i.e., Consolidated Tribal Health Project, Inc., Greenville Rancheria's Community Clinic, Karuk Tribal Health Clinic, Lake County Tribal Health Consortium, Inc., Pit River Health Service, Inc., Round Valley Indian Health Center, Sycuan Medical Clinic, Toiyabe Indian Health Project, Inc., and Tule River Indian Health Center, Inc.) or at an urban family practice clinic at the University of California Davis Medical Center in Sacramento, CA. The study was conducted according to a protocol approved by the Office of Human Research Protection at the University of California Davis School of Medicine and in accordance to the tenets of the Declaration of Helsinki. The study was approved by the University of California Davis Office of Human Research Protection.
All patients seen in the above medical clinics with diabetes mellitus who have not had a dilated fundus examination within the past year, as reported by the patient and confirmed by available medical records, were included in the study. They were imaged at their respective outpatient primary care clinic using nFP. A single nonstereoscopic 45° image of the disc and macula of both eyes was taken using a digital 10-megapixel camera by trained medical personnel. The imager was allowed to re-image an eye if the imager determined the quality was poor owing to reasons such as patient blink, alignment, or poor fixation. A single best-quality image in a given session for each patient was uploaded for remote interpretation. The rural clinics used the Topcon (Tokyo, Japan) TRC-NW6S nonmydriatic retinal camera linked to a 10-megapixel Nikon (Tokyo) D80 camera and uploaded the images to an electronic database called EyePACS picture archive communication system (Fig. 1). The urban clinic used the Nidek (Fremont, CA) AFC-210 nonmydriatic fundus camera with 10-megapixel resolution, and the images were also uploaded to an online electronic imaging database called ANKA (Fig. 2). All digital images, from both rural and urban settings, were reviewed by the same retinal specialist from the University of California Davis Eye Center (S.S.P.) using the same liquid crystal display monitor of 1280×800 resolution. The findings were documented electronically into EyePACS for rural sites and in the patient's electronic medical record for the urban center. The diagnosis of diabetic retinopathy was made by the presence of any signs of diabetic retinopathy including dot-blot hemorrhage, microaneurysms, cotton wool spots, exudates, and abnormal neovascularization. Other incidental fundus photograph findings, other than diabetic retinopathy, were also documented by the reviewer.
Fig. 1.
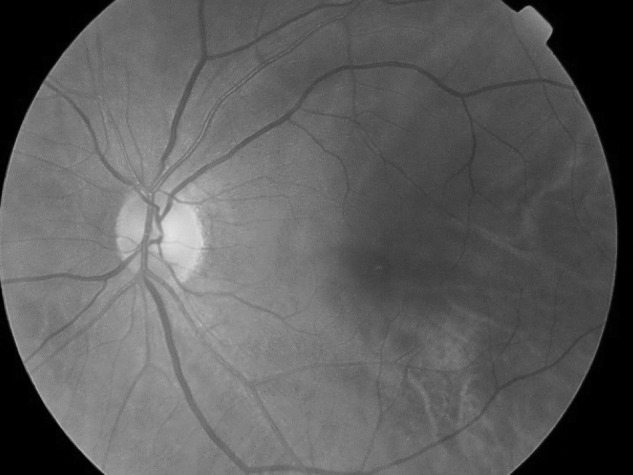
Normal 45° fundus photograph of a diabetic patient screened at a rural medical clinic in the California Native American Indian reservations, using a Topcon TRC-NW6S nonmydriatic fundus camera with 10-megapixel resolution, remotely accessible via the EyePACS picture archive communication system.
Fig. 2.
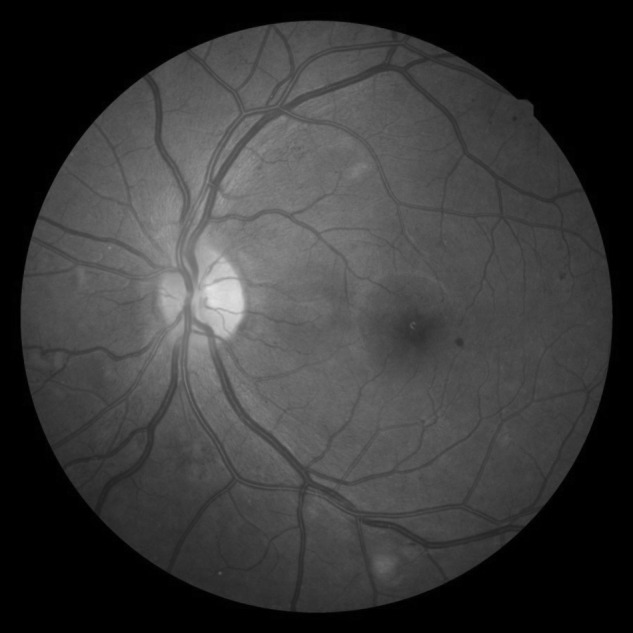
Diabetic retinopathy diagnosed with a 45° fundus photograph of a diabetic patient screened at the family practice clinic at the University of California Davis Medical Center, using a Nidek AFC-210 nonmydriatic fundus camera with 10-megapixel resolution, remotely accessible via ANKA.
In this chart review, the patient's demographic information including ethnicity and medical information, including hemoglobin A1c (HbA1c), which was obtained either within 3 months of the time of nFP or the most recent value available from the time of clinical encounter were recorded. Data handling, calculations, and statistical analysis using unpaired t tests and chi-square tests were performed using Microsoft® (Redmond, WA) Excel® with the WinSTAT® Add-In. A statistically significant difference was defined as p<0.05.
Results
The demographics of all subjects with diabetes mellitus screened for diabetic retinopathy using nFP in rural and urban sites can be found in Table 1. In total, 872 patients from the rural sites and 517 patients from the urban center were included in the study. The mean age of the subjects evaluated from the rural sites was 52.9±26.4 years (range, 1 month–91 years). Similarly, the mean age of the subjects seen in the urban center was 57.2±25.8 years (range, 27–93 years). In the rural sites, 59.2% of subjects were female. In the urban center, 55.5% of subjects were female.
Table 1.
Demographics of Subjects from Rural Versus Urban Settings Screened for Diabetic Retinopathy Using Nonmydriatic Fundus Photography
| RURAL SITES | URBAN CENTER | P VALUE | |
|---|---|---|---|
| Total number of subjects | 872 | 517 | — |
| Age [mean±SD (range)] | 52.9±26.4 (1–91) | 57.2±25.8 (27–93) | <0.001 |
| Gender (% female) | 59.2% | 55.5% | 0.37 |
| Ethnicity (%) | |||
| American Indian and Alaska Native | 60.1% | 1.4% | <0.001 |
| White, non-Hispanic | 3.0% | 27.3% | |
| Black | 0.1% | 21.9% | |
| Hispanic or Latino | 0.1% | 19.3% | |
| Asian | 0.2% | 17.0% | |
| Native Hawaiian and other Pacific Islander | 0.1% | 2.9% | |
| Unspecified | 36.4% | 10.3% | |
SD, standard deviation.
Among subjects from rural sites, ethnic origins of the subjects were 60.1% Native American Indian and/or Alaskan, 3.0% white non-Hispanic, 0.2% Asian, 0.1% black, 0.1% Native Hawaiian and other Pacific Islander, 0.1% Hispanic or Latino, and 36.4% unspecified. Among subjects from the urban academic center, ethnic origins of the subjects were 27.3% white non-Hispanic, 21.9% black, 19.3% Hispanic or Latino, 17.0% Asian, 2.9% Native Hawaiian and other Pacific Islander, 1.4% Native American Indian and/or Alaskan, and 10.3% unspecified.
The results of the fundus photograph interpretations from both rural and urban settings are summarized in Table 2. Among eyes imaged, 17.6% of the photographs from rural sites and 14.3% of the photographs from the urban academic center were of poor (or insufficient) quality for interpretation. This included photographs that were exceedingly blurry or decentered (such as an image that visualized the macula but did not include the optic nerve). This classification did not include those images that were lost in electronic transmission (i.e., 5.2% from the urban setting and 0.1% from rural sites). Among the images of adequate quality from the rural sites, the fundus photographs showed evidence of fundus abnormalities in 26.7% of the patients, 12.6% had signs of diabetic retinopathy, and 14.1% had fundus abnormalities other than diabetic retinopathy. Among subjects from the urban setting with fundus images of adequate quality for interpretation, 43.9% had fundus abnormalities, 29.6% had signs of diabetic retinopathy, and 14.3% had fundus abnormalities other than diabetic retinopathy. The difference in prevalence of diabetic retinopathy between images obtained from the rural clinics compared with that of the urban clinic was statistically significant (p<0.001). In contrast, there was no difference in the prevalence of diagnosing other fundus abnormalities among the rural and urban population except for hypertensive retinopathy and optic nerve head cupping (p<0.05), with the latter being the most common fundus abnormality noted other than diabetic retinopathy. In this study, optic nerve head cupping was defined as having a cup-to-disc ratio of >0.5 or asymmetrical optic nerve head cupping of >0.2 between the two eyes. Optic nerve head cupping in one or both eyes was seen in 10.5% of patients from the rural sites and 7.6% from the urban setting. Other fundus abnormalities noted with nFP included optic nerve disease, retinal scarring, pigmented lesions, and other vascular and vitreous pathology (Table 3).
Table 2.
Summary of Retrospective Review of Results of Diabetic Retinopathy Screening Using Nonmydriatic Fundus Photography
| RURAL SITES | URBAN SITE | P VALUE | |
|---|---|---|---|
| Total number of subjects | 872 | 517 | — |
| Fundus photographs with adequate image qualitya | 1,437 (82.4%) | 886 (85.7%) | <0.05 |
| Normal fundus | 639 (73.3%) | 290 (56.1%) | <0.001 |
| Signs of diabetic retinopathy | 110 (12.6%) | 153 (29.6%) | <0.001 |
| Fundus abnormalities other than diabetic retinopathy | 123 (14.1%) | 74 (14.3%) | 0.94 |
Image quality was deemed adequate if either good or excellent quality as per the retinal specialist's interpretation.
Table 3.
Incidental Fundus Abnormalities Found During Diabetic Retinopathy Screening Using Nonmydriatic Fundus Photography
| RURAL SITES | URBAN CENTER | P VALUE | |
|---|---|---|---|
| Total number of fundus photographs | 1,744 | 1,034 | — |
| Hypertensive retinopathya | 1 (0.1%) | 6 (0.6%) | <0.05 |
| Optic nerve cupping (cup:disc >0.5) | 183 (10.5%) | 79 (7.6%) | <0.05 |
| Optic nerve disease unspecifiedb | 11 (0.6%) | 7 (0.7%) | 0.81 |
| Retinal scarringc | 18 (1.0%) | 13 (1.3%) | 0.46 |
| Pigmented lesionsd | 2 (0.1%) | 3 (0.3%) | 0.17 |
| Vascular pathologye | 7 (0.4%) | 5 (0.5%) | 0.77 |
| Vitreous pathologyf | 3 (0.2%) | 3 (0.3%) | 0.68 |
| Otherg | 6 (0.3%) | 5 (0.5%) | 0.55 |
Includes copper wiring, silver wiring, flame hemorrhages.
Optic nerve drusen, optic nerve pallor, optic nerve gliosis, myelinated nerve fiber layer.
Pan retinal photocoagulation; macular/retinal scarring, presumed ocular histoplasmosis syndrome.
Congenital hypertrophy of the retinal pigment epithelium, choroidal nevi, fundus heterochromia.
Retinal vein occlusion; sclerosed vessels, lipemic vessels, arteriovenous nicking.
Posterior vitreous detachment, vitreous hemorrhage.
Epiretinal membrane, amelanotic neovascular membrane, fleck crystals.
Because there was a significant difference in the incidence of diabetic retinopathy being diagnosed by nFP between the rural and urban patient populations, the HbA1c values of these two patient populations were compared (Table 4). Because the HbA1c values for the rural population were only accessible via data entered into EyePACS, only 16.7% of patients seen in the rural clinic sites had an HbA1c measurement available for interpretation within 3 months of nFP imaging, in contrast to the 96.9% of patients seen in the urban clinic whose HbA1c values were accessible via electronic medical records. Nonetheless, the mean HbA1c of patients seen in the rural sites was 8.3±2.1%, which was comparable to the value of 8.3±2.2% noted among patients from the urban setting.
Table 4.
Summary of Hemoglobin A1c Screening and Prevalence of Diabetic Retinopathy in Patients Seen in Remote Rural Medical Clinics Versus an Urban Medical Center
| RURAL SITES | URBAN CENTER | P VALUE | |
|---|---|---|---|
| Total number of subjects | 872 | 517 | — |
| Number of HbA1c availablea | 146 (16.7%) | 501 (96.9%) | <0.001 |
| HbA1c value [mean±SD (range)] | 8.3±2.1 (5–16.7) | 8.3±2.2 (5.3–13.9) | 0.85 |
| Number of diabetic retinopathy | 110 (12.6%) | 153 (29.6%) | <0.001 |
Hemoglobin A1c (HbA1c) serum test provided within 3 months of nonmydriatic fundoscopic photography imaging.
SD, standard deviation.
A summary of HbA1c levels around the time of imaging and the prevalence of diabetic retinopathy in all clinic sites combined as sorted by ethnicity can be found in Table 5. Diabetic retinopathy was detected in 12.1% of Native American Indian and/or Alaskan, 25.8% of white non-Hispanic, 30.7% of African American or black, 26.7% of Hispanic or Latino, 34.4% of Asian, 31.3% of Native Hawaiian and other Pacific Islander, and 15.7% of subjects with unreported racial origins. The difference in the prevalence of diabetic retinopathy among ethnic populations was statistically significant (p<0.001) through chi-squared analysis. It is of note that >90% of Native American Indian and/or Alaskan and unspecified ethnicity subjects were from rural sites in this study.
Table 5.
Racial Differences in Hemoglobin A1c and Prevalence of Diabetic Retinopathy in Urban and Rural Sites Combined at the Time of Fundus Photography
| ETHNICITY | NUMBER OF SUBJECTS | HBA1C [MEAN±SD (RANGE)] | NUMBER (%) WITH DR |
|---|---|---|---|
| American Indian and Alaska Native | 531 (38.2%) | 8.5±2.3 (5.3–16.2) | 64 (12.1%) |
| White non-Hispanic | 167 (12.0%) | 7.9±2.0 (5–14) | 43 (25.8%) |
| Black | 114 (8.2%) | 8.5±2.4 (5.1–16.7) | 35 (30.7%) |
| Hispanic or Latino | 101 (7.3%) | 8.8±2.3 (5.6–16.1) | 27 (26.7%) |
| Asian | 90 (6.5%) | 8.2±2.1 (5.3–14.6) | 31 (34.4%) |
| Native Hawaiian and other Pacific Islander | 16 (1.2%) | 8.4±1.7 (5.9–11.2) | 5 (31.3%) |
| Unspecified | 370 (26.6%) | 7.9±1.9 (5.4–13.1) | 58 (15.7%) |
DR, diabetic retinopathy; HbA1c, hemoglobin A1c; SD, standard deviation.
Table 6 shows the distribution of subjects screened among the nine different rural clinics. Some variations in image quality were noted depending on the volume of screened subjects, but the majority of images were good quality from all the clinics. Although some variations were noted among the clinics in terms of mean HbA1c and proportion of eyes being diagnosed with diabetic retinopathy during screening, all sites had <24% prevalence of retinopathy and mean HbA1c of <8.9%.
Table 6.
Distribution of Patients and Findings Among the Nine Rural Medical Clinics
| RURAL SITE | NUMBER OF SUBJECTS | SIGNS OF DR | % ADEQUATE IMAGE QUALITY | % HBA1C AVAILABLE | HBA1C [MEAN±SD (RANGE)] |
|---|---|---|---|---|---|
| Consolidated Tribal Health Project, Inc. | 47 | 5 (10.6%) | 88.3% | 91.5% | 8.1±1.8 (5.6–13.2) |
| Greenville Rancheria's Community Clinic | 55 | 4 (7.3%) | 60.0% | 20.0% | 7.1±2.2 (5.5–13.5) |
| Karuk Tribal Health Clinic | 36 | 0 | 92.9% | 48.6% | 7.9±1.9 (5.9–11.4) |
| Lake County Tribal Health Consortium, Inc. | 138 | 33 (23.9%) | 93.5% | 43.5% | 8.8±2.2 (5.3–13.9) |
| Pit River Health Service, Inc. | 46 | 6 (13.0%) | 65.2% | 8.7% | 7.7±0.2 (7.6–7.9) |
| Round Valley Indian Health Center | 199 | 16 (8.0%) | 72.6% | 0.0% | NA |
| Sycuan Medical Clinic | 11 | 1 (9.1%) | 59.1% | 81.9% | 8.9±2.7 (5.8–13.3) |
| Toiyabe Indian Health Project, Inc. | 284 | 44 (15.5%) | 90.9% | 0.0% | NA |
| Tule River Indian Health Center, Inc. | 57 | 1 (1.75%) | 76.3% | 3.5% | 6.8±0.5 (6.4–7.1) |
HbA1c, hemoglobin A1c; NA, not available; SD, standard deviation.
Discussion
An annual dilated eye examination is generally recommended among diabetic patients in order to screen for diabetic retinopathy. More frequent dilated examination is warranted in the event of more severe retinopathy such that appropriate treatment can be administered before vision is affected.18 Unfortunately, adherence to recommended annual eye examinations is unacceptably low in both rural and urban settings.19,20 The poor screening rate in remote rural areas can be partially attributed to limited access to eye care specialists. In urban settings where access to an eye care specialist is not an issue among patients with health insurance coverage, the reason for poor rate of diabetic retinopathy screening is likely related to nonadherence, possibly resulting from poor patient awareness. Among our urban study population, transportation should not have been an issue because the medical clinic and the eye clinic are located in the same building. Because most patients with diabetes mellitus in both rural and urban settings generally visit their primary care physician regularly for medication refills, for example, this study assessed the diagnostic value of using nFP in the primary care setting for diabetic retinopathy screening as a first step to improve the rate of screening for diabetic retinopathy in both rural and urban settings.
Nonmydriatic fundus cameras allow for acquisition of high-quality digital fundus images.10,21 A single central 45° fundus image can be obtained without pharmacologic dilation by noncertified photographers with minimal training because the cameras have autofocusing capacity to image the disc and macula. In our study, the ease of use was evident by the high percentage of images that were of adequate quality (i.e., good or excellent) for interpretation from both rural and urban locations (82.4% and 85.7%, respectively). However, it is noteworthy that different cameras were used by the urban clinic (Nidek AFC-201) and the rural clinics (Topcon TRC-NW6S). Despite the same image resolution of 10 megapixels and angle of imaging, there may be inherent differences in the camera and the camera software (e.g., contrast, brightness, etc.) that theoretically may make the ease in diagnosing retinopathy differ. However, this is unlikely because, as shown by comparing Figures 1 and 2, comparable image quality was obtained with the two cameras for diagnosing retinopathy.
The present study evaluated the diagnostic value of nFP in detecting diabetic retinopathy. All screened patients were triaged for follow-up eye examination with an eye care provider based on the fundus photographic findings. Those with no signs of retinopathy were triaged for an annual eye exam, whereas those with detectable retinopathy were seen by an eye care provider within weeks to months, depending on the findings. Among our urban patient population, we recently showed a good correlation between the degree of retinopathy noted on the single 45° fundus image and findings on follow-up dilated eye examination.18 This is in contrast to some previous studies suggesting that a single central 45° fundus image may have good sensitivity and specificity to determine absence or presence of diabetic retinopathy but may not be as good for grading the severity.21–23 By using the presence of macular hard exudates as a surrogate marker for possible diabetic macular edema, it has been estimated that 95% of eyes with suspected diabetic macular edema could be identified with nFP.24
We initially hypothesized that diabetic retinopathy might be more common among diabetic patients screened in rural medical clinics than in an urban academic center because the rural sites would have limited access to eye care providers.25 The results of our study revealed the opposite finding. The diabetic retinopathy detection rate was much higher among diabetic patients screened in the urban clinic compared with the rural clinics (29.6% versus 12.6%, respectively) despite no obvious differences in glycemic control in both groups based on the available HbAlc levels (mean HbA1c, 8.3% in both groups). The higher incidence of retinopathy in the urban clinic population may reflect the older mean age of the urban patient population group in our study (p<0.001) or differences in ethnic composition of the two study populations.26 Although Native American Indians and/or Alaskans have been reported to have the highest prevalence of diabetes mellitus,27 the prevalence of diabetic retinopathy detected in this study population was only 12%, much lower than that noted in the more ethnically diverse urban population. Whether this is due to differences in socioeconomic status or insurance coverage of the two study populations is unknown. The patients seen in the rural sites all have medical coverage provided by the California Indian Health Service, whereas those patients seen in the urban medical center often had coverage limited to MediCal or MediCare insurance.
Differences in patient adherence to healthcare recommendations may also play a role in affecting our findings. The diabetic patients screened for retinopathy in the remote rural medical clinics were patients who had limited access to eye care providers. In contrast, the diabetic patients screened in the urban academic medical clinic had access to eye care providers but who may be nonadherent to recommended annual dilated eye examination. It is known that patients who are poorly compliant likely also have poor control of their diabetes.28 Although no difference in HbA1c was noted between the two study populations in our study, the result may be misleading because we had access to the HbA1c levels in only a small subset of the rural study population for our analysis.
The prevalence of diabetic retinopathy being diagnosed with nFP was similar to those reported by the Centers for Disease Control and Prevention recently,1 but lower in both our study populations (rural and urban) compared with reports from several years ago.3 Cummings et al.29 found the prevalence of diabetic retinopathy in a rural eastern North Carolina community to be as high as 40.9% among their study patients, much higher than our rural cohort. A similarly high incidence was reported by Murgatroyd et al.21 These observations may highlight possible regional differences among rural and urban communities even within the United States, or they may also highlight a possible recent trend toward a decrease in incidence of retinopathy with improved glycemic control. It is therefore unknown whether the observations made in our study can be extrapolated to other communities. Other confounding variables in all the aforementioned studies include selection bias, chronicity of disease, compliance to treatment, or access to eye care providers.
There were several other incidental findings noted during our nFP screening for diabetic retinopathy. Among them, optic nerve head cupping, suspicious for glaucoma, was the most common in both rural (10.5%) and urban sites (7.6%). The usefulness of nFP for diagnosing diseases other than diabetic retinopathy, such as glaucoma suspects,30 retinopathy of prematurity, age-related macular degeneration, or other vitreoretinal diseases, is being investigated.
There are other limitations of our study worth noting. First, although both groups of patients were screened using a 10-megapixel fundus camera, the camera and the server used to download and view the images were not identical between the urban sites and the rural sites. In addition, because this is a retrospective study, we had limited access to the medical history of the study population, including the duration of diabetes and history of glycemic control. Such information may correlate better with incidence of diabetic retinopathy in our study population rather than a single HbA1c measurement alone.31
In summary, this study shows that teleophthalmology can be a useful method for screening diabetic patients for retinopathy in both urban and rural settings.15 A single 45° nFP does not replace a comprehensive eye examination, but it is a useful tool for accessing diabetic patients who are not getting the recommended eye exam, so that they can be triaged to the necessary eye care provider for a complete evaluation and treatment as needed.18 Although this photographic screening has been used traditionally to screen diabetic patients in rural remote areas without access to eye care providers, our study suggests that it may be also useful in urban settings where a significant portion of diabetic patients with retinopathy do not receive the recommended screening eye examination.
Acknowledgments
The authors thank Dr. David Springer and Steve Viramontes, PHN, of the California Indian Health Service for their assistance with this study. The authors also thank Dr. Thuy Pham who performed some of the initial chart reviews. This study was supported in part by a Research to Prevent Blindness unrestricted departmental grant and a University of California Davis telemedicine grant.
Disclosure Statement
No competing financial interests exist. The sponsor or funding organization had no role in the design or conduct of this research.
References
- 1.Centers for Disease Control and Prevention 2011 national diabetes fact sheet. Available at www.cdc.gov/diabetes/pubs/factsheet11.htm (last accessed May16, 2013)
- 2.Bradford CA. Basic ophthalmology, 8th ed. San Francisco: American Academy of Ophthalmology, 2004:166–170 [Google Scholar]
- 3.National Institutes of Health Diabetic retinopathy: Causes and risk factors. Available at http://nihseniorhealth.gov/diabeticretinopathy/causesandriskfactors/02.html (last accessed August27, 2009)
- 4.American College of Physicians, American Diabetes Association, American Academy of Ophthalmology Screening guidelines for diabetic retinopathy.Ann Intern Med 1992;116:683–685 [PubMed] [Google Scholar]
- 5.Diabetic Retinopathy Clinical Research Network (DRCR.net), Beck RW, Edwards AR, et al. . Three-year follow-up of a randomized trial comparing focal/grid photocoagulation and intravitreal triamcinolone for diabetic macular edema. Arch Ophthalmol 2009;127:245–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diabetic Retinopathy Clinical Research Network; Writing Committee, Aiello LP, Beck RW, Bressler NM, et al. . Rationale for the Diabetic Retinopathy Clinical Research Network treatment protocol for center-involved diabetic macular edema. Ophthalmology 2011;118:e5–e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Early Treatment Diabetic Retinopathy Study report number 9: Early photocoagulation for diabetic retinopathy. Ophthalmology 1991;98: 766–785 [PubMed] [Google Scholar]
- 8.Early Treatment Diabetic Retinopathy Study report number 1: Photocoagulation for diabetic macular edema. Arch Ophthalmol 1985;103:1796–1806 [PubMed] [Google Scholar]
- 9.American Academy of Ophthalmology Preferred practice pattern: Diabetic retinopathy. San Francisco: American Academy of Ophthalmology, 1998. [Google Scholar]
- 10.Gómez-Ulla F, Fernandez MI, Gonzalez F, Rey P, Rodriguez M, Rodriguez-Cid MJ, et al. . Digital retinal images and teleophthalmology for detecting and grading diabetic retinopathy. Diabetes Care 2002;25:1384–1389 [DOI] [PubMed] [Google Scholar]
- 11.Healthcare Effectiveness Data and Information Set Report (HEDIS) 2011. Available at www.Sanfordhealthplan.org/ClassLibrary/Page/Images/files/HEDIS_Report_2011.pdf (last accessed May16, 2013)
- 12.Hartnett ME, Key IJ, Loyacano NM, et al. . Perceived barriers to diabetic eye care. Arch Ophthalmol 2005;123:387–391 [DOI] [PubMed] [Google Scholar]
- 13.Boucher MC, Desroches G, Garcia-Salinas R, Kherani A, Maberley D, Olivier S, Oh M, Stockl F. Teleophthalmology screening for diabetic retinopathy through mobile imaging units in Canada. Can J Ophthalmol 2008;43:658–668 [DOI] [PubMed] [Google Scholar]
- 14.Schoenfeld ER, Greene JM, Wu SY, Leske MC. Patterns of adherence to diabetes vision care guidelines: Baseline findings from the Diabetic Retinopathy Awareness Program. Ophthalmology 2001;108:563–571 [DOI] [PubMed] [Google Scholar]
- 15.Au A, Gupta O. The economics of telemedicine for vitreoretinal diseases. Curr Opin Ophthalmol 2011;22:194–198 [DOI] [PubMed] [Google Scholar]
- 16.Li Z, Wu C, Olayiwola JN, et al. . Telemedicine-based digital retinal imaging vs standard ophthalmologic evaluation for the assessment of diabetic retinopathy. Conn Med 2012;76:85–90 [PubMed] [Google Scholar]
- 17.Tuulonen A, Ohinmaa T, Alanko HI, et al. . The application of teleophthalmology in examining patients with glaucoma: A pilot study. J Glaucoma 1999;8:367–373 [PubMed] [Google Scholar]
- 18.Shah SU, Seibles J, Park SS. Photographic diabetic retinopathy screening in an urban family practice clinic: Effect on compliance to eye examination. Ophthalmic Surg Lasers Imaging 2011;42:383–389 [DOI] [PubMed] [Google Scholar]
- 19.Lee PP, Feldman ZW, Ostermann J, Brown DS, Sloan FA. Longitudinal rates of annual eye examinations of persons with diabetes and chronic eye diseases. Ophthalmology 2003;110:1952–1959 [DOI] [PubMed] [Google Scholar]
- 20.Kirkman MS, Caffrey HH, Williams SR, Marrero DG. Impact of a program to improve adherence to diabetes guidelines by primary care physicians. Diabetes Care 2002;25;11:1946–1951 [DOI] [PubMed] [Google Scholar]
- 21.Murgatroyd H, Ellingford A, Cox A, Binnie M, Ellis JD, MacEwen CJ, Leese GP. Effect of mydriasis and different field strategies on digital image screening of diabetic eye disease. Br J Ophthalmol 2004;88:920–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin DY, Blumenkranz MS, Brothers RJ, Grosvenor DM. The sensitivity and specificity of single-field nonmydriatic monochromatic digital fundus photography with remote image interpretation for diabetic retinopathy screening: A comparison with ophthalmoscopy and standardized mydriatic color photography. Am J Ophthalmol 2002;134:204–213 [DOI] [PubMed] [Google Scholar]
- 23.Vujosevic S, Benetti E, Massignan F, Pilotto E, Varano M, Cavarzeran F, Avogaro A, Midena E. Screening for diabetic retinopathy: 1 and 3 nonmydriatic 45-degree digital fundus photographs vs 7 standard early treatment diabetic retinopathy study fields. Am J Ophthalmol 2009;148:111–118 [DOI] [PubMed] [Google Scholar]
- 24.Bresnick GH, Mukamel DB, Dickinson JC, Cole DR. A screening approach to the surveillance of patients with diabetes for the presence of vision-threatening retinopathy. Ophthalmology 2000;107:19–24 [DOI] [PubMed] [Google Scholar]
- 25.Leese GP, Ahmed S, Newton RW, Jung RT, Ellingford A, Baines P, Roxburgh S, Coleiro J. Use of mobile screening unit for diabetic retinopathy in rural and urban areas. BMJ 1993;306:187–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sivaprasad S, Gupta B, Crosby-Nwaobi R, Evans J. Prevalence of diabetic retinopathy in various ethnic groups: A worldwide perspective. Surv Ophthalmol 2012;28:1–24 [DOI] [PubMed] [Google Scholar]
- 27.Silver K, Williams M, Macario E. The National Eye Health Education Program: Increasing awareness of diabetic eye disease among American Indians and Alaska Natives. Ethn Dis 2006;16:920–925 [PubMed] [Google Scholar]
- 28.López-Simarro F, Brotons C, Moral I, Cols-Sagarra C, Selva A, Aguado-Jodar A, Miravet-Jiménez S. Inertia and treatment compliance in patients with type 2 diabetes in primary care. Med Clin (Barc) 2012;138:377–384 [DOI] [PubMed] [Google Scholar]
- 29.Cummings DM, Morrissey S, Barondes MJ, Rogers L, Gustke S. Screening for diabetic retinopathy in rural areas: The potential of telemedicine. J Rural Health 2001;17:25–31 [DOI] [PubMed] [Google Scholar]
- 30.Yogesan K, Constable IJ, Barry CJ, et al. . Evaluation of a portable fundus camera for use in the teleophthalmologic diagnosis of glaucoma. J Glaucoma 1999;8:297–301 [PubMed] [Google Scholar]
- 31.Silpa-Archa S, Sukhawarn R. Prevalence and associated factors of diabetic retinopathy in Chandrubeksa Hospital, Directorate of Medical Services, Royal Thai Air Force. J Med Assoc Thai 2012;95(Suppl 4):S43–S49 [PubMed] [Google Scholar]


