Significance
Experimental use of near-threshold stimuli is regarded as an important approach to study neural processes leading to conscious access (defined in operational terms as “reportability”). Looking to what extent prestimulus periods contribute to the variability of perceptual states has become increasingly popular, frequently pointing to an increased excitability of relevant sensory regions. Here, we aim to extend this “local” perspective by a network approach. A framework called “Windows to Consciousness” is introduced. We postulate that along with an enhanced excitability, preestablished pathways of information flow are a crucial ingredient, determining whether an upcoming near-threshold stimulus will be consciously perceived. Using magnetoencephalography combined with state-of-the-art source-imaging approaches, we are able to report supportive evidence.
Keywords: alpha oscillations, graph theory, WIN2CON
Abstract
Which aspects of our sensory environment enter conscious awareness does not only depend on physical features of the stimulus, but also critically on the so-called current brain state. Results from magnetoencephalography/EEG studies using near-threshold stimuli have consistently pointed to reduced levels of α- (8–12 Hz) power in relevant sensory areas to predict whether a stimulus will be consciously perceived or not. These findings have been mainly interpreted in strictly “local” terms of enhanced excitability of neuronal ensembles in respective cortical regions. The present study aims to introduce a framework that complements this rather local perspective, by stating that the functional connectivity architecture before stimulation will predetermine information flow. Thus, information computed at a local level will be distributed throughout a network, thereby becoming consciously accessible. Data from a previously published experiment on conscious somatosensory near-threshold perception was reanalyzed focusing on the prestimulus period. Analysis of spectral power showed reduced α-power mainly in the contralateral S2 and middle frontal gyrus to precede hits, thus overall supporting the current literature. Furthermore, differences between hits and misses were obtained on global network (graph theoretical) features in the same interval. Most importantly, in accordance with our framework, we could show that the somatosensory cortex is “more efficiently” integrated into a distributed network in the prestimulus period. This finding means that when a relevant sensory stimulus impinges upon the system, it will encounter preestablished pathways for information flow. In this sense, prestimulus functional connectivity patterns form “windows” to conscious perception.
An increasing amount of evidence is accumulating underscoring the fact that perception of external stimuli is not an invariant process, but crucially depends on the current brain state at the time the stimulus impinges on the system. The term “brain state” is rather vaguely defined and may be characterized as fluctuating “transient equilibrium conditions, which contains all aspects of past history that are useful for future use” (1; but see also ref. 2). In functional terms, brain states form neural predispositions to generate a specific output [e.g., a conscious (i.e., reportable) percept or an action]. Such behaviorally relevant brain states are present at multiple time scales, ranging from very slow (e.g., the circadian rhythm) to very fast [e.g., within an α-cycle of ∼100 ms (3)]. However, which aspects of neuronal activity actually form a brain state is a complicated issue and critically depends on the behavior under investigation.
For studying neural processes that support conscious perception [here operationally defined as capability of participant to report a stimulus, e.g., verbally or via button press (4)], the use of stimuli matched to perceptual threshold (hence, near-threshold stimuli, NT) is a popular approach (a more comprehensive overview of paradigms and pragmatic issues in defining conscious access to sensory information in an experimental context is given by ref. 4). In NT experiments strong trial-to-trial variability is achieved, with ∼50% of the stimuli being consciously perceived. Importantly, in these types of experiments the term “consciousness” is reduced to a reportable sensation. Because physical differences between stimuli can be excluded as a determining factor, intrinsic neural processes at the time of stimulation will be decisive whether an NT stimulus will be perceived or go unnoticed. In EEG/magnetoencephalography (MEG) studies using visual stimuli, the strength of prestimulus ongoing oscillatory activity mainly in the α-band has been consistently reported to reflect behavioral predispositions, whether an upcoming stimulus will be perceived or not: for example conscious [i.e., reportable perception of visual stimuli (5–7) or transcranial magnetic stimulation-induced visual percepts (phosphenes) (8)] are preceded by relatively reduced levels of α-power in posterior brain regions. Together with other research, this has been interpreted to support the so-called functional inhibition hypothesis (9, 10) of α. Accordingly the strength of α-power is proposed to be indicative of the excitability of the respective neural tissue, with low α-power indicating high excitability. Thus, within this “local” framework, the notion would be that excitability (indexed by α-power) fluctuates from trial-to-trial, so that on some occasions physically weak stimuli will suffice to overcome the inhibition and ignite relevant sensory regions as a consequence (e.g., refs. 8, 11, and 12).
Notably, interpreting prestimulus α-effects in NT experiments in the aforementioned local perspective is to some extent detached from major neurocognitive model frameworks of conscious perception (4, 13), which strongly emphasize “global”—in particular frontoparietal—networks. Fluctuating patterns of prestimulus brain connectivity have been reported, albeit rarely and so far only outside the context of NT experiments (e.g., refs. 14–16). Here, we present a tactile NT study (reanalysis of ref. 17) in which we conceptualize the prestimulus period as a “window to consciousness” (hence the name of our framework: WIN2CON). Our central claim is that local prestimulus excitability differences (expressed via differences in α-power) in “essential nodes” [i.e., cortical regions that selectively encode distinct aspects of conscious perception (18, 19)] are a necessary but not sufficient requirement for an NT stimulus to become consciously reportable. In addition to local increases in excitability, relevant cortical regions mostly located in secondary and association areas (depending on the task at hand) need to be functionally connected to higher-order brain regions described by some as a Global (neuronal) Workspace (20, 21). In this sense, enhanced connectivity of an essential node [e.g., in this study, as suggested by the previous analysis by Wühle et al. (17), the secondary somatosensory cortex, S2; for the issue of whether S1 is an essential node, see ref. 22] to brain structures, which render information consciously accessible, constitute predefined or privileged pathways along which neural information can propagate when confronted with an appropriate stimulus. In the case of an NT stimulus, the architecture of such a prestimulus network will therefore be crucial. On a conceptual level, our idea of the local–global interplay in the prestimulus period integrates a core notion of the so-called gating-by-inhibition hypothesis (10): local up- and down-regulation of α-power constitutes a mechanism of routing information throughout a distributed network; relatively high levels of α-power should be accompanied by an overall decoupling of this respective area, and vice versa.
To investigate this hypothesis, in the present MEG NT study we complemented conventional oscillatory power (i.e., local synchronization) analysis with detailed analysis of functional connectivity patterns in source space. For this purpose we used graph theory (23) to derive patterns of abstract properties of prestimulus functional networks a global and spatially more fine-grained (i.e., nodal) level. Furthermore, a seed-based analysis was used to uncover specific coupling patterns of S2. We hypothesized in particular that, besides differences in local α-power, diverse connectivity indices should reveal that S2 is more efficiently connected to distributed regions (e.g., those of the Global Workspace), when an upcoming NT tactile stimulus reaches consciousness.
Results
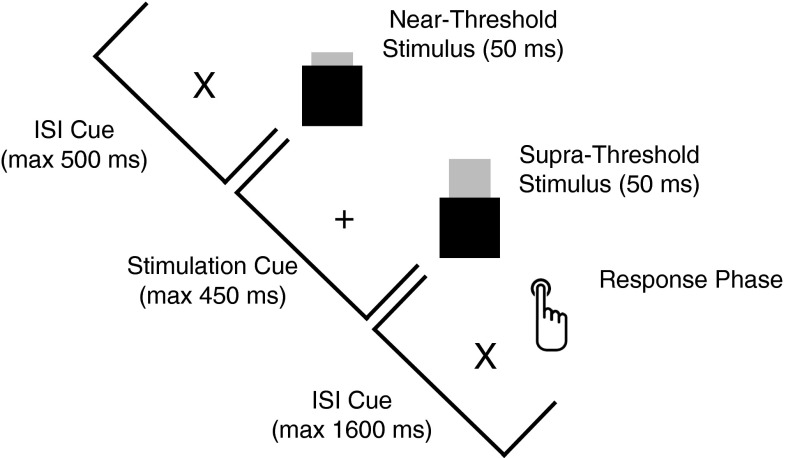
The present study aimed at testing the generic WIN2CON framework on preexisting data published by Wühle et al. (17). The experiment is described in Materials and Methods and can be read in detail in the original paper (17). In this study, we focused on the critical NT (Fig. 1) condition that, because of an adaptive staircase procedure, lead to a rough balance between hits and misses. In brief, the onset of a trial was indicated by a visual cue, which was followed by a brief (50 ms) NT tactile stimulus. A second tactile stimulus of identical duration followed after an interstimulus interval of 450 ms, which was suprathreshold (maximum stimulation intensity) on each trial. In a response phase (indicated by a second visual cue 400 ms after the suprathreshold stimulus, maximum duration 1,600 ms), the participant indicated the quantity of tactile stimuli via button press. Because the original experiment also included sham trials without any first stimulus, participants could not be certain about the exact amount of stimuli. After a brief summary of the behavioral data, we first focus on effects of local synchronization in the α-band, thus enabling us to relate our findings to previous reports in the literature, particularly in the visual modality. This summary is followed by a detailed overview of differences in global as well as local graph-theoretical properties between hits and misses in the NT condition. Finally, the spatial connectivity pattern of S2 to the rest of the network completes the analysis.
Fig. 1.
Setup of a trial in the critical NT condition. Switch of the visual cue from an “X” to a “+” indicated the beginning of the stimulation period. Participants indicated the number of tactile stimuli via button press in a period when the cue switched back again from a “+” to an “X.” Note that although two stimuli were always presented, participants only noticed one (i.e., a miss) stimulus on ∼50% of the trials.
Behavioral Data.
Overall accuracy on the control trials, where either one or two clearly perceptible, suprathreshold stimuli were presented, was very high (>92%), indicating a high level of compliance on the participants side. The adaptive staircase procedure ensured a balance between hits (M ± SE; 49.14 ± 1.05%) and misses (50.86 ± 1.05%). Between participants average NT-intensities varied from 9.07–31.14% of the maximum stimulation intensity, indicating a strong interindividual variability. On average, small but systematic differences could be observed for the stimulation intensities between hits and misses; however, an F test shows that the average intensity difference between hits and misses is well in the range of random fluctuations of stimulus intensities because of the variation of the threshold across the experiment (see figure 4b in ref. 17).
Perception-Related α-Power Effects.
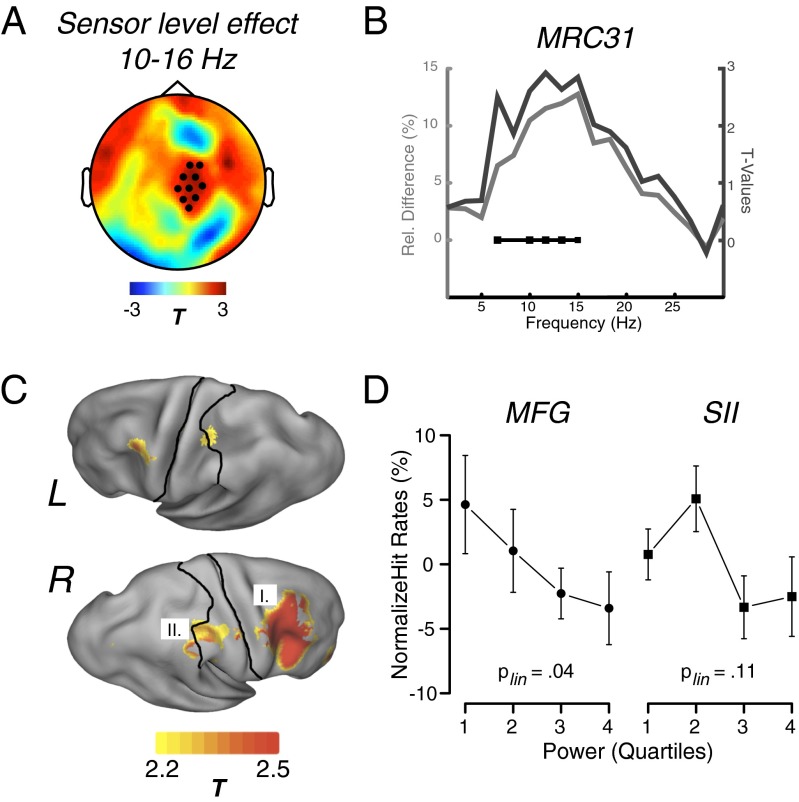
Because the grand average indicated a maximum sensor level effect at 12/13 Hz, spectral estimates averaged over a range between 10–16 Hz in a 600-ms time-window before the NT stimulus was entered into a nonparametric cluster-based permutation test, contrasting misses vs. hits. This analysis revealed one positive cluster (P = 0.05) over central sensors with a rightward lateralization (i.e., contralateral to the stimulated left hand) (Fig. 2A). As hypothesized, this finding indicates relatively enhanced power within this frequency range in the prestimulus period when an upcoming NT tactile stimulus is missed. To gain a more detailed insight into the spectral pattern of this hypothesis-conform effect, the grand-average as well as the uncorrected statistical values are displayed in Fig. 2B for a representative right central sensor. This analysis illustrates that the actual effect is broad, extending from the lower α- into the lower β-range (∼7–15 Hz), with relative differences in power ranging between 10% and 15%. Despite being aware that the effect does not perfectly overlap with the classic textbook definition of α, we will use this term throughout the rest of the manuscript for simplicity.
Fig. 2.
Oscillatory power effects. (A) Before misses, α-power is relatively enhanced over right central sensors. Filled circles mark sensors belonging to a significant cluster as identified via a nonparametric permutation test. (B) Statistical values and relative power differences between misses and hits (positive values meaning more power for misses) for a representative sensor in the aforementioned cluster. Connected squares indicate (uncorrected) significant differences between the conditions. (C) Source-level difference for 12 ± 5 Hz indicates that in particular the right S2 and posterior portions of the MFG drive the described effect. Borders of the somatosensory cortex are drawn on the inflated surfaces (both created using CARET; http://brainvis.wustl.edu/wiki/index.php/Main_Page). (D) Normalized hit rates displayed as a function of binned single trial power (1 = lowest power; 4 = highest power) for the right MFG and S2. Note that for S2, the linear trend was weak overall.
The sensor level analysis was also performed to optimally inform our source space analysis using a frequency-domain beam-forming technique (Materials and Methods) applied to the aforementioned time and frequency (12 ± 5 Hz) window. Confirming the sensor-level data, effects were mainly right-lateralized even though similar patterns could be identified in roughly homolog cortical regions of the left (ipsilateral to upcoming stimulation) hemisphere (Fig. 2C). Differences were characterized by enhanced α-power when participants missed an upcoming NT stimulus. Although, as hypothesized, the effect was present in S2, a descriptively even larger effect in the same direction (i.e., misses > hits) could be observed for posterior portions of the right middle frontal gyrus (MFG), strongly overlapping with premotor areas (e.g., BA6). To scrutinize the relationship between α-power and behavior in more detail, single-trial power values were extracted for two grid points in these respective regions and sorted according to their magnitude. Normalized hit rates are shown for four bins, ranging from low (1) to high (4) α-power. Although for MFG a linear decrease (P = 0.04) (Fig. 2D, Left) of normalized hit rate was observable with increasing α-power, the relationship was more complex for the right S2 (Fig. 2D, Right). Even though, on average, this region significantly differentiates between misses and hits, the linear trend is only weak (P = 0.11), whereas some nonlinear elements appear to be present as well (cubic trend: P = 0.06). The latter observation partially overlaps with a previous report (24), which also described a rather nonlinear relationship between single-trial –sorted α-power and somatosensory detection.
To summarize this part, as hypothesized on a level of “local” activity, misses were preceded by relatively enhanced power in the α-range lateralized to S2 contralateral to the stimulated finger and posterior MFG. For S2, however, single-trial analysis suggests nonlinear processes to influence the relationship between α-power and behavior. In the following subsections of Results we address diverse aspects of the functional connectivity architecture in the prestimulus period.
Global Graph Properties.
Even though the framework outlined in the introduction makes specific predictions with regards to connectivity states of essential nodes (here: right S2) in the prestimulus time period, at a first level of analysis, we compared global network (graph theoretical) (Materials and Methods) properties between misses and hits. As a first omnibus measure to determine frequencies of interests, we turned to small-worldedness. This measure relates the level of clustering to the distances (path length) in a graph and can therefore be driven by increases of clustering or decreases of distance (a small-world network is characterized by high clustering and short distances). Subsampling the data (every third frequency) and entering into a t test with Bonferroni correction, this analysis revealed an effect (P = 0.007) at 17 Hz (i.e., slightly above the power effects) with increased small-worldedness preceding misses (Fig. 3A) [interestingly, a frequency virtually identical to the one disclosed by Hipp et al. for large parts of the analysis (25)]. To elucidate the nature of this effect, global clustering as well as measures of long-range integration (distance and efficiency) (Materials and Methods) were analyzed separately for 17 Hz. This analysis revealed that the small-worldedness is likely to be driven by the prevailing network before misses, being overall more clustered than the one before hits (P = 0.007). With regards to distance, no overall statistically significant difference was observed for hits compared with misses (P = 0.23), with both conditions attaining normalized distance values close to random networks. However, another measure of global integration—efficiency (a measure much related to distance, however more robust to outliers; see Materials and Methods)—indicates shorter communication pathways in the prestimulus period for hits (P = 0.04). Therefore, on a global level, prestimulus networks before misses can be described in terms of more local integration, whereas networks before hits are rather characterized by global integration.
Fig. 3.
Depiction of global and local graph-theoretical findings. (A) Relative increase of small worldedness at ∼17 Hz for misses in the prestimulus period, implying enhanced clustering or a reduction of distance or both. (B) The effect in A is most likely driven by an enhanced clustering for misses (Left), as indicated by the normalized clustering coefficient. No significant difference was observed with regards to distance (Center). However, efficiency was increased significantly for this condition in the prestimulus period (Right), indicating a more integrated global network before hits. (C) Surface mapping of local graph-theoretical metrics (misses minus hits) indicates, in particular for the right S2 (this study’s region of interest), relatively increased clustering and long-range integration (distance/efficiency metric) for upcoming hits. The local clustering result (C, Left) seems initially at odds with the global-clustering result (B, Left); however, massive relative increases for misses were observed in cingulate cortex, which likely drives the global effect (see Fig. S1). Dotted lines indicate the borders of the somatosensory cortex.
Local Graph Properties.
Whereas global graph-theoretical measures characterize distinct features of an entire network, several measures can also be calculated locally (i.e., at the level of individual nodes). For the previously determined frequency of interest (17 Hz; see above), we computed local clustering, distance, and efficiency, and contrasted misses vs. hits. Results are displayed in Fig. 3C, masked at P < 0.05 (uncorrected) for visualization purposes. A critical prediction of the WIN2CON framework for NT experiments is that relevant essential nodes—in the present case S2—are overall more strongly integrated, signifying preestablished pathways of information flow from these regions to other brain regions. This hypothesis is confirmed by the global integration measures distance (higher for misses) and efficiency (lower for misses) (Fig. 3C, Center and Left). Although some differences can be noted between these two measures (e.g., with regards to the right occipital cortex; note that efficiency is considered to be the overall more robust measure), both measures show conforming effects in the right S2, with the focus of the effect shifted slightly anterior compared with the power effect (Fig. 2C). A further (unpredicted) conforming effect for both measures could be identified for the left anterior temporal cortex, encompassing inferior frontal areas for the efficiency measure. Note that the local effects on the distance measure could be identified despite an absence of global effects on this measure, meaning that integration of individual nodes can differ meaningfully between conditions, whereas the average path length within the entire graph remains unchanged. Interestingly, local clustering effects could be observed in very similar regions (Fig. 3C): note the striking overlap in particular between the effects for clustering and those observed for nodal efficiency (Fig. 3C, Left and Right, respectively). Interestingly, and nonconforming to the overall relatively increased clustering observed for misses on the global measure (Fig. 3B, Left), local clustering indicated relatively enhanced clustering for hits in the aforementioned regions, in particular S2. The global clustering effect appears to be mainly driven by massive relative increases for misses in the cingulate cortex, with a rightward lateralization (Fig. S1). Cingulate regions have been implicated to constitute a highly interconnected region (26), which may also have contributed to some extent to the global clustering effect. To summarize this part, the most important finding is that S2 (here contralateral to the stimulated hand)—a putative essential node for conscious somatosensory perception—exhibits relatively increased levels of integration at a local level (increased clustering) as well as over long distances (reduced distance/increased efficiency) in the prestimulus period when an upcoming NT stimulus is detected (hits).
Secondary Somatosensory Cortex Connectivity Pattern.
The main finding so far, conforming with our overall theoretical framework, was the demonstration that S2 (i.e., a putative essential node for conscious tactile perception) appears to be more integrated into a widespread network when an upcoming NT stimulus is consciously reportable. The present analysis gives a descriptive overview of cortical regions in which connectivity differences (imaginary coherence at 17 Hz) between misses and hits can be observed when S2 is used as a seed region. In agreement with the aforementioned efficiency/distance effects, Fig. 4 clearly depicts that before hits, S2 is more strongly connected to a widely distributed set of brain regions. The strongest effect in this sense was observed for left anterior temporal/inferior frontal regions, spatially strongly overlapping with the aforementioned local graph-theoretical effects. Less-pronounced effects were also detectable in approximately homolog areas ipsilateral to the seed region. Increased connectivity was furthermore observed between the right S2 and right as well as left sensorimotor regions, including right premotor areas (BA 6), the left and right inferior parietal lobe, left and right anterior cingulate gyrus, right MFG, left angular gyrus (BA 39), and left posterior temporal cortex. The only brain region that exhibited a reversed pattern of connectivity to the right S2 in the prestimulus period was the right posterior cingulate gyrus/precuneus: this region was more strongly connected to the seed region before misses.
Fig. 4.
Depiction of functional connectivity differences (misses vs. hits) at 17 Hz in the prestimulus period using the right S2 as seed region (see center image). Relatively increased coupling can be seen before hits between the seed and a widely distributed set of brain regions, overall conforming with local graph-theoretical findings (Fig. 3C). The only exception to this larger picture is the right posterior cingulate gyrus/precuneus, which is more strongly connected to the right S2 before misses.
Discussion
Contrasting brain responses to stimuli close to perceptual threshold has been frequently used in the quest to identify the neuronal correlates of consciousness, with most studies showing that the NT stimulus has to engage a network of mainly frontal-parietal regions to become reportable (see ref. 4 for a review). However, a growing amount of studies—in particular in the visual domain—demonstrates that ongoing brain activity in prestimulus periods can already determine the perceptual fate of an upcoming stimulus. Even though other methods have been applied, such as functional MRI (fMRI) (e.g., ref. 27), most support for this idea comes from human electrophysiology, where brain activity can be sampled at high temporal resolution. These studies consistently point to a crucial role of prestimulus α-power (currently α-phase is also under intensive investigation, but beyond the scope of this manuscript; see refs. 28 and 29). In the context of NT experiments, the common observation is that before hits, brain regions involved in processing the upcoming stimulus exhibit relatively reduced α-power compared with misses. In accordance with the functional-inhibition hypothesis (9, 10), this has been mostly interpreted as a sign of altered local excitability (i.e., with reduced α-levels signifying enhanced excitability, whereas high levels of α putatively represent states of relative inhibition) (11). Overall, our effects on oscillatory power are in line with this hypothesis: before misses relatively enhanced power could be observed in the α- to lower β-band in right sensory and premotor regions (i.e., contralateral to the upcoming NT stimulus). However, specifically in the somatosensory domain there have been divergent reports, which will be discussed in the subsequent section. The WIN2CON framework outlined in the introduction, however, exceeds existing notions based on local excitability by stating—in compliance to the gating-by-inhibition hypothesis—that the level of α-activity is related to the extent the respective region is integrated into a distributed network. With regards to the prestimulus period, the specific prediction would be that an essential node with relatively low α-power will entertain a greater amount of functional connections than with high levels of α-power (see ref. 30 for a test of this prediction during visual stimulus processing). Thus, when a NT stimulus arrives, it will encounter “preestablished” nonrandom routes, reducing the degree-of-freedom of information flow determining stimulus’ future behavioral fate. In the present context we mean the likelihood to become consciously accessible, as indicated by the subject’s report. Our results provide strong evidence for our proposed framework.
Local Activity Effects.
Before elaborating on the more central connectivity effects, the α-power effects will be put into perspective to similar electrophysiological experiments performed in the somatosensory as well as the visual modality, for which the greatest body of evidence exists. Early evidence from an EEG study by Ergenoglu et al. (5) using NT visual stimuli showed that relatively reduced α-power at posterior electrodes preceded detected stimuli, a finding that has been frequently replicated since (12, 14; for a nonconforming finding, see ref. 31; the latter study, however, combined a detection task within a spatial attention paradigm). Further studies using transcranial magnetic stimulation to elicit phosphenes also point to lower levels of α preceding a conscious visual percept (8). A recent report by Lange et al. (7) convincingly indicates that reduced posterior α precedes both, correct as well as incorrect (i.e., illusory) visual percepts, thus implying that the level of α does not necessarily reflect the veridicality of perception, but rather the excitability of the respective neuronal ensemble. This notion is fully compatible with the aforementioned functional inhibition hypothesis. Motivated by findings in tinnitus (a pathological conscious auditory percept), we have arrived at similar conclusions for the auditory modality (32, 33). This very basic feature of α-oscillations may explain to some extent its sensitivity for various kinds of cognitive manipulations, such as attention or working memory (for review, see ref. 34; see below for more on this issue).
In agreement with the NT findings in the visual modality, we were capable of finding prestimulus differences in the higher α- to lower β-range, with lower power preceding hits. This finding was evident mainly in contralateral S2 as well as MFG, and to a far weaker extent (and only visible in source analysis; see Fig. 2C) in homologous ipsilateral regions. Overall, this finding supports the notion described in the introduction that S2 is in a relatively increased excitable state before the arrival of the NT stimulus in case of conscious perception. Our power effects add to a domain with rather heterogenous findings. Although reduced prestimulus α-power facilitating conscious perception in the visual domain can be considered a fairly robust finding, in the somatosensory domain there have been diverging reports. For example, although Schubert et al. (35) and Jones et al. (22) (albeit for S1) report similar findings as in the present study, Linkenkaer-Hansen et al. [36; for a conforming EEG study see also Zhang and Ding (37)] has reported a rather complex nonlinear (inverted-U shape) relationship between α-power in the somatosensory cortex and detection performance. At parietal sensors, the authors observed a positive linear relationship. Regarding the quadratic trend, the authors argue with a stochastic resonance-type mechanism, which predicts optimal processing of an upcoming stimulus at intermediate noise levels. Besides the very obvious differences in the experimental design, an important difference should be noted between this study and the present report: in Linkenkaer-Hansen et al. (36) NT stimuli could arrive at both hands separately or simultaneously in a nonpredictable fashion, making the task overall more demanding. This result may account for the posterior positive relationship between α and performance (i.e., suppression of distracting visual input), which was not observed in the present study. Furthermore, it has to be stated that the present conclusions are mainly drawn from the hits vs. misses contrast. When depicting detection performance sorted on the basis of single-trial α-power (Fig. 2), it becomes obvious that in S2—although an overall high vs. low power behavioral difference is present—the relationship is not perfectly linear as, for example in the right MFG. Thus, our findings may not be totally at odds with those in Linkenkaer-Hansen et al. (36) and subtle details in the experimental design may actually shape the specific outcome. For example, Haegens et al. (11) report that although for the majority of assessed brain regions, including S2, lower α-power correlated with increased firing and high α-power with reduced firing, the relationship was quadratic in the case of S1. Thus, differing contributions of S1 and S2 to a behavioral task may lead to more complex association patterns with behavior, if the separation (on sensor as well as source level) of the relative contributions does not work perfectly. An unpredicted area exhibiting a global hits vs. misses α-effect, as well a linear relationship to behavior, was the right MFG. In a previous fMRI study (38) it could be shown that premotor activity relates to the grading of conscious experience, with clear conscious experience going along with the strongest blood-oxygen level-dependent signal. However, we are unaware of reports demonstrating premotor prestimulus effects in the context of NT stimuli. At the moment, we can only speculate about the functional relevance. One possibility is that the effect relates to the formation of unconscious motor plans (e.g., ref. 39). More related to the present finding is a recent report by De Lange et al. showing the influence of perceptual decision making on motor/premotor oscillatory activity (40). This effect was already present in a prestimulus phase on trials as well, when participants were not explicitly cued with respect to the upcoming target. Thus, our results may indicate shifted starting points in a perceptual decision-making context when the actual NT stimulus arrives. Even though this is appealing as an interpretation, a caveat is that responses were given with the right hand (i.e., ipsilateral to the observed effects). Future studies should explicitly map response categories to one hand to allow a better comparison of effects to those reported on perceptual decision making. An alternative possibility would be that right MFG effects mediate prestimulus attentional processes that may be fluctuating on a trial-by-trial basis. The relationship of our effects to “attention” will be treated below in a separate section.
To conclude, although some differences to the somatosensory NT study by Linkenkaer-Hansen et al. (36) exist, our local activation results are very compatible with the overall reported pattern of increased hit rate if a NT stimulus is preceded by low α-power. Importantly, taken together with previous MEG/EEG studies in the visual domain, our result illustrates how regionally specific prestimulus effects are, making more trivial explanations, such as overall arousal, an unlikely explanation.
Beyond Local Processing: Prestimulus Functional Networks as Windows to Conscious Perception.
The majority of neuroscientific theoretical frameworks on conscious perception acknowledge the outstanding importance of long-range connections in making locally computed information available in a distributed network. The involvement of frontoparietal areas—which entertain feedback connections to early sensory regions—have been especially frequently reported as a hallmark feature distinguishing conscious from nonconscious states (13, 41). This notion is backed up by several experimental works using diverse neuroimaging techniques (4), but clinical studies on populations with disturbed consciousness [e.g., vegetative state (42)] also underline the critical involvement of frontal and parietal areas in generating conscious states. Thus, activity patterns that represent distinct features of the sensory environment will not reach conscious access if remaining locally confined. Despite the importance of this notion when discussing the neuronal mechanisms underlying consciousness, it has hardly impacted EEG/MEG studies focusing on prestimulus periods (see for example ref. 43 for one of the rare EEG studies showing parietal involvement preceding perceptual switches using bistable visual stimuli). Indeed, the most common finding, a prestimulus α-difference in relevant sensory regions (8, 12, 14), has been usually interpreted strictly in terms of local excitability modulations: for example, in visual NT experiments visual cortical areas are in a relatively preexcited state before hits compared with misses. Thus, a weak stimulus impinging upon relevant sensory areas will have an enhanced probability of creating an output. However, another at least complementary option—at the heart of the WIN2CON framework proposed here—is that hits are preceded by enhanced functional connectedness of relevant “essential node” sensory regions (see introductory discussion) to downstream cortical areas, thus forming preestablished pathways of information flow. Few works along these lines have been performed using fMRI (see e.g., ref. 44). In particular, in the somatosensory domain, Ploner et al. (16) were able to show that prestimulus functional connectivity strength between the anterior insular cortex and brainstem periaqueductal gray predicted whether a subsequent noxious stimulus was perceived as painful or not. For the domain of NT perception however, we are not aware of studies using time-sensitive methods, such as MEG/EEG, to address the issue of prestimulus functional connectivity states [for example, in working memory task (45)].
Conforming to our theoretical framework, we are capable of showing that task-relevant secondary somatosensory regions exhibit increased local (clustering) as well as long-range (degree, efficiency) connectedness before hits. This finding nicely confirms the aforementioned gating-by-inhibition hypothesis, allowing the notion that “spontaneously” fluctuating α-activity may shape functional networks in a manner as, for example, induced by a cue (46) or stimulus (30). However, diverging results were also identified: for example, in the right MFG, where strong α-power effects could be found, we were not able to find effects on any of the relevant graph-theoretical measures. In a same vein, strong effects on graph-theoretical measures were consistently found for left anterior temporal/inferior frontal areas (indicating stronger connectedness before hits); however, α-power effects were absent. Thus, it appears that α-power, as a measure of local excitability, does not universally reflect the level of functional connectivity of the respective brain region. It is possible that the outlined association may mainly hold for sensory and primary motor areas, a matter that requires deeper scrutiny. Using the right S2 as seeding region, however, reveals that the aforementioned regions are more highly connected to S2 before hits. Further regions that entertain stronger prestimulus connectivity before hits include frontal as well as parietal regions. This finding implies that NT perception is not only more likely with increased preestablished connectedness of the relevant sensory region, but that the functional connectivity pattern needs to include core regions of the so-called neuronal workspace (20). Further studies will show if this statement can be generalized beyond the somatosensory modality. Interestingly, within the overall pattern of enhanced connectivity with S2 before hits, the posterior cingulate cortex exhibits a decoupling to the right S2 before misses. This region has been implicated to be a core region of the default-mode network (47) and a decoupling of sensory regions may be a prerequisite for an external orientation of attention, even though this interpretation is necessarily very speculative. Importantly, however, this example illustrates that despite their utility, graph-theoretical measures are abstract measures that may mask some of the fine-pattern details. This aspect may also be exemplified by the small-worldedness metric that was overall increased at 17 Hz before misses, an effect mainly driven by global-clustering level. Studying the local clustering values, however, indicates that the global effect is the result of a very strong relative increase before misses in the cingulate cortex, whereas other relevant brain regions outlined above (e.g., right S2) exhibit increased clustering before hits. This finding underlines the importance of closely observing several levels when looking at functional architectures, from the global perspective (summarizing specific properties of large networks in a single number) down to specific patterns using seed regions.
Relationship to “Attention.”
The main claim of our “windows” metaphor—that is, conscious access (using an operational definition; see, for example, ref. 4) to NT stimuli require distinct prestimulus functional network architectures—may be criticized for not explicitly equating such brain states with certain psychological states or cognitive constructs. It may, for example, be asserted that the reported patterns “simply” reflect attention. Indeed, the relationship and even distinction between “attention” and “consciousness” is a controversial issue; however, a consensus among several major neuroscientific theorists now assumes them to be in principle separate entities (13, 41, 48). In an influential taxonomy of diverse states of conscious access, Dehaene et al. hold that although attention can be directed independent of a stimulus becoming conscious (“subliminal perception”), full conscious access can only be achieved with top-down attention present (41). Whereas for NT stimuli this relationship appears intuitive, impressive psychological experiments have also demonstrated that clearly suprathreshold stimuli can go unnoticed in the absence of attention (49), even in cases in which very salient stimuli are introduced into a visual scene (50).
Thus, in particular because our focus is on the prestimulus period when neural activity related to the NT stimulus is not yet present, one may rightly ask whether our prestimulus patterns reflect attentional differences between hits and misses. As mentioned earlier, α-reductions in regions processing upcoming attended locations/stimuli is a robust finding in EEG/MEG studies. We are not aware of such studies relating attentional α modulations to graph-theoretical effects (in particular efficiency). However, based on the “gating-by-inhibition” hypothesis (10), very similar associations as found in this study would be predicted; that is, an attended region becoming more connected, whereas an unattended region processing a distractor might become decoupled (for such a pattern in a different experimental context, see ref. 30). A suggestive argument in favor of an attentional interpretation is the involvement of the right MFG, which has been reported to be a core region of the dorsal attention network (51). Naturally however, the involvement of a specific brain region cannot be directly taken as proof for the involvement of a specific cognitive process. Additionally, other arguments point out a more complex picture. For example, whereas attention usually goes along with improved (more “veridical”) perception, some studies show the involvement of sensory α in illusory perception (e.g., refs. 7, 32, and 52). Furthermore, studies that attempted to dissociate the “attentional” from the “awareness” aspect by combining a visual NT task within a cued attention paradigm have reported nonconsistent findings when it comes to α: for example, although Wyart and Tallon-Baudry were able to show postcue α-power effects for hits vs. misses only for the attended side (53), Busch and VanRullen (54) generally reported a posterior hits vs. misses main effect for α-power independent of attention (see main effect in figure S5 of ref. 54). Last but not least, the presence of α-modulations in diverse experimental contexts makes its reduction to a single cognitive construct unlikely.
To conclude this topic, although some valid arguments can be brought up that our prestimulus power and connectivity effects are somehow related to trial-by-trial attentional fluctuations, the question is too complex to be resolved based on our study or plausibility arguments alone. Systematic studies investigating time-resolved network properties following attentional manipulations will be needed. In any case, because this work is concerned with predispositions of NT stimulus perception (41), an attentional interpretation of our findings would cause no contradictions to our framework.
Limitations and Conclusions.
Certainly, our study holds some limitations; for example, it could be stated that the adaptation of intensity on a trial-by-trial basis for maintaining a balance between perceptual states leads to a systematic difference in physical intensities between the conditions. Although this is indeed the case on average, Wühle et al. (17) were able to show that the within-condition variability is significantly higher than between-condition variability, thus showing that the overlap is stronger than the actual difference. In addition, fixing the threshold before the experiment could promote the factor time as a confound. Using a fixed-threshold hit rate might decrease or increase over time by fatigue or sensory learning, respectively. In any case, we would argue that adapting intensity on a trial-by-trial basis is not favorable for uncovering prestimulus effects and that, experimentally, this approach is rather conservative with respect to our purposes. Apart from this experimental issue, it has to be stated that at the moment no statements can be drawn to what extent S2 connectivity changes may be a consequence of local α-power modulations or actually its cause. Furthermore, as outlined above, clear assignments of psychological functions (e.g., attention) to fluctuating ongoing brain-activity patterns would be premature.
Despite these shortcomings, the most important contribution of our study is to extend conceptually as well as experimentally a network perspective on conscious perception to the prestimulus period, thereby expanding our view on what constitutes a brain state. To conclude, our data support the view that beyond local excitability, conscious perception of NT stimuli requires distinct functional connectivity patterns in the prestimulus period. Preestablished functional connectivity between sensory and frontoparietal areas likely favors the conscious processing of an upcoming weak sensory stimulus by constituting privileged pathways along which locally processed information can spread. In terms of connectedness, relevant sensory regions appear to be marked by high clustering (indicating highly specialized local computations), as well as high efficiency (indicating strong long-range integration of local computations). Because this happens in the absence of a stimulus, we consider this to be an important predisposition of conscious perception.
Materials and Methods
Participants.
Overall, 12 healthy volunteers (8 women; range 21–25 y) participated in the experiment. All participants were right-handed as assessed by the Edinburgh Inventory (55). Before the experiment, participants gave written informed consent. The procedures of the experiment were approved by the ethics committee of the Medical faculty of the University of Tübingen and were in accordance with the Declaration of Helsinki.
Stimulus Material and Procedure.
The basic set-up of a trial is depicted in Fig. 1. Throughout the experiment, a fixation cue (∼1° of visual angle) was presented in the middle of a screen placed 1 m in front of the participant. During the intertrial interval, this cue consisted of a gray “X” that switched to a “+” symbol 500 ms before the onset of the first of two somatosensory stimuli. The visual symbol switched back 400 ms following the offset of the second stimulus and the task of the participant was to report the amount of tactile stimuli presented on each trial. Critically, two tactile stimuli (50-ms duration; half a cycle of a sine-wave) were always applied to the left index finger, of which the second was consistently clearly above the perceptual threshold (maximum output of stimulator), whereas the first stimulus could either be a near-threshold or above-threshold stimulation [see original report by Wühle et al. (17)]. In an additional condition, sham stimuli were applied in which only a clicking sound mimicking the stimulator sound were presented as first stimulus. The interstimulus interval between the tactile stimuli was set to 450 ms. In the present study, we focused the analysis on the critical NT condition: that is, although two stimuli were always presented, participants equiprobably reported having perceived either one or two stimuli.
Tactile stimulation was achieved by protruding four pins of a Braille element (2 × 2 arrangements) simultaneously via activation of piezo-crystals (Metec). When stimulation was turned off, the rods were hidden under the contact area. Because of the sine-wave shape of the stimulation maximum, protrusion of the rods was reached after ∼25 ms. To minimize auditory and visual information about the stimulation, white noise was presented via insert-earphones and the participant’s left hand was covered with a blanket. The NT intensity of the stimulus was implemented using a simple one-up/one-down staircase procedure (step size 1%). The first stimulus was set to a fixed intensity level of 10% of the maximum protrusion. The staircase procedure was upheld throughout the entire experiment to assure NT stimulation and counterbalanced data. Even though this procedure leads on average to a minimal but systematic difference between hits and misses, this bias was well within the range of the general intertrial variability as confirmed by an F test (see ref. 17 for a detailed treatment). Furthermore (see also Discussion), with regards to prestimulus intervals, this average systematic difference would rather obscure prestimulus effects: that is, our results are likely to be a conservative estimate of the “true” prestimulus influence. To assure that only trials were included in which the staircase procedure converged onto relatively stable values, the first 50 trials were discarded. The experiment consisted overall of four blocks with 200 trials each, in which condition order was randomized. The critical NT condition was presented on 400 trials, leading to approximately 200 hit and 200 miss trials in each individual.
MEG Recording.
Measurements were performed inside a magnetically shielded room (Vakuumschmelze) in a seated position. Signals were acquired using a 151-channel whole-head MEG system (VSM Medtech) and digitized at 625 Hz using a low-pass antialiasing filter of 208 Hz. Data were filtered offline with a 40-Hz low-pass and a 1-Hz high-pass filter and subsequently epoched into trials of 2.55-s length, starting 250 ms before the visual cue indicating the onset of the stimulation period (see above).
Data and Statistical Analysis.
Data and statistical analysis focused on a period of 600 ms before the onset of the NT stimulus, which was segmented from each trial. Data from one sensor was discarded from all participants because of excessive noise. To remove direct current (DC) drifts, data from single trials were detrended by subtracting the average offset from every single sampling point. Cleaning of data were performed in several steps. (i) First hits and misses data were concatenated and inspected for extreme outlying trials, usually containing unphysiological artifacts such as channel jumps, and so forth. These trials were removed and (ii) data subsequently downsampled to 300 Hz before entering it into an independent component analysis (ICA) (56). Component topographies and time courses were inspected and components capturing eye movements (blinks and horizontal eye movements) or heart-beat–related activity were identified (maximum of five components per participant). Component weights gained from the concatenated data were then applied separately to the hit and miss trials, artifact components removed, and raw data reconstructed excluding the respective artifactual data. (iii) Finally, ICA cleaned data were visually inspected and remaining artifactual trials removed manually. Before subsequent analysis, trial numbers between hits and misses were equalized to avoid any systematic influence of absolute trial numbers on the results.
Artifact cleaned data were then analyzed on a sensor level in a frequency domain using multitaper spectral estimation (57) by applying orthogonal Slepian tapers to each epoch (frequency smoothing: ±3 Hz). The tapered epochs were subsequently Fourier-transformed in a 2- to 30-Hz range and the power estimates averaged over trials. An exploratory look at the grand average relative differences between misses and hits (Fig. 2 A and B) implied a ∼10–15% increase for misses in the relevant frequency range of interest (α- extending to lower β-range; see the introduction) at sensors contralateral to the upcoming stimulation. This hypothesis-conform pattern was therefore passed on to a nonparametric cluster-based permutation test (58) that efficiently handles the issue of multiple comparisons. In brief, spectral estimates between 10 and 16 Hz were averaged, and misses were contrasted with hits via a dependent-samples t test. The resulting statistical values were thresholded at P < 0.05 and summed up in contiguous spatial clusters to yield a Tsum. To estimate the probability of each cluster, spectral estimates of misses and hits were swapped randomly and the aforementioned statistical procedure repeated. On each of the overall 1,000 permutations, the maximal Tsum was kept, thereby obtaining a distribution of cluster values under the null hypothesis that the two perceptual categories do not differ. For the resulting significant cluster (Results), the statistical analysis was repeated without correction for multiple comparisons to obtain a clearer picture of the detailed spectral profile of the effect (Fig. 4B), which was subsequently used to optimize our source level analysis.
A frequency domain beam-former (Dynamic Imaging of Coherent Sources) (59) was used to estimate probable generators for the sensor-level effect. Grid points were placed equidistantly at 1 cm into each brain volume and leadfields were calculated for each participant from a single-shell model of the brain (60). Together with the cross-spectral density matrix estimated for 12 ± 5 Hz (a range covering our sensor-level effect) using multitaper FFT, spatial filters were computed for each grid point from the concatenated hits and misses data using regularization factor of 10%. The beam-former filters are designed such that they optimally pass information from one location while suppressing activity from other regions (61). These filters were then used to estimate the power within the aforementioned frequency range at each grid point for each condition separately. Source-level spectral estimates were then normalized from individual headspace onto a Montreal Neurological Institute template brain for purposes of group statistics (misses vs. hits) and visualization (using surface renderings implemented in CARET and thresholded at P < 0.05; http://brainvis.wustl.edu/wiki/index.php/Caret:About). To better estimate the pattern of the relationship between power in the α-β range, spatial filters (procedure analog to the one outlined above) were computed for two locations (our a priori region of interest, right S2, and additionally in a data-driven manner the right MFG, which turned out to be very prominent) and single-trial power estimates were calculated. These values were then sorted according to magnitude and divided into four bins. For each bin, we then computed the normalized hit rate (analogous to ref. 12) by normalizing the hit rate in each bin by the average hit rate over all bins. Binned power values were then used as a predictor of normalized hit rate in a regression analysis to evaluate the probability of linear, quadratic, and cubic relationships. This analysis was performed using R (www.r-project.org).
All-to-all connectivity in source space was computed according to following procedure: (i) Sensor level cross-spectral density (CSD) was calculated for the aforementioned prestimulus period between 3–30 Hz in steps of 1 Hz. (ii) A common lcmv beam-former filter (61) was computed for concatenated data (hits and misses), band-pass filtered between 3–30 Hz. (iii) This common filter was applied separately to the CSD matrices for each condition to obtain the source level CSD matrices (source × source × frequency; an approach analogous to ref. 62). (iv) From these data structures, we derived the imaginary coherence (IC) (63), a conservative measure of phase synchronization that is putatively insensitive to volume conduction effects. This process is favorable for estimation of some graph-theoretical properties: although, for example, degree can be contrasted between two conditions, thereby removing some parts trivially caused by volume conduction, other measures cannot be sensibly interpreted from a contrast (such as small-worldedness) will be therefore remain highly biased. However, before graph theory can be applied, the all-to-all connectivity matrix needs to be thresholded to yield a so-called adjacency matrix. In the majority of studies this matrix is binary, with zeros indicating absence of a functional connection; a 1 indicates its presence. In absence of a “true” criterion, thresholds are necessarily arbitrary. We approached this issue by calculating the adjacency matrix for thresholds between 0.01 and 0.1 (note that obviously absolute values are smaller for IC and that, furthermore, for some graph-theoretical measures the graph needs to be fully connected) and then deriving small-worldedness of the entire graph. This measure, introduced by refs. 64 and 65, can be derived from the ratio of the normalized clustering coefficient (i.e., dividing the empirically observed values by one derived from a corresponding random network) to the normalized distance (or also called path length). Although the clustering coefficient measures the density of connections of a node to its neighbors (averaged over nodes to obtain the value for the entire graph; i.e., putatively representing local integration), distance measures the amount of steps (i.e., connections or edges) it takes from one node to an arbitrary other node. Thus, the latter measure characterizes the aspect of local integration. A small-worldedness value larger than one therefore indicates that a network can be characterized by both: that is, high clustering as well as short distance. Because small-worldedness should represent a property of naturally occurring networks such as the brain, we chose the threshold value that maximized this metric (here: IC ≥ 0.07) for the computation of all other global as well as local metrics. As indicated above, clustering coefficient and distance are measures that can be computed for the entire graph or locally for each individual node, which can then be interpolated onto a structural image. We additionally computed global and local efficiency, a measure that is in principle inversely related to distance but less sensitive to outliers. Higher efficiency values indicate globally that a network is in principle more integrated (because overall distances are low) and locally that a specific node is more highly integrated with the rest of the network. With regards to statistical analysis, we focused our analysis on 17 Hz, for which we identified an overall small-worldedness difference (Pcorrected < 0.05) between misses and hits in the prestimulus period (Results). Paired t tests were then applied to clustering coefficient, distance (both normalized for the global measures), and efficiency to the global as well as local measures. Images of local measures were subsequently thresholded at a P < 0.05. We mainly hypothesized that relatively increased power for misses in the α-range in S2 should be accompanied by a reduced capacity of this region to distribute information to other brain regions, putatively reflected by reduced efficiency and enhanced distance (see introductory paragraphs). Because these “local” measures are still a summary measure for the relation of the node to the entire network, we finalized the analysis by mapping out the spatial pattern of connectivity differences between misses and hits by using S2 as a seeding region. Connectivity patterns were contrasted by paired t test and the resulting images thresholded at P < 0.05.
All aspects of offline data analysis were performed using the Matlab-based FieldTrip toolbox (66) or custom-made functions build on top of this toolbox. Graph-theoretical measures were computed interfacing the appropriate FieldTrip data structures with the Brain Connectivity Toolbox (67).
Supplementary Material
Acknowledgments
This work was supported by European Research Council Grant WIN2CON, ERC StG 283404 (to N.W.), German Research Council Grant DFG LI 1904/1-1 and DFG Werner Reichardt Centre for Integrative Neuroscience (to C.B.), and the German Federal Ministry of Education and Research funded Neurofocus project (C.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1317267111/-/DCSupplemental.
References
- 1.Buzsaki G. Rhythms of the Brain. Oxford: Oxford Univ Press; 2006. [Google Scholar]
- 2.Koenig T, et al. A deviant EEG brain microstate in acute, neuroleptic-naive schizophrenics at rest. Eur Arch Psychiatry Clin Neurosci. 1999;249(4):205–211. doi: 10.1007/s004060050088. [DOI] [PubMed] [Google Scholar]
- 3.VanRullen R, Macdonald JS. Perceptual echoes at 10 Hz in the human brain. Curr Biol. 2012;22(11):995–999. doi: 10.1016/j.cub.2012.03.050. [DOI] [PubMed] [Google Scholar]
- 4.Dehaene S, Changeux JP. Experimental and theoretical approaches to conscious processing. Neuron. 2011;70(2):200–227. doi: 10.1016/j.neuron.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 5.Ergenoglu T, et al. Alpha rhythm of the EEG modulates visual detection performance in humans. Brain Res Cogn Brain Res. 2004;20(3):376–383. doi: 10.1016/j.cogbrainres.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Romei V, Gross J, Thut G. On the role of prestimulus alpha rhythms over occipito-parietal areas in visual input regulation: Correlation or causation? J Neurosci. 2010;30(25):8692–8697. doi: 10.1523/JNEUROSCI.0160-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lange J, Oostenveld R, Fries P. Reduced occipital alpha power indexes enhanced excitability rather than improved visual perception. J Neurosci. 2013;33(7):3212–3220. doi: 10.1523/JNEUROSCI.3755-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romei V, et al. Spontaneous fluctuations in posterior alpha-band EEG activity reflect variability in excitability of human visual areas. Cereb Cortex. 2008;18(9):2010–2018. doi: 10.1093/cercor/bhm229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: The inhibition-timing hypothesis. Brain Res Brain Res Rev. 2007;53(1):63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Jensen O, Mazaheri A. Shaping functional architecture by oscillatory alpha activity: Gating by inhibition. Front Hum Neurosci. 2010;4:186. doi: 10.3389/fnhum.2010.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haegens S, Nácher V, Luna R, Romo R, Jensen O. α-Oscillations in the monkey sensorimotor network influence discrimination performance by rhythmical inhibition of neuronal spiking. Proc Natl Acad Sci USA. 2011;108(48):19377–19382. doi: 10.1073/pnas.1117190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Dijk H, Schoffelen JM, Oostenveld R, Jensen O. Prestimulus oscillatory activity in the alpha band predicts visual discrimination ability. J Neurosci. 2008;28(8):1816–1823. doi: 10.1523/JNEUROSCI.1853-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamme VA. Zap! Magnetic tricks on conscious and unconscious vision. Trends Cogn Sci. 2006;10(5):193–195. doi: 10.1016/j.tics.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Hanslmayr S, et al. Prestimulus oscillations predict visual perception performance between and within subjects. Neuroimage. 2007;37(4):1465–1473. doi: 10.1016/j.neuroimage.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Keil J, Müller N, Ihssen N, Weisz N. On the variability of the McGurk effect: Audiovisual integration depends on prestimulus brain states. Cereb Cortex. 2012;22(1):221–231. doi: 10.1093/cercor/bhr125. [DOI] [PubMed] [Google Scholar]
- 16.Ploner M, Lee MC, Wiech K, Bingel U, Tracey I. Prestimulus functional connectivity determines pain perception in humans. Proc Natl Acad Sci USA. 2010;107(1):355–360. doi: 10.1073/pnas.0906186106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wühle A, Mertiens L, Rüter J, Ostwald D, Braun C. Cortical processing of near-threshold tactile stimuli: An MEG study. Psychophysiology. 2010;47(3):523–534. doi: 10.1111/j.1469-8986.2010.00964.x. [DOI] [PubMed] [Google Scholar]
- 18.Crick F, Koch C. Are we aware of neural activity in primary visual cortex? Nature. 1995;375(6527):121–123. doi: 10.1038/375121a0. [DOI] [PubMed] [Google Scholar]
- 19.Zeki S, Aglioti S, McKeefry D, Berlucchi G. The neurological basis of conscious color perception in a blind patient. Proc Natl Acad Sci USA. 1999;96(24):14124–14129. doi: 10.1073/pnas.96.24.14124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dehaene S, Kerszberg M, Changeux JP. A neuronal model of a global workspace in effortful cognitive tasks. Proc Natl Acad Sci USA. 1998;95(24):14529–14534. doi: 10.1073/pnas.95.24.14529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baars BJ. The conscious access hypothesis: Origins and recent evidence. Trends Cogn Sci. 2002;6(1):47–52. doi: 10.1016/s1364-6613(00)01819-2. [DOI] [PubMed] [Google Scholar]
- 22.Jones SR, Pritchett DL, Stufflebeam SM, Hämäläinen M, Moore CI. Neural correlates of tactile detection: A combined magnetoencephalography and biophysically based computational modeling study. J Neurosci. 2007;27(40):10751–10764. doi: 10.1523/JNEUROSCI.0482-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bullmore E, Sporns O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10(3):186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- 24.Linkenkaer-Hansen K, Nikulin VV, Palva JM, Kaila K, Ilmoniemi RJ. Stimulus-induced change in long-range temporal correlations and scaling behaviour of sensorimotor oscillations. Eur J Neurosci. 2004;19(1):203–211. doi: 10.1111/j.1460-9568.2004.03116.x. [DOI] [PubMed] [Google Scholar]
- 25.Hipp JF, Hawellek DJ, Corbetta M, Siegel M, Engel AK. Large-scale cortical correlation structure of spontaneous oscillatory activity. Nat Neurosci. 2012;15(6):884–890. doi: 10.1038/nn.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagmann P, et al. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6(7):e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sadaghiani S, Hesselmann G, Kleinschmidt A. Distributed and antagonistic contributions of ongoing activity fluctuations to auditory stimulus detection. J Neurosci. 2009;29(42):13410–13417. doi: 10.1523/JNEUROSCI.2592-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanrullen R, McLelland D. What goes up must come down: EEG phase modulates auditory perception in both directions. Front Psychol. 2013;4:16. doi: 10.3389/fpsyg.2013.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathewson KE, et al. Pulsed out of awareness: EEG alpha oscillations represent a pulsed-inhibition of ongoing cortical processing. Front Psychol. 2011;2:99. doi: 10.3389/fpsyg.2011.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Popov T, Miller GA, Rockstroh B, Weisz N. Modulation of α power and functional connectivity during facial affect recognition. J Neurosci. 2013;33(14):6018–6026. doi: 10.1523/JNEUROSCI.2763-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Babiloni C, Vecchio F, Bultrini A, Luca Romani G, Rossini PM. Pre- and poststimulus alpha rhythms are related to conscious visual perception: A high-resolution EEG study. Cereb Cortex. 2006;16(12):1690–1700. doi: 10.1093/cercor/bhj104. [DOI] [PubMed] [Google Scholar]
- 32.Weisz N, Moratti S, Meinzer M, Dohrmann K, Elbert T. Tinnitus perception and distress is related to abnormal spontaneous brain activity as measured by magnetoencephalography. PLoS Med. 2005;2(6):e153. doi: 10.1371/journal.pmed.0020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weisz N, Hartmann T, Müller N, Lorenz I, Obleser J. Alpha rhythms in audition: Cognitive and clinical perspectives. Front Psychol. 2011;2:73. doi: 10.3389/fpsyg.2011.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jensen O, Bonnefond M, VanRullen R. An oscillatory mechanism for prioritizing salient unattended stimuli. Trends Cogn Sci. 2012;16(4):200–206. doi: 10.1016/j.tics.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Schubert R, Haufe S, Blankenburg F, Villringer A, Curio G. Now you’ll feel it, now you won’t: EEG rhythms predict the effectiveness of perceptual masking. J Cogn Neurosci. 2009;21(12):2407–2419. doi: 10.1162/jocn.2008.21174. [DOI] [PubMed] [Google Scholar]
- 36.Linkenkaer-Hansen K, Nikulin VV, Palva S, Ilmoniemi RJ, Palva JM. Prestimulus oscillations enhance psychophysical performance in humans. J Neurosci. 2004;24(45):10186–10190. doi: 10.1523/JNEUROSCI.2584-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Ding M. Detection of a weak somatosensory stimulus: Role of the prestimulus mu rhythm and its top-down modulation. J Cogn Neurosci. 2010;22(2):307–322. doi: 10.1162/jocn.2009.21247. [DOI] [PubMed] [Google Scholar]
- 38.Christensen MS, Ramsøy TZ, Lund TE, Madsen KH, Rowe JB. An fMRI study of the neural correlates of graded visual perception. Neuroimage. 2006;31(4):1711–1725. doi: 10.1016/j.neuroimage.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 39.D’Ostilio K, Garraux G. Automatic stimulus-induced medial premotor cortex activation without perception or action. PLoS ONE. 2011;6(2):e16613. doi: 10.1371/journal.pone.0016613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Lange FP, Rahnev DA, Donner TH, Lau H. Prestimulus oscillatory activity over motor cortex reflects perceptual expectations. J Neurosci. 2013;33(4):1400–1410. doi: 10.1523/JNEUROSCI.1094-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dehaene S, Changeux JP, Naccache L, Sackur J, Sergent C. Conscious, preconscious, and subliminal processing: A testable taxonomy. Trends Cogn Sci. 2006;10(5):204–211. doi: 10.1016/j.tics.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 42.Laureys S, Schiff ND. Coma and consciousness: Paradigms (re)framed by neuroimaging. Neuroimage. 2012;61(2):478–491. doi: 10.1016/j.neuroimage.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 43.Britz J, Landis T, Michel CM. Right parietal brain activity precedes perceptual alternation of bistable stimuli. Cereb Cortex. 2009;19(1):55–65. doi: 10.1093/cercor/bhn056. [DOI] [PubMed] [Google Scholar]
- 44.Sadaghiani S, et al. Intrinsic connectivity networks, alpha oscillations, and tonic alertness: A simultaneous electroencephalography/functional magnetic resonance imaging study. J Neurosci. 2010;30(30):10243–10250. doi: 10.1523/JNEUROSCI.1004-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palva JM, Monto S, Kulashekhar S, Palva S. Neuronal synchrony reveals working memory networks and predicts individual memory capacity. Proc Natl Acad Sci USA. 2010;107(16):7580–7585. doi: 10.1073/pnas.0913113107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weisz N, Müller N, Jatzev S, Bertrand O. Oscillatory alpha modulations in right auditory regions reflect the validity of acoustic cues in an auditory spatial attention task. Cereb Cortex. 2013 doi: 10.1093/cercor/bht113. in press. [DOI] [PubMed] [Google Scholar]
- 47.Raichle ME, et al. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koch C, Tsuchiya N. Attention and consciousness: Two distinct brain processes. Trends Cogn Sci. 2007;11(1):16–22. doi: 10.1016/j.tics.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 49.Simons DJ, Chabris CF. Gorillas in our midst: Sustained inattentional blindness for dynamic events. Perception. 1999;28(9):1059–1074. doi: 10.1068/p281059. [DOI] [PubMed] [Google Scholar]
- 50.Simons DJ. Monkeying around with the gorillas in our midst: Familiarity with an inattentional-blindness task does not improve the detection of unexpected events. Iperception. 2010;1(1):3–6. doi: 10.1068/i0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 52.Leske S, et al. The strength of alpha and beta oscillations parametrically scale with the strength of an illusory auditory percept. Neuroimage. 2013 doi: 10.1016/j.neuroimage.2013.11.014. in press. [DOI] [PubMed] [Google Scholar]
- 53.Wyart V, Tallon-Baudry C. Neural dissociation between visual awareness and spatial attention. J Neurosci. 2008;28(10):2667–2679. doi: 10.1523/JNEUROSCI.4748-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Busch NA, VanRullen R. Spontaneous EEG oscillations reveal periodic sampling of visual attention. Proc Natl Acad Sci USA. 2010;107(37):16048–16053. doi: 10.1073/pnas.1004801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 56.Jung TP, et al. Imaging brain dynamics using independent component analysis. Proc IEEE Inst Electr Electron Eng. 2001;89(7):1107–1122. doi: 10.1109/5.939827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Percival DB, Walden AT. Spectral Analysis for Physical Applications: Multitaper and Conventional Univariate Techniques. Cambridge, UK: Cambridge Univ Press; 1993. [Google Scholar]
- 58.Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164(1):177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 59.Gross J, et al. Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proc Natl Acad Sci USA. 2001;98(2):694–699. doi: 10.1073/pnas.98.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nolte G. The magnetic lead field theorem in the quasi-static approximation and its use for magnetoencephalography forward calculation in realistic volume conductors. Phys Med Biol. 2003;48(22):3637–3652. doi: 10.1088/0031-9155/48/22/002. [DOI] [PubMed] [Google Scholar]
- 61.Van Veen BD, van Drongelen W, Yuchtman M, Suzuki A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans Biomed Eng. 1997;44(9):867–880. doi: 10.1109/10.623056. [DOI] [PubMed] [Google Scholar]
- 62.Besserve M, Martinerie J, Garnero L. Improving quantification of functional networks with EEG inverse problem: Evidence from a decoding point of view. Neuroimage. 2011;55(4):1536–1547. doi: 10.1016/j.neuroimage.2011.01.056. [DOI] [PubMed] [Google Scholar]
- 63.Nolte G, et al. Identifying true brain interaction from EEG data using the imaginary part of coherency. Clin Neurophysiol. 2004;115(10):2292–2307. doi: 10.1016/j.clinph.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 64.Strogatz SH. Exploring complex networks. Nature. 2001;410(6825):268–276. doi: 10.1038/35065725. [DOI] [PubMed] [Google Scholar]
- 65.Humphries MD, Gurney K. Network ‘small-world-ness’: A quantitative method for determining canonical network equivalence. PLoS ONE. 2008;3(4):e0002051. doi: 10.1371/journal.pone.0002051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011;2011:156869. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rubinov M, Sporns O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage. 2010;52(3):1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.