Significance
When hornworm (Manduca sexta) larvae feed on Nicotiana attenuata plants in native habitats, more disappear at night when they feed on transgenic, nicotine-free N. attenuata plants because wolf spiders (Camptocosa parallela) selectively prey on nicotine-free larvae. When larvae consume nicotine-replete plants, their midgut-expressed cytochrome P450 6B46 (CYP6B46) is upregulated. The larvae in which CYP6B46 is silenced by plant-mediated RNAi excrete more of their ingested nicotine. Silencing CYP6B46 impairs the mechanisms of passing ingested nicotine from the midgut to the hemolymph to be exhaled from the spiracles during spider attack. Spiders are deterred by this nicotine-rich halitosis. Thus, the CYP6B46-silenced larvae exhale less nicotine and become ready spider prey, demonstrating that CYP6B46 functions to repurpose the normally excreted nicotine for defense.
Keywords: alkaloid, Coyote tobacco, Lepidoptera, reverse genetics, tobacco hornworm
Abstract
Manduca sexta (Ms) larvae are known to efficiently excrete ingested nicotine when feeding on their nicotine-producing native hostplant, Nicotiana attenuata. Here we describe how ingested nicotine is co-opted for larval defense by a unique mechanism. Plant-mediated RNAi was used to silence a midgut-expressed, nicotine-induced cytochrome P450 6B46 (CYP6B46) in larvae consuming transgenic N. attenuata plants producing MsCYP6B46 dsRNA. These and transgenic nicotine-deficient plants were planted into native habitats to study the phenotypes of larvae feeding on these plants and the behavior of their predators. The attack-behavior of a native wolf spider (Camptocosa parallela), a major nocturnal predator, provided the key to understanding MsCYP6B46’s function: spiders clearly preferred CYP6B46-silenced larvae, just as they had preferred larvae fed nicotine-deficient plants. MsCYP6B46 redirects a small amount (0.65%) of ingested nicotine from the midgut into hemolymph, from which nicotine is exhaled through the spiracles as an antispider signal. CYP6B46-silenced larvae were more susceptible to spider-attack because they exhaled less nicotine because of lower hemolymph nicotine concentrations. CYP6B46-silenced larvae were impaired in distributing ingested nicotine from midgut to hemolymph, but not in the clearing of hemolymph nicotine or in the exhalation of nicotine from hemolymph. MsCYP6B46 could be a component of a previously hypothesized pump that converts nicotine to a short-lived, transportable, metabolite. Other predators, big-eyed bugs, and antlion larvae were insensitive to this defense. Thus, chemical defenses, too toxic to sequester, can be repurposed for defensive functions through respiration as a form of defensive halitosis, and predators can assist the functional elucidation of herbivore genes.
Plants produce a pharmacopeia of potent chemical defenses that prevent the attack of unadapted herbivores and thwart the growth of adapted ones. Frequently, lepidopteran herbivores co-opt these diet-acquired toxins for their own defensive purposes. The eastern tent caterpillar (Malacosoma americanum) regurgitates hydrogen cyanide and benzaldehyde ingested from their cyanogenic hostplants when attacked by ants (1). The Atala butterfly (Eumaeus atala) acquires a toxic azoxyglycoside from its cycad hosts and becomes unpalatable to bird and ant predators (2). Similarly, rattlebox moths (Utetheisa ornatrix) co-opt pyrrolizidine alkaloids that their larvae sequester while feeding on rattlebox legume hostplants (Crotalaria spp.) to deter predatory spiders (3). Prey frequently advertise their toxic status with warning colorations, odors, and behaviors, and predators readily learn these aposematic signals to avoid consuming toxic prey (4). The molecular mechanisms of how herbivores co-opt plant defenses for their own defense remain largely unexplored.
The pyridine alkaloid nicotine is a defense metabolite of several Nicotiana spp. Nicotine is extremely effective against herbivores because of its ability to poison the essential neuromuscular junction common to all animals that use muscles to move: the acetylcholine receptor (5, 6). Nicotiana spp. hostplants respond to the herbivore attack with large increases in nicotine accumulation (7). However, the tobacco hornworm (Manduca sexta, Ms), a specialist lepidopteran herbivore that feeds on nicotine-producing Nicotiana plants, tolerates doses of nicotine that are lethal for unadapted herbivores (8). More endoparasitoid wasps (Cotesia congregata) emerged as adults from parasitized M. sexta larvae fed on low nicotine varieties of cultivated tobacco than from larvae fed on nicotine-rich varieties (9). The generalist predatory argentine ant (Iridomyrmex humilis) also preferred M. sexta larvae reared on artificial diets (AD) without nicotine over those reared on high nicotine diets, and were deterred by topical nicotine treatments (10). These results suggest that M. sexta larvae might be able to use this diet-derived toxin for their own protection. How this happens remains a mystery, as the larvae’s resistance of ingested nicotine does not appear to include sequestration and storage of this toxin.
The exact mechanisms responsible for M. sexta’s nicotine resistance remain unclear, but both efficient excretion and metabolism appear to be involved. Some researchers have focused on the polar metabolites of nicotine, such as cotinine and the N-oxides of both nicotine and cotinine, which are commonly found in the urine and blood of human smokers (8, 11, 12); cytochrome P450s (CYPs) are thought to mediate nicotine’s oxidation to these metabolites (8, 11, 13–15), but other researchers have been unable to find the oxides in M. sexta’s excretions and propose that nicotine is rapidly excreted without modification (16–18). Although this theory is widely accepted, most studies have not been able to recover all of the ingested nicotine in the frass and nicotine can be found in the hemolymph of larvae feeding on nicotine-containing diets. Hence, within these physiological limits of M. sexta’s excretory-based tolerance lie opportunities for the defensive use of nicotine. Whether nicotine-resistance and co-option are regulated by a common mechanism remains unknown.
Here we examine how M. sexta larvae co-opt diet-ingested nicotine for their own defense. In a previous unbiased microarray study, we found that a midgut-expressed cytochrome P450 (CYP6B46) was strongly down-regulated in larvae that were fed genetically modified hostplants with suppressed nicotine production (19, 20). To evaluate if this CYP6B46 is involved in nicotine resistance and co-option, we used a reverse genetics approach, plant-mediated RNA interference (PMRi) (20, 21), to silence this gene in larvae feeding on nicotine-containing, native coyote tobacco (Nicotiana attenuata) hostplants transformed to harbor the silencing construct. Lepidopteran herbivores appear to lack the RNA-dependent RNA polymerase required to sustain gene silencing by RNAi; however, a continuous supply of double-stranded (ds)RNA administered via the hostplant (or diet) effectively silences genes in these herbivores (21, 22).
N. attenuata plants were transformed with an expression vector containing a 300-bp fragment of CYP6B46 in an inverted repeat (ir) orientation. Continuous dsRNA ingestion efficiently silenced CYP6B46 in the midguts of larvae feeding on these plants in a highly target-sequence–specific manner, as the most similar CYP expressed in larval midguts, CYP6B45, was not cosilenced (20). These PMRi plants were planted into the native habitat of both hostplant and larvae, the Great Basin Desert, Utah, which teems with larval predators—such as bugs, mantids, ants, antlions, spiders, and lizards—but lacks the Argentine ants and C. congregata endoparasitoids previously reported to be nicotine-sensitive. One of these predators, a wolf spider [Camptocosa parallela (Lycosidae)], selectively attacked CYP6B46-silenced larvae just as it did larvae feeding on nicotine-free hostplants. The particular predatory behavior of these spiders revealed the function of MsCYP6B46 in externalizing ingested nicotine for defensive use. The combination of natural history studies and the plant- and herbivore-reverse genetic procedures can fruitfully dissect the molecular mechanisms governing the tritrophic interactions.
Results
Wolf Spiders Avoid Nicotine-Fed Larvae in Nature.
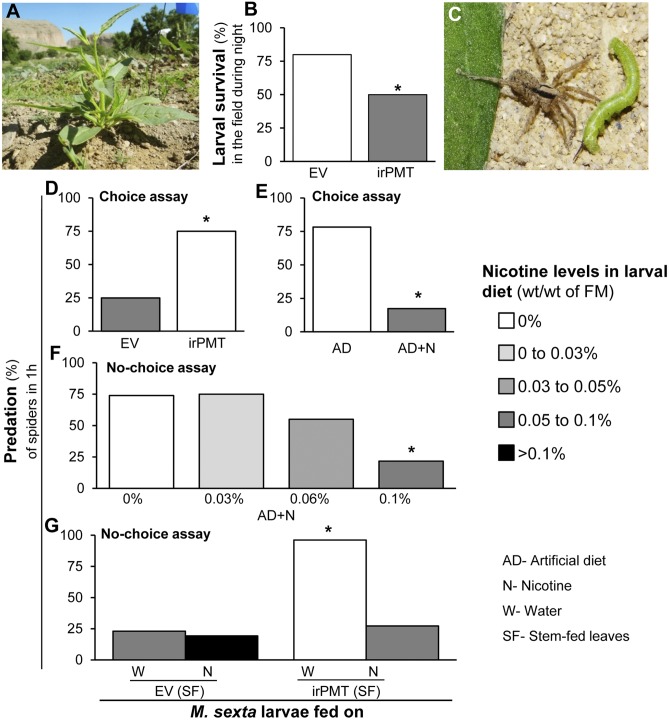
To investigate the effect of hostplant nicotine on the survival of M. sexta, we transplanted stably transformed N. attenuata plants silenced in nicotine production and accumulation (inverted-repeat putrescine N-methyl transferase, irPMT) (23) into a field plot in Utah (Fig. 1A). Survival rates of M. sexta larvae feeding on nicotine-deficient and -producing plants in the predator-rich field were monitored, and we found that fewer irPMT-fed larvae survived than did larvae feeding on control plants producing WT levels of nicotine [empty vector (EV)-fed], especially during the nights (Fig. 1B and SI Appendix, Table S1); during the day, no significant differences in survivorship were found [EV = 76%; irPMT = 72% (n = 50 larvae per line)]. We hypothesized that this difference in survivorship resulted from the selective predation of a night-active nicotine-sensitive predator. We surveyed the N. attenuata field plantation during subsequent nights. We found wolf spiders during nighttime surveys of the N. attenuata field plantation (Fig. 1C) (density 1.55 ± 0. 05 individuals per square meter). We tested these spiders in choice and no-choice assays (SI Appendix, Fig. S1 A–C) with M. sexta larvae fed on foliage or AD of different nicotine contents (Fig. 1 D–F). C. parallela strongly preferred larvae fed irPMT plants over those fed EV plants and similarly, AD-reared larvae over those reared on nicotine-containing AD (Fig. 1 D–F). The nicotine-sensitivity of this spider was confirmed in no-choice assays, in which the predation rate was found to decrease with increasing nicotine concentrations in the larval diet (depicted by the shading of the bars in all figures) (Fig. 1F). Larvae fed nicotine-supplemented [stem-fed (SI Appendix, Fig. S1D)] irPMT leaves (irPMT+N) were preyed upon at rates similar to those of larvae on plants with WT-levels of nicotine (Fig. 1G). From these results, we conclude that the spiders were deterred by M. sexta larvae’s ingested nicotine or a metabolic product thereof.
Fig. 1.
Spiders are deterred by nicotine-fed larvae. (A) Nicotiana attenuata growing in the Great Basin Desert, Utah. (B) Nocturnal survival (%) of larvae feeding on nicotine-containing EV and nicotine-deficient (irPMT) plants in the field (n = 50 larvae per line). (C) Spider attacking M. sexta larva. Spider predation (%) in the choice assay (1 h) on second-instar M. sexta larvae feeding on (D) EV (n = 16) and irPMT (n = 16) plants and (E) AD (n = 23) and AD containing 0.1% of nicotine (AD+N) (n = 23). Spider predation (%) in no-choice assays (1 h) with M. sexta larvae feeding on: (F) AD containing 0 (n = 23), 0.03 (n = 20), 0.06 (n = 20), and 0.1% (n = 23) nicotine (AD+N), or (G) water (W) or 1 mM nicotine (N) stem-fed (24 h) EV and irPMT leaves (n = 26 in all of the treatments). Asterisks indicate significant differences (P ≤ 0.05) by Fisher’s exact test on the frequencies as well as percentages. Shading of the bars reflects relative nicotine concentration of the larval diet throughout all figures. Hence, the bar shading provides the important information for the interpretation of transcripts, larval nicotine excretion, and the hemolymph-nicotine data presented in the subsequent figures.
Silencing M. sexta’s Nicotine-Induced CYP6B46 by PMRi.
That midgut CYP6B46 transcript accumulation was elicited specifically in response to nicotine ingestion was confirmed using larvae fed control (EV plants with WT nicotine levels) and nicotine-deficient irPMT plants (Fig. 2A), and further confirmed in additional experiments with larvae fed irPMT plants stem-fed water (irPMT+W) or nicotine solutions (irPMT+N) (SI Appendix, Fig. S2A) and AD lacking nicotine or AD containing 0.1% nicotine (AD+N) (SI Appendix, Fig. S2B). To understand MsCYP6B46’s function in larval nicotine metabolism, we created a transgenic line of N. attenuata to silence the expression of CYP6B46 in M. sexta larvae feeding on these plants, using PMRi (20, 21).
Fig. 2.
Silencing larval CYP6B46 dramatically affects spider predation. (A) CYP6B46 transcript levels (relative to ubiquitin) in midguts of first-instar larvae feeding on WT and irPMT N. attenuata plants (F1,8 = 9.984, P ≤ 0.05, n = 5). (B) Schematic representation of plant-mediated RNAi: pSOL8 binary vector constructed to express 300-bp dsRNA of MsCYP6B46 in N. attenuata and trophic transfer of CYP6B46 dsRNA from plant to larvae. (C) CYP6B46 transcript levels (relative to ubiquitin) in various tissues (foregut, midgut, hindgut, hemolymph, Malpighian tubules, cuticle with fat body and spiracle) of fourth-instar larvae feeding on EV (E), irCYP (C), and irPMT (P) plants (F20,84 = 487.2, P ≤ 0.0001, n = 5). (D) Spider predation (%) in no-choice assays on larvae fed EV and irCYP leaves (n= 26). Asterisks and small letters above the bars in A and C indicate significant differences determined by one-way ANOVAs; asterisk in D indicates significant differences (P ≤ 0.05) by Fisher's exact test. See Fig. 1 legend for the codes for the bar-shading.
We transformed N. attenuata plants with the recombinant vector containing two 300-bp fragments of MsCYP6B46 in an inverted-repeat orientation to create stable transgenic irCYP6B46 (irCYP) plants (homozygous for a single genomic insertion) that synthesized dsRNA of MsCYP6B46 under control of a strong CaMV promotor (Fig. 2B). irCYP plants were indistinguishable from isogenic WT plants in growth, morphology and secondary metabolite content (SI Appendix, Table S2); most importantly, they had nicotine contents equivalent to those of WT plants when grown in both the field and glasshouse (SI Appendix, Fig. S2C). When M. sexta larvae ingested leaves from these irCYP plants and consequently CYP6B46 dsRNA (Fig. 2B), dramatic (95%) sequence-specific silencing of larval CYP6B46 was observed in their midguts, the tissue with the highest transcript accumulation levels (Fig. 2C). Transcript abundance was also significantly reduced in the foregut, hindgut, hemolymph, and Malpighian tubules, which had much lower basal levels of expression than did the midguts (Fig. 2C). Although strong silencing was observed in internal larval tissues, silencing of the very low basal levels of expression in the skin, fat body, and spiracles was not evident, suggesting that the PMRi procedure may be most effective in tissues most exposed to ingested leaf material. These results clearly demonstrated that larvae feeding on irCYP plants were strongly silenced in their midgut CYP6B46 expression, and for brevity, we refer to these as “CYP-silenced” larvae.
In Situ CYP6B46 Silencing Increases Larval Susceptibility to Predatory Spiders.
We planted irCYP plants into a field plot in the plant’s native habitat, infested them with larvae, and compared larval survivorship with those infesting WT control plants. CYP-silenced larvae survived similarly poorly on these plants as normal larvae had on the nicotine-deficient irPMT plants during night time [EV = 80%; irCYP = 50% (n = 50 larvae per line)]. We hypothesized that this difference was because of the selective predation of the nicotine-sensitive wolf spiders. In no-choice assays with larvae fed individually on irCYP and EV plants, spiders consumed significantly more CYP-silenced larvae than control larvae (Fig. 2D, and Movies S1 and S2). These bioassay results suggested a role of nicotine ingestion and CYP6B46 expression in M. sexta’s spider-deterrence.
CYP6B46’s Role in M. sexta’s Processing of Nicotine.
The spider’s predation behavior clearly established an association between CYP6B46 expression and M. sexta’s spider-deterrence abilities. To evaluate this association, we quantified the previously reported (8, 11) oxidation products of nicotine [cotinine, cotinine N-oxide (CNO) and nicotine 1-N-oxide (NNO)] in the frass of control and CYP-silenced larvae. We developed a sensitive and accurate U(H)PLC-microToF mass spectrometer-based procedure using internal standards to quantify nicotine, cotinine, and their N-oxides with a limit of detection of 0.25 ng (nicotine) and 0.5 ng (cotinine, CNO, and NNO) and a high efficiency of extraction (>90%) of these compounds from frass (SI Appendix, Fig. S3). As had been previously reported by Self et al. (17) almost 50 y ago, we found no evidence for metabolites other than nicotine in the frass.
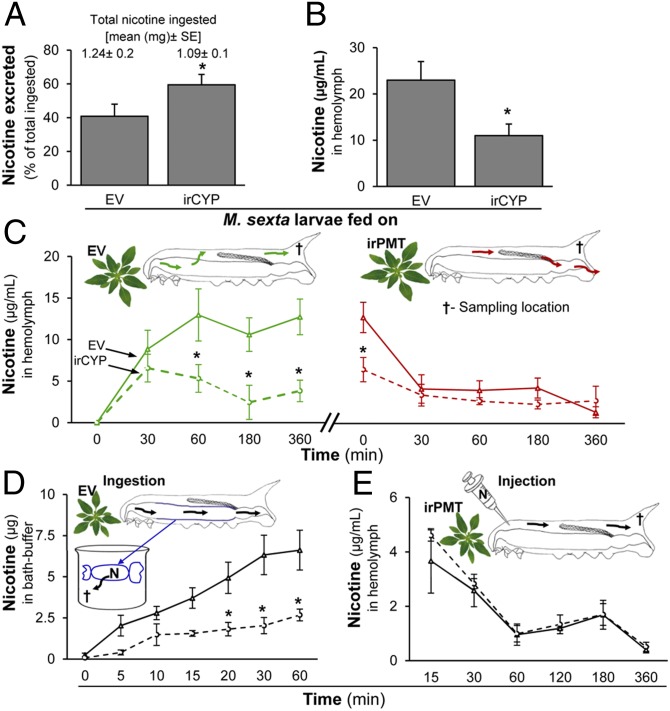
Because nicotine oxidation products were not found, we hypothesized that the food intake of the CYP-silenced larvae was lower than the controls, thereby lowering the overall food- and consequently nicotine-content of their body and making them susceptible to the spiders. To test this theory, we adopted the mass-balance approach of the Waldbauer assays (24, 25) to quantitatively evaluate the flux of nicotine through larval bodies. We compared the mass of food and nicotine ingested and excreted by the CYP-silenced and EV-fed larvae, which were not different (SI Appendix, Fig. S4 and Table S3). However, we observed that during this 24-h assay, although the EV- and irCYP-fed larvae ingested the same amount of nicotine in the foliage they consumed, CYP-silenced larvae excreted significantly more (∼20%) in their frass than did EV-fed control larvae (Fig. 3A and SI Appendix, Table S3).
Fig. 3.
Effect of CYP6B46 silencing on larval nicotine excretion and nicotine flux in larval body. (A) Nicotine excreted (percent of total ingested) by fourth-instar EV- or irCYP-feeding larvae (experimental details in SI Appendix, Fig S4) [(mean ± SE) F1,12 = 8.77, P ≤ 0.05, n = 8]. (B) Nicotine in hemolymph of fourth-instar EV- or irCYP-feeding larvae (F1,4 = 106.6, P ≤ 0.01, n = 5). (C) Kinetics of nicotine absorption by (green arrows in larval body and green lines in graph) and discharge from (red arrows and red lines) the hemolymph of control and CYP-silenced fourth-instar larvae (experimental details in SI Appendix, Fig. S5A). (D) Kinetics of nicotine discharge from excised midguts (containing ingested host-plant diet) of control and CYP-silenced fourth instar larvae; nicotine entering the bathing solution (sodium phosphate buffer + 0.3 M sorbitol, pH 7.0) was measured at regular intervals, up to 60 min. (E) Kinetics of nicotine discharge from hemolymph of control and CYP-silenced fourth-instar larvae, after injecting 0.001% nicotine (of FM) into the hemolymph to determine if CYP-silenced larvae discharge nicotine from their hemolymph at rates different from that of controls. Asterisks indicate significant differences determined by one-way ANOVA (P ≤ 0.05). See Fig. 1 legend for the bar-shading codes.
CYP6B46 Silencing Alters Nicotine Efflux from Midgut to Hemolymph.
The results of the nicotine-Waldbauer assays revealed that CYP-silenced larvae retained less nicotine in their body and excreted more in their frass. We examined larval hemolymph and found that the hemolymph of CYP-silenced larvae contained 47% less nicotine than did the controls (Fig. 3B). We developed a procedure to multiply sample the hemolymph of individual larvae as they fed first on nicotine-free irPMT plants, were switched to nicotine-containing EV plants, and were returned to irPMT plants to compare the nicotine dynamics in the hemolymph of control and CYP-silenced larvae as they ingested realistic doses of dietary nicotine (SI Appendix, Fig. S5). Previous work had shown that larvae clear a majority of ingested or injected nicotine within 6 h (17), and this time interval was used between the dietary switches.
Hemolymph nicotine levels rapidly increased after larvae were switched to nicotine diets to attain steady-state levels within 30–60 min. CYP-silenced larvae attained steady-state levels that were ∼70% (wt/vol) lower than those of EV-fed larvae [after 360 min, EV = 12.7 ± 2.1 (mean ± SE) µg/mL and CYP-silenced: 3.9 ± 1.2 (mean ± SE) µg/mL] (Fig. 3C). When switched back to nicotine-free plants, hemolymph values of both larval types returned to similar levels within 30 min (Fig. 3C). These results demonstrate that CYP6B46 expression dramatically influences the flux of nicotine into the hemolymph from the ingested food, but not its clearance from the hemolymph once the ingestion ceases.
To further examine both inferences, we conducted two additional experiments. In the first experiment, size-matched control and CYP-silenced larvae were fed EV plants for 6 h before their midguts were dissected, sutured, and sealed at both the ends without loss of gut contents, and incubated in a bath containing a neutral pH buffer (matching hemolymph) for 60 min (Fig. 3D). Midguts of CYP-silenced larvae released ∼60% (wt/vol) less nicotine into the bath buffer, compared with midguts of control larvae (Fig. 3D); moreover, none of the polar nicotine metabolites were detected in the bath buffer, suggesting an important role for CYP6B46 in the transfer of nicotine into the hemolymph. To test the second inference about CYP6B46’s lack of role in the clearance of nicotine from the hemolymph, we bypassed the midgut-associated function of CYP6B46 by injecting a physiologically realistic quantity of nicotine directly into the hemolymph of control and CYP-silenced larvae feeding on nicotine-free plants (Fig. 3E). Again, we found no significant difference in the clearance of nicotine from the hemolymph of both groups of larvae over 6 h (Fig. 3E), and again no evidence of polar nicotine metabolites, consistent with the results of the previous feeding experiment.
Spider Predatory Behavior Revealed That Larvae Externalize Ingested Nicotine.
The nicotine levels in the hemolymph of CYP-silenced larvae were clearly lower than those of control larvae feeding on the same nicotine-containing diets; could the lower hemolymph nicotine levels account for the large increase in nicotine excretion in the frass? In 24 h, when both normal and CYP-silenced larvae consumed ∼1.1 mg of nicotine in their diets, CYP-silenced larvae excreted ∼0.13 mg more nicotine than did control caterpillars in their frass (SI Appendix, Table S3). The amount of nicotine in hemolymph of a control larva is only 23 ± 4.0 µg/mL (mean ± SE); considering that the larvae used for the Waldbauer assays weighed 1 ± 0.25 g (mean ± SE) and contained 300 ± 60 µL (mean ± SE) hemolymph, the hemolymph of control larvae accounts for ∼7.2-µg nicotine, which is ∼0.65% of the ingested 1.1-mg nicotine. Thus, retention of the large amount of unaccounted nicotine (∼740 µg in control and ∼370 µg in the CYP-silenced larvae) by the hemolymph or its excretion through the hemolymph during a 24-h feeding period seemed unlikely. Hence, we returned to our observations of spider predatory behavior for a clue about the missing nicotine.
Spiders usually assess their prey after capture by tapping it with chemosensory endowed legs and palps (26) (Movies S1–S3). Wolf spiders were clearly rejecting nicotine-fed larvae before penetrating their prey with their mandibles to inject their mixture of digestive enzymes and poisons; this suggested that larvae externalize some fraction of their ingested nicotine. We washed larvae feeding on different nicotine-containing diets and found no evidence for surface externalization of the ingested nicotine that couldn’t be attributed to direct surface contamination (SI Appendix, Fig. S6A). This finding motivated us to explore if larvae emit some fraction of their ingested nicotine into the headspace. We analyzed the headspace of these larvae and found significant quantities of nicotine. We compared the headspace of control and CYP-silenced larvae that had ingested the same amount of nicotine in their food and found that the headspace of control larvae contained fourfold more nicotine [22 ± 3.0 ng (mean ± SE)] than the headspace of CYP-silenced larvae [5 ± 1.0 ng (mean ± SE)] (Fig. 4A, and SI Appendix, S6 B and C).
Fig. 4.
CYP6B46 silencing reduces larval nicotine emission and increases spider predation, which can be complemented by volatile nicotine perfuming. (A) Emission of ingested nicotine (Left) [(mean ± SE) F2,6 = 36.14, P ≤ 0.0005, n = 3] by the fourth-instar larvae feeding on EV and irCYP plants and emission of injected nicotine (Right) [(mean ± SE) n = 3] by the nicotine-free control and CYP-silenced fourth-instar larvae. Nicotine adsorbed on the PDMS tube attached to a spiracle of the fourth-instar control or CYP-silenced larvae (each weighing 7.0 ± 0.25 g): (B) after feeding on nicotine containing leaves for 1 h [(mean ± SE) F1,9 = 5.82, P ≤ 0.05, n = 5] or (C) after injecting 0.001% nicotine (of FM) [(mean ± SE) n = 5]. (D) M. sexta larva with attached PDMS tubes for the volatile nicotine trapping from the spiracle (Sp) and cuticle (Cu). (E) Spider predation (%) in a no-choice assay on M. sexta larvae fed on EV, irPMT, and irCYP plants with water or nicotine perfuming (n = 15 per treatment); experimental details are given in SI Appendix, Fig. S7B. Asterisks above the bars in A–C indicate significant differences determined by one-way ANOVA; asterisks above the bars in E indicate significant differences (P ≤ 0.05) by Fisher’s exact test. See Fig. 1 legend for the bar-shading codes.
CYP6B46-Silencing Inhibits the Spiracular Release of Nicotine and Makes Larvae Vulnerable to Spider Predation.
To evaluate if the headspace reflected the differences in hemolymph nicotine observed between CYP-silenced and control larvae, we injected nicotine into their hemolymph of both control and CYP-silenced larvae after rendering them both nicotine-free by feeding them on irPMT plants; the headspace nicotine levels of these injected larvae did not differ significantly between control and CYP-silenced larvae and were in the same range as normal nicotine-fed larvae (Fig. 4A). Again, none of the nicotine-metabolites could be detected in the headspace of larvae.
To understand how hemolymph nicotine levels could translate into headspace emissions, we developed a procedure to specifically quantify nicotine emissions from larval spiracles, the mouth equivalents of a caterpillar, and the lung equivalent, the tubular tracheal system that ramifies throughout the larvae body supplying oxygen via microscopic tracheoles. We glued small segments of polydimethylsiloxane (PDMS) adsorptive tubes either directly over spiracles or onto the adjacent skin (Fig. 4D) and quantified nicotine emissions at different times from AD-fed larvae into which we had just injected nicotine into their hemolymph. Nicotine emissions from the spiracles increased dramatically, tracking the expected increase in hemolymph concentrations (SI Appendix, Fig. S7A).
We next compared spiracular nicotine emissions of control and CYP-silenced larvae and found that the difference in headspace emissions corresponded to their spiracular emissions (Fig. 4B). To test whether this spiracular emission was controlled by CYP6B46 activity in larval midguts, we injected nicotine into CYP-silenced and control larvae to equalize their hemolymph nicotine concentrations and found their spiracular nicotine emissions to be equivalent (Fig. 4C).
Finally, to evaluate whether the difference in spiracular nicotine emission could account for the spider feeding preferences, we conducted no-choice assays in which the headspaces of irPMT-, irCYP-, and EV-fed larvae were perfumed with water or amounts of nicotine that were only five-times that found in larval headspace that accumulates in a 50-mL chamber enclosing one larva for 1 h (SI Appendix, Fig. S7 B and C). This concentration is likely a conservative estimate of the amount of nicotine emitted by the larvae during a spider-prey encounter. Indeed, when the headspace of spider-preferred irPMT- or CYP-fed larvae (which were not emitting nicotine beforehand) was perfumed with nicotine, spider predation decreased by 64% (P ≤ 0.05) (Fig. 4E). Clearly, dietary nicotine is only used defensively by larvae with a fully active CYP6B46. A similarly negative effect on the spider’s predatory behavior was also observed in assays performed with AD-fed larvae with and without headspace perfuming (SI Appendix, Fig. S7D).
Other Abundant Predators Are Not Deterred by M. sexta’s Dietary Nicotine.
Apart from spiders, many other predators frequently explore N. attenuata plants in its native habitat in search of food. It is plausible that M. sexta’s nicotine exhaling mechanism could be effective against a broad spectrum of predators. The most frequently observed diurnal predator in the field plot is the big-eyed bug Geocoris pallens (Lygaeidae) (27, 28) (3.7 ± 0.1 individuals per square meter in 2013). To evaluate the effect of dietary nicotine on G. pallens predation, we conducted no-choice assays in which EV-, irPMT-, or irCYP-fed second-instar larvae were offered to G. pallens; these diurnal predators did not differentiate between the larvae fed on different plants (SI Appendix, Table S4).
Another locally abundant generalist predator is the antlion larvae [Myrmeleon carolinus (Myrmeleontidae)]. Antlions construct funnel-shaped pits in sandy soil adjacent to N. attenuata plants in the field plantation and are known for their ability to avoid toxin-containing tissues of their prey (29). Because nicotine is present throughout the body of M. sexta larvae, we hypothesized that antlions would reject larvae fed nicotine-containing diets. EV-, irPMT-, and irCYP-fed second-instar larvae were dropped into separate antlion pits and their response (feeding or rejection) was recorded. These predators also did not differentiate between larvae fed on the different diets (SI Appendix, Table S4).
Discussion
Cytochrome P450 genes are ubiquitous and occur in large families (30). These genes play important ecological and evolutionary roles in the interactions of plants and their attackers, as they are frequently involved in the detoxifications of diverse xenobiotics by mediating the reactions like N- or S-oxidation, hydroxylation, epoxidation, and O-, N-, or S- dealkylation (31, 32). The particular CYP that we found to be strongly regulated in response to dietary nicotine in M. sexta larvae, MsCYP6B46, is a member of the CYP6B enzyme family, which is well known for its unique role in the perception of signaling molecules of plant-defense responses, in addition to its more conventional role in the detoxification of plant defenses (33–36). Our functional analysis of the nicotine-induced MsCYP6B46 stumbled after not finding the expected polar metabolites of nicotine in larval tissues and frass or from heterologous expression assays, but was revived when the behavior of a native predator revealed an unexpected organismic-level function of MsCYP6B46. All organisms in their natural environments, and particularly plants, as they lie at the base of all terrestrial food chains, are carefully scrutinized by literally thousands of other organisms with very different, frequently highly hostplant-tuned sensory modalities; the phenotyping services that competitors, pathogens, herbivores, pollinators, predators, and the plethora of different types of mutualists that interact with an organism lacking the expression of a particular gene, allow for an unbiased “ask the ecosystem” approach for the discovery of the function of this gene at an organismic level.
Planting the nicotine-deficient irPMT N. attenuata plants into the plant’s native habitat enabled the identification of a nicotine-sensitive predator, the wolf spider. M. sexta larvae silenced in CYP6B46 expression were more attacked and consumed by these spiders even though they consumed equivalent amounts of nicotine compared with control larvae (Fig. 2). The prey assessment behavior of this predator indicated that it was deterred by a factor related to the larvae’s external surface or its headspace. Recently, we discovered that M. sexta larvae emit branched chain aliphatic acids by hydrolyzing the O-acyl sugars that they acquire from the glandular trichomes of N. attenuata (37); this motivated us to explore how larvae might externalize a fraction of their ingested nicotine. As we were budgeting the nicotine flux through the larvae, we found that CYP-silenced larvae had lower hemolymph- and higher frass-nicotine levels, so we examined the dynamics of nicotine in the hemolymph after switching larvae from or to the foliage of nicotine-containing plants. CYP6B46 mediated the transition of nicotine from the guts into the hemolymph, but not the clearance of nicotine from the hemolymph (Fig. 3). Finally, the analysis of larval headspace and the spider’s response to nicotine in larval headspace clearly showed that silencing MsCYP6B46 interfered with the larvae’s ability to pass the ingested nicotine from midgut to hemolymph, resulting in its reduced exhalation from the spiracles during spider attack.
Although the CYPs have been mainly thought to mediate the oxidation of nicotine (11, 14), none of the nicotine oxides were detected in hemolymph, frass, excised guts, and larval headspace of WT-fed and CYP-silenced larvae [which is consistent with the findings of Self et al. (17)]. Thus, it is possible that instead of producing these stable nicotine oxides, midgut-based CYP6B46 may convert nicotine to a short-lived metabolite that is readily pumped to the hemolymph and reconverts to nicotine immediately on entering the hemolymph, as originally proposed by Morris (38). Hence, our results are consistent with a previous hypothesis (38) that CYP6B46 converts nicotine to a short-lived metabolite, which is pumped across the insect gut into the hemolymph and rapidly converted back into nicotine. This hypothesis is consistent with the fact that M. sexta larvae contain various pumps that clear the nervous system of different alkaloids and dyes (6, 16). Considering the biochemically validated functions of insect CYPs (30, 31), it is unlikely that CYP6B46 itself acts as a nicotine-pump, but it could be part of a multicomponent pump, which includes transporters that could use the large midgut-hemolymph pH gradient as a driving force. Previously, a prominent role of Malpighian tubule-based pumps had been demonstrated in alkaloid excretion (39, 40). Here, the CYP-silenced larvae differed from controls only in their flux of nicotine from the midgut to hemolymph; although CYP6B46 was silenced by PMRi in the Malpighian tubules, the clearance rates of “injected” nicotine from the hemolymph in control and CYP-silenced larvae were similar (Fig. 3) and the spiracular release of nicotine was proportional to hemolymph nicotine levels (Fig. 4 and SI Appendix, Fig. S7A). These results clearly demonstrate that the role of the Malpighian tubules in regulating hemolymph nicotine levels is CYP6B4-independent and they do not play a regulatory role in nicotine exhalation.
CYP6B46 silencing neither affects larval growth nor mortality (20); it does not even affect the larval food intake and excretion (SI Appendix, Table S3). This finding suggests that the CYP6B46-mediated nicotine efflux from the midgut could have evolved as a defense-specific mechanism, which may even be independent of the larvae’s rapid excretion based nicotine tolerance. Whether it was a secondary innovation evolving after the basic excretory machinery was in place could be explored in other nicotine-tolerant taxa within the sphingid clade by studying the role of this CYP in different species that tolerate nicotine ingestion but do not feed on nicotine-containing hostplants (8).
Another question raised by our results regards the predator-specificity of this nicotine-mediated defense. Notably, the wolf spiders and orb-weaving spiders are known for their sensitivity to alkaloids and so to alkaloid-ingested lepidopteran prey (3, 41). In various geographic regions harboring several different species of wolf spiders, M. sexta feeds on different alkaloid-rich solanaceous host plants; thus it is possible that an alkaloid-mediated defense is a general strategy against the alkaloid sensitive wolf spiders. However, the exhalation-based defense will clearly only be effective with volatile alkaloids, such as nicotine. Because hemolymph nicotine concentration is regulated by the CYP6B46-mediated efflux of midgut nicotine to the larval hemolymph, it is also likely responsible for the differential survival of C. congregata endoparasitoids (42) that are susceptible to high nicotine concentrations of larval hemolymph. It is possible that the nicotine exhalation behavior accounts for the selective larval predation by the nicotine-sensitive ants (10). Similarly, it would be interesting to understand how both G. pallens and the M. carolinus larvae cope with the small amounts of nicotine that they ingest when consuming larvae.
Taking these data together, our work demonstrates how predators can assist the process of elucidating the function of herbivore genes. It also reveals a mechanism for elevating the nicotine concentration of the hemolymph and headspace in the exceptionally nicotine-tolerant M. sexta larvae that frequently face the nicotine-sensitive enemies, like spiders and hemolymph-dwelling endoparasitoids.
Materials and Methods
Plant Material.
N. attenuata 30× inbred seeds, which were originally collected in 1988 from a native population in Utah, were used for the generation of Agrobacterium tumefaciens-mediated stable transgenic lines by the procedure described in Krügel et al. (43). Seeds were germinated on sterile Gamborg B5 medium (Duchefa) after 1 h of treatment with diluted smoke (House of Herbs) and 1 µM GA3 (Roth) (43). Ten days after germination, seedlings were transferred into Teku pots containing a peat-based substrate, and after an additional 10–12 d, the plantlets were transplanted into individual 1-L pots with the same substrate. In the glasshouse, plants were grown at 24 °C to 26 °C, relative humidity ∼60%, and supplemented with light from 400- and 600-W sodium lamps (Philips) for 16 h (44).
irPMT [NaPMT National Center for Biotechnology Information (NCBI) accession no. AF280402] N. attenuata plants (A-03-108-3), fully characterized in ref. 23, were used as a nicotine-free hostplant to feed M. sexta larvae. Previously characterized N. attenuata transgenic line irCYP (A-09-30-2) was used to silence M. sexta’s CYP6B46 (MsCYP6B46 NCBI accession no. GU731529) by PMRi. Generation of irCYP line and silencing of M. sexta larval genes were reported previously (20). An EV-transformed plant line (A-04-266-3) was used as transgenic control plant (45).
Field experiments were conducted at Lytle Ranch Preserve in Santa Clara, Utah, 84765 (37°08′45′′N, 114°01′11′′W) 2004 to June 2013. Seeds of N. attenuata irPMT and irCYP lines were imported and released in accordance with several Animal and Plant Health Inspection Service notifications (SI Appendix, Table S5). Planting of transgenic lines in the field plot was performed as described in Kessler et al. (46).
M. sexta Larvae.
Eggs of the in-house reared M. sexta were stored in a growth chamber (Snijders Scientific) at 26 °C/16-h light, 24 °C/8-h dark, until the larvae hatched; these larvae were used for all of the glasshouse-related experiments. For field experiments, M. sexta eggs were provided by North Carolina State University (Raleigh, NC) in 2004/2005 and by Carol Miles (Department of Biological Sciences, Binghamton University, Binghamton, NY) in 2012.
In various experiments, AD (47) was fed to the larvae. This process enabled us to control dietary nicotine concentrations, whenever required; it also enabled us to rear larvae free from any influence of the hostplant.
Survivorship Assays with M. sexta Larvae Feeding on EV, irPMT, or irCYP Plants.
In 2013, M. sexta larvae were fed on EV, irCYP, or irPMT plants until the second instar. Fifty larvae from each N. attenuata line were placed on the plants of the same respective line (three larvae per plant) that were planted across a predator-rich field in a random spatial array. To quantify survival during the day, larvae were placed on plants in the field at 6:00 AM and the number of larvae surviving on each plant was counted at 8:00 PM; to quantify nocturnal survival, larvae were placed on plants in the field at 8:00 PM and surviving larvae were counted at 6:00 AM. Survivorship assays were also conducted in 2004 and 2012; their details are given in the SI Appendix.
Predator Abundance in the Field.
In 2013, predators were counted in 1-m2 quadrats randomly placed in the field plot; C. parallela individuals were counted from 20 quadrats, whereas G. pallens individuals were counted from 15 quadrats.
Spider Predation Assays.
C. parallela spiders were collected from in and around the N. attenuata field plantation where they were particularly abundant. Spiders were placed individually in chambers and starved for 12 h before all assays. Each assay was conducted for up to 1 h with late second-instar larvae. Spiders were never reused in experiments. Spider’s choice or predation was recorded only if the spider consumed the entire larva within the duration of the assay.
Choice assay.
One test (irPMT/irCYP/AD+N fed) and one control (EV/AD fed) larvae were placed with a single spider inside the polypropylene container (60 cc) (SI Appendix, Fig. S1A). Each spider was allowed only one choice during the 1-h assay period. Spiders’ choices of larvae were expressed in terms of the percentage of spiders that chose larvae from each test treatment.
No-choice assay.
One larva was enclosed with one spider in each assay container (SI Appendix, Fig. S1B, and Movies S1–S3). Assays with test and respective control larvae were always performed simultaneously. Percentage of larvae preyed on by the spider in 1 h was calculated for each treatment group of larvae.
No-choice assay with perfuming.
In each assay, two larvae feeding on the same N. attenuata line or on the same AD combination were placed in two separate assay containers. Each container contained an Eppendorf tube containing a cotton swab moistened with 500 µL of 1-mM nicotine (Sigma-Aldrich) or 500-µL water (control) and its opening was covered with perforated parafilm. One spider was placed in each container and was monitored for 1 h. For each larval treatment group, the percentage of larvae preyed on by the spider was calculated for each perfuming treatment.
RNA Isolation and Quantitative Real-Time PCR.
One-day-old larvae were used to examine the changes in CYP6B46 transcript abundance in response to nicotine ingestion. Midguts of these larvae were dissected; midguts of five larvae were pooled to produce one sample. Hemolymph, Malpighian tubules, foregut, midgut, and hindgut, were collected from the fourth-instar larvae, as reported previously (20); in addition, pieces of larval cuticle (with associated fat bodies) from spiracular and nonspiracular regions were collected. To quantify CYP6B46 silencing efficiency and conduct tissue-specific CYP6B46 transcript profiling, fourth-instar larvae were used.
In the nicotine flux determination experiments, to render the larvae nicotine-free or to feed them the same diet as that of control larvae, fourth-instar CYP-silenced larvae were fed (for 6–12 h) on irPMT or EV plants, respectively. To evaluate if the CYP silencing persisted in such CYP-silenced non-irCYP–feeding larvae, CYP6B46 transcripts were profiled in midguts after both control and CYP-silenced larvae fed on irPMT or EV plants for 24 h.
RNA isolation was performed using TRIzol reagent (Invitrogen), according to the manufacturer’s protocol. Quantitative real-time PCR to measure CYP6B46 transcript levels was performed as reported by Kumar et al. (20). Ubiquitin was used as an internal control to normalize the abundance of CYP6B46 transcripts.
Waldbauer Assays for Nicotine Budgeting.
Waldbauer assays to budget the ingested and excreted nicotine in control and CYP-silenced M. sexta larvae were performed as described previously (24, 25, 48). A schematic flowchart of Waldbauer assay protocol is shown in SI Appendix, Fig. S4 (SI Appendix, SI Materials and Methods).
Kinetics of Nicotine Flux in Larvae.
Schematic flowchart of the experimental procedure for the monitoring of the nicotine kinetics in larval hemolymph is shown in SI Appendix, Fig. S5A. All larvae used in this analysis were in the fourth instar and of similar masses (7.0 g ± 0.25 g). Hemolymph (2 µL) of each larva was collected at 0, 30, 60, 180, and 360 min by clipping the tip of the larval horn.
Afflux of ingested nicotine to hemolymph.
Controls and CYP-silenced larvae were rendered nicotine-free by feeding them on irPMT plants for 6 h. To restart the nicotine flux from midgut to hemolymph, these larvae were fed leaves from EV or irCYP plants (having equivalent nicotine contents), respectively, for 6 h and the amount of leaf mass consumed was quantified. Only the larvae that fed continuously during these 6 h were used in the analysis. Nicotine concentrations of the collected hemolymph samples (for control and CYP-silenced larvae, for each time-interval, n = 5) were measured by U(H)PLC/ESI-QTOF-MS (microTOF QII) (46) (SI Appendix, SI Materials and Methods).
Efflux of ingested nicotine from hemolymph.
Larvae that were used to monitor the nicotine afflux to hemolymph were fed on leaves of their respective plant lines for 24 h. The larvae were then transferred to nicotine-free irPMT plants to terminate nicotine ingestion and to begin the gradual clearing of nicotine from midgut and hemolymph. Nicotine concentrations of the collected hemolymph samples (for control and CYP-silenced larvae, for each time-interval, n = 5) were measured by U(H)PLC/ESI-QTOF-MS (microTOF QII).
Efflux of injected nicotine from hemolymph.
This experiment was conducted for determining the kinetics of nicotine discharged from the hemolymph of control and CYP-silenced larvae, in the absence of the flux of ingested nicotine from midgut into the hemolymph. Guts and hemolymph of EV- and irCYP-feeding larvae were rendered nicotine-free by feeding these larvae on irPMT plants for 6 h. Nicotine (70 ± 2.5 µg) was injected to the hemolymph of each of these larvae to attain a concentration of 0.001% of fresh mass (FM). Injection volume was constant (50 µL) for every larva and the dorsal point between fifth and sixth body segments was used as the injection site. After injection, hemolymph samples were collected (for control and CYP-silenced larvae, for each time-interval, n = 5) and their nicotine concentrations were measured by U(H)PLC/ESI-QTOF-MS (microTOF QII).
Nicotine efflux from the dissected midgut.
To understand if the CYP silencing in the midguts of irCYP-fed larvae influenced the kinetics of nicotine efflux from the midgut, we conducted the assays using excised midguts of control and CYP-silenced larvae. EV- and irCYP-fed fourth-instar larvae were fed on EV plants for 6 h to ensure that their gut-contents had the same nicotine concentration and that the results of the assay were not influenced by variation in food material. Midguts of these larvae were dissected so that the food content of the midgut remained intact. Ends of each midgut were sealed with clamps and the sealed midgut was carefully submerged in 500 µL of bathing buffer (NaPO4 pH 7.0 and 0.3 M sorbitol) in a 30-mm Petri plate. Midguts that were punctured during the dissection or had lost food material were not used. A 50-µL aliquot of bath-buffer was collected at 0, 5, 10, 15, 20, 30, and 60 min (for control and CYP-silenced larvae, for each time-interval, n = 5). Nicotine concentrations of these collected bath-buffer samples were measured by HPLC/ESI-Q3-MS (Varian 1200).
Volatile Nicotine Trapping.
Volatile nicotine was measured by adsorbing it on the pieces (2 mm of PDMS tubing; Reichelt Chemietechnik).
Measuring nicotine in larval headspace.
Nicotine in larval headspace was trapped for 1 h in a sealed and ventilated glass vial (5 cc), having a PDMS tube suspended in the headspace from the seal with the help of a solid needle (SI Appendix, SI Materials and Methods and Fig. S6B).
Headspace-nicotine during the no-choice assays with perfuming.
Nicotine in the container of no-choice assays with perfuming was trapped for 1 h on the PDMS tube suspended in the chamber; adsorbed nicotine was extracted and quantified using HPLC/ESI-Q3-MS (Varian 1200). (SI Appendix, SI Materials and Methods).
Trapping nicotine emitted from spiracle and cuticle.
To evaluate if the levels of nicotine exhaled from larval spiracles varied with respect to hemolymph nicotine concentration, different amounts of nicotine (0.001, 0.002, and 0.004% of FM) were injected into the hemolymph of AD-fed larvae (7.0 ± 0.25 g FM). Injection volume was kept constant (50 µL) for every larva and the dorsal point between the fifth and sixth body segments was used as the injection site (SI Appendix, SI Materials and Methods). To measure nicotine emitted from spiracles, a PDMS tube was glued around the spiracle without disturbing its opening movement, using instant-adhesive (Fig. 4D). PDMS tubes were attached around the spiracle for 2 h, after which they were carefully detached for nicotine extraction. A similar procedure was used to trap nicotine emitted from the spiracles of nicotine-injected (0.002% of FM) control and CYP-silenced larvae. However, to trap the spiracle-emitted nicotine ingested by the control and CYP-silenced larvae (which had fed on EV or irCYP plants, respectively), PDMS tubes were attached around the spiracles of washed larvae. These larvae were fed on irPMT leaves for 2 h before the attachment of the PDMS tubes.
To measure nicotine emitted from the cuticle of AD-fed larvae, PDMS tubes were attached to the dorsal tip of the cuticle. In each larva, only one spiracle was sampled; therefore, the total amount of nicotine emitted by each larva was estimated by multiplying the amount of nicotine on one PDMS tube by 18 (total number of spiracles/ larva). Nicotine adsorbed on PDMS tubes attached to spiracle or cuticle was quantified using the internal standard (SI Appendix, SI Materials and Methods).
Extraction and Quantification of Nicotine.
Leaf and larval frass.
Homogenized leaf material and larval frass were extracted in extraction buffer A [60% methanol (vol/vol) containing 0.05% glacial acetic acid] and were chromatographed on Agilent-HPLC 1100 as described previously (49); relative concentration of nicotine was estimated using the standard curve of external nicotine standards (SI Appendix, SI Materials and Methods).
Hemolymph, bath-buffer, and PDMS tube.
Two microliters hemolymph or 50 µL bath-buffer or the sampled piece of PDMS tube was mixed with 50 µL extraction buffer B [60% methanol (vol/vol), 0.05% glacial acetic acid and 5 ng d3-nicotine (Cambridge Isotope Laboratories) as an internal standard]. These mixtures were centrifuged at 13.4 × g for 20 min, at 4 °C. Clear supernatant was collected and analyzed using a HPLC/ESI-Q3-MS (Varian 1200) with 35V capillary voltage as described previously (46).
Analysis of Cotinine, CNO and NNO.
The limits of detection for nicotine, cotinine, CNO and NNO were determined using U(H)PLC/ESI-qTOF-MS. The efficiency of extraction of each compound from frass was determined by spiking varying amounts in the frass, extracting it, and then quantifying it. These compounds were then detected and quantified from the frass or hemolymph of control or CYP-silenced larval samples (SI Appendix, SI Materials and Methods).
Statistical Analyses.
Significance (P ≤ 0.05) of the binary results of all survivorship and predation assays was tested in contingency tables using Fisher’s exact test. These results were normalized by calculating the percentages for each column only to set the upper y-axis limit to uniform 100% level so that the data from all of the assays can be easily visually compared. Percentages were also analyzed by the Fisher’s exact test and the significance applicable to both the frequencies and percentages is shown in the figures. All of the other quantitative data were subjected to one-way ANOVAs and the statistical significance (P ≤ 0.05) was determined using Fisher’s least-significant difference post hoc tests.
Supplementary Material
Acknowledgments
We thank the Brigham Young University for use of their awesome Lytle Ranch Preserve field station; Danny Kessler, Tanja Bloss, Anne Adler, and Celia Diezel for help with the field experiments; Drs. Sirsha Mitra and Shuqing Xu for statistical help; Matthias Schoettner for unflagging support with the analytics; the Max Planck Institute for Chemical Ecology glasshouse team for nurturing the plants; Drs. Charles Dondale, Rod Crawford, and Paula Cushing for spider identification; and René Feyereisen for useful comments on MsCYP6B46 annotation and function. This work was supported in part by a doctoral fellowship from Deutscher Akademischer Austauschdienst (to P.K.); European Research Council advanced Grant, ClockworkGreen 293926 (to I.T.B.); Global Research Laboratory Program 2012055546 of the National Research Foundation of Korea; Human Frontier Science Program Grant RGP0002/2012; and the Max Plank Society.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See QnAs on page 1226.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1314848111/-/DCSupplemental.
References
- 1.Peterson SC, Johnson ND, Leguyader JL. Defensive regurgitation of allelochemicals derived from host cyanogenesis by eastern tent caterpillars. Ecology. 1987;68(5):1268–1272. [Google Scholar]
- 2.Bowers MD, Larin Z. Acquired chemical defense in the lycaenid butterfly, Eumaeus atala. J Chem Ecol. 1989;15(4):1133–1146. doi: 10.1007/BF01014817. [DOI] [PubMed] [Google Scholar]
- 3.Eisner T, Eisner M. Unpalatability of the pyrrolizidine alkaloid containing moth, Utetheisa ornatrix, and its larva, to wolf spiders. Psyche (Stuttg) 1991;98(1):111–118. [Google Scholar]
- 4.Bowers MD. Aposematic Caterpillars: Life-Styles of the Warningly Colored and Unpalatable. New York: Chapman and Hall; 1993. [Google Scholar]
- 5.Matsuda K, Kanaoka S, Akamatsu M, Sattelle DB. Diverse actions and target-site selectivity of neonicotinoids: structural insights. Mol Pharmacol. 2009;76(1):1–10. doi: 10.1124/mol.109.055186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray CL, Quaglia M, Arnason JT, Morris CE. A putative nicotine pump at the metabolic blood-brain barrier of the tobacco hornworm. J Neurobiol. 1994;25(1):23–34. doi: 10.1002/neu.480250103. [DOI] [PubMed] [Google Scholar]
- 7.Baldwin IT. The alkaloidal responses of wild tobacco to real and simulated herbivory. Oecologia. 1988;77(3):378–381. doi: 10.1007/BF00378046. [DOI] [PubMed] [Google Scholar]
- 8.Wink M, Theile V. Alkaloid tolerance in Manduca sexta and phylogenetically related sphingids (Lepidoptera: Sphingidae) Chemoecology. 2002;12(1):29–46. [Google Scholar]
- 9.Thorpe KW, Barbosa P. Effects of comsumption of high and low nicotine tobacco by Manduca sexta (Lepidoptera: Sphingidae) on survival of gregarious endoparasitoidCotesia congregata (Hymenoptera: Braconidae) J Chem Ecol. 1986;12(6):1329–1337. doi: 10.1007/BF01012352. [DOI] [PubMed] [Google Scholar]
- 10.Cornelius ML, Bernays EA. The effect of plant chemistry on the acceptability of caterpillar prey to the Argentine ant Iridomyrmex humilils (Hymenoptera, Formicidae) J Insect Behav. 1995;8(5):579–593. [Google Scholar]
- 11.Snyder MJ, Walding JK, Feyereisen R. Metabolic fate of the allelochemical nicotine in the tobacco hornworm Manduca sexta. Insect Biochem Mol Biol. 1994;24(8):837–846. [Google Scholar]
- 12.Hukkanen J, Jacob P, 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57(1):79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 13.Isman M. A Physiological Perspective. New York: Chapman and Hall; 1992. [Google Scholar]
- 14.Snyder MJ, Hsu EL, Feyereisen R. Induction of cytochrome P-450 activities by nicotine in the tobacco hornworm, Manduca sexta. J Chem Ecol. 1993;19(12):2903–2916. doi: 10.1007/BF00980591. [DOI] [PubMed] [Google Scholar]
- 15.Snyder MJ, Stevens JL, Andersen JF, Feyereisen R. Expression of cytochrome P450 genes of the CYP4 family in midgut and fat body of the tobacco hornworm, Manduca sexta. Arch Biochem Biophys. 1995;321(1):13–20. doi: 10.1006/abbi.1995.1362. [DOI] [PubMed] [Google Scholar]
- 16.Maddrell SHP, Gardiner BOC. Excretion of alkaloids by malpighian tubules of insects. J Exp Biol. 1976;64(2):267–281. doi: 10.1242/jeb.64.2.267. [DOI] [PubMed] [Google Scholar]
- 17.Self LS, Guthrie FE, Hodgson E. Adaptation of tobacco hornworms to the ingestion of nicotine. J Insect Physiol. 1964;10(6):907–914. [Google Scholar]
- 18.Self LS, Guthrie FE, Hodgson E. Metabolism of nicotine by tobacco-feeding insects. Nature. 1964;204(495):300–301. doi: 10.1038/204300a0. [DOI] [PubMed] [Google Scholar]
- 19.Govind G, et al. Unbiased transcriptional comparisons of generalist and specialist herbivores feeding on progressively defenseless Nicotiana attenuata plants. PLoS ONE. 2010;5(1):e8735. doi: 10.1371/journal.pone.0008735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar P, Pandit SS, Baldwin IT. Tobacco rattle virus vector: A rapid and transient means of silencing manduca sexta genes by plant mediated RNA interference. PLoS ONE. 2012;7(2):e31347. doi: 10.1371/journal.pone.0031347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mao YB, et al. Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat Biotechnol. 2007;25(11):1307–1313. doi: 10.1038/nbt1352. [DOI] [PubMed] [Google Scholar]
- 22.Gordon KHJ, Waterhouse PM. RNAi for insect-proof plants. Nat Biotechnol. 2007;25(11):1231–1232. doi: 10.1038/nbt1107-1231. [DOI] [PubMed] [Google Scholar]
- 23.Steppuhn A, Gase K, Krock B, Halitschke R, Baldwin IT. Nicotine’s defensive function in nature. PLoS Biol. 2004;2(8):E217. doi: 10.1371/journal.pbio.0020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rayapuram C, Baldwin IT. Using nutritional indices to study LOX3-dependent insect resistance. Plant Cell Environ. 2006;29(8):1585–1594. doi: 10.1111/j.1365-3040.2006.01534.x. [DOI] [PubMed] [Google Scholar]
- 25.Waldbauer GP. The Consumption and Utilization of Food by Insects. London: Academic; 1982. pp. 229–288. [Google Scholar]
- 26.Foelix RF. Chemosensitive hairs in spiders. J Morphol. 1970;132(3):313–333. doi: 10.1002/jmor.1051320306. [DOI] [PubMed] [Google Scholar]
- 27.Kessler A, Baldwin IT. Defensive function of herbivore-induced plant volatile emissions in nature. Science. 2001;291(5511):2141–2144. doi: 10.1126/science.291.5511.2141. [DOI] [PubMed] [Google Scholar]
- 28.Schuman M, Barthel K, Baldwin IT. Herbivory-induced volatiles function as defenses increasing fitness of the native plant Nicotiana attenuata in nature. eLife. 2012;1(1):e00007. doi: 10.7554/eLife.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eisner T, Baldwin IT, Conner J. Circumvention of prey defense by a predator: Ant lion vs. ant. Proc Natl Acad Sci USA. 1993;90(14):6716–6720. doi: 10.1073/pnas.90.14.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feyereisen R. Arthropod CYPomes illustrate the tempo and mode in P450 evolution. Biochim Biophys Acta. 2011;1814(1):19–28. doi: 10.1016/j.bbapap.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 31.Bergé JB, Feyereisen R, Amichot M. Cytochrome P450 monooxygenases and insecticide resistance in insects. Philos Trans R Soc Lond B Biol Sci. 1998;353(1376):1701–1705. doi: 10.1098/rstb.1998.0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coon MJ, Vaz ADN, Bestervelt LL. Cytochrome P450 2: Peroxidative reactions of diversozymes. FASEB J. 1996;10(4):428–434. doi: 10.1096/fasebj.10.4.8647341. [DOI] [PubMed] [Google Scholar]
- 33.Li XC, Baudry J, Berenbaum MR, Schuler MA. Structural and functional divergence of insect CYP6B proteins: From specialist to generalist cytochrome P450. Proc Natl Acad Sci USA. 2004;101(9):2939–2944. doi: 10.1073/pnas.0308691101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petersen RA, Zangerl AR, Berenbaum MR, Schuler MA. Expression of CYP6B1 and CYP6B3 cytochrome P450 monooxygenases and furanocoumarin metabolism in different tissues of Papilio polyxenes (Lepidoptera: Papilionidae) Insect Biochem Mol Biol. 2001;31(6–7):679–690. doi: 10.1016/s0965-1748(00)00174-0. [DOI] [PubMed] [Google Scholar]
- 35.Zhou JL, Zhang GR, Zhou Q. Molecular characterization of cytochrome P450 CYP6B47 cDNAs and 5′-flanking sequence from Spodoptera litura (Lepidoptera: Noctuidae): Its response to lead stress. J Insect Physiol. 2012;58(5):726–736. doi: 10.1016/j.jinsphys.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 36.Li XC, Schuler MA, Berenbaum MR. Jasmonate and salicylate induce expression of herbivore cytochrome P450 genes. Nature. 2002;419(6908):712–715. doi: 10.1038/nature01003. [DOI] [PubMed] [Google Scholar]
- 37.Weinhold A, Baldwin IT. Trichome-derived O-acyl sugars are a first meal for caterpillars that tags them for predation. Proc Natl Acad Sci USA. 2011;108(19):7855–7859. doi: 10.1073/pnas.1101306108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morris CE. Uptake and metabolism of nicotine by the CNS of a nicotine-resistant insect, the tobacco hornworm (Manduca sexta) J Insect Physiol. 1983;29(11):807–817. [Google Scholar]
- 39.Glendinning JI, Slansky F. Consumption of a toxic food by caterpillars increases with dietary exposure- support for a role of induced detoxification enzymes. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1995;176(3):337–345. [Google Scholar]
- 40.Snyder MJ, Glendinning JI. Causal connection between detoxification enzyme activity and consumption of a toxic plant compound. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1996;179(2):255–261. doi: 10.1007/BF00222792. [DOI] [PubMed] [Google Scholar]
- 41.Eisner T, Meinwald J. Alkaloid-Derived Pheromones and Sexual Lelection in Lepidoptera. Florida: Academic; 1987. [Google Scholar]
- 42.Barbosa P, et al. Plant allelochemicals and insect parasitoids: Effects of nicotine on Cotesia congregata and Hyposoter annulipes. J Chem Ecol. 1986;12:1319–1328. doi: 10.1007/BF01012351. [DOI] [PubMed] [Google Scholar]
- 43.Krügel T, Lim M, Gase K, Halitschke R, Baldwin IT. Agrobacterium-mediated transformation of Nicotiana attenuata, a model ecological expression system. Chemoecology. 2002;12(4):177–183. [Google Scholar]
- 44.Halitschke R, Baldwin IT. Antisense LOX expression increases herbivore performance by decreasing defense responses and inhibiting growth-related transcriptional reorganization in Nicotiana attenuata. Plant J. 2003;36(6):794–807. doi: 10.1046/j.1365-313x.2003.01921.x. [DOI] [PubMed] [Google Scholar]
- 45.Schwachtje J, Kutschbach S, Baldwin IT. Reverse genetics in ecological research. Plos One. 2008;3(2):e1543. doi: 10.1371/journal.pone.0001543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kessler D, et al. Unpredictability of nectar nicotine promotes outcrossing by hummingbirds in Nicotiana attenuata. Plant J. 2012;71(4):529–538. doi: 10.1111/j.1365-313X.2012.05008.x. [DOI] [PubMed] [Google Scholar]
- 47.Waldbauer GP, Yamamoto RT, Bowers WS. Laboratory rearing of tobacco hornworm Protoparce sexta (Lepidoptera- Sphingidae) J Econ Entomol. 1964;57(1):93–95. [Google Scholar]
- 48.Cresswell JE, Merritt SZ, Martin MM. The effect of dietary nicotine on the allocation of assimilated food to energy metabolism and growth in fourth-instar larvae of the southern armyworm, Spodoptera eridania (Lepidoptera: Noctuidae) Oecologia. 1992;89(3):449–453. doi: 10.1007/BF00317425. [DOI] [PubMed] [Google Scholar]
- 49.Keinänen M, Oldham NJ, Baldwin IT. Rapid HPLC screening of jasmonate-induced increases in tobacco alkaloids, phenolics, and diterpene glycosides in Nicotiana attenuata. J Agric Food Chem. 2001;49(8):3553–3558. doi: 10.1021/jf010200+. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.