Significance
Activation of the complement system, a network of circulating and surface-bound molecules, is known to enhance humoral immunity. However, paradoxically, the lack of some complement components (mainly C1q and C4), but not C3, is associated with the development of autoimmunity. Prior studies have suggested that this association could be explained by the role of complement in the disposal of dying cells that are a potential source of autoantigens. This study demonstrates that C3 bound to dying cells can direct the intracellular route of the cargo and modulate the subsequent T-cell response to antigens displayed on dying cells. These results uncover a new role of C3 and have important implications for our understanding of the role of complement in health and disease.
Abstract
Apoptotic cells are a source of autoantigens and impairment of their removal contributes to the development of autoimmunity in C1q deficiency. However, the lack of complement component 3 (C3), the predominant complement opsonin, does not predispose to autoimmunity, suggesting a modifying role of C3 in disease pathogenesis. To explore this hypothesis, here we investigated the role of C3 in the T-cell response to apoptotic cell-associated antigens. By comparing the phagosome maturation and the subsequent MHC class II presentation of a peptide derived from the internalized cargo between C3-deficient or C3-sufficient dendritic cells, we found that C3 deficiency accelerated the fusion of the apoptotic cargo with lysosomes. As a result, C3 deficiency led to impaired antigen-specific T-cell proliferation in vitro and in vivo. Notably, preopsonization of the apoptotic cells with C3 activation fragments rectified the trafficking and T-cell stimulation defects. These data indicate that activated C3 may act as a “chaperone” in the intracellular processing of an apoptotic cargo and, thus, may modulate the T-cell response to self-antigens displayed on dying cells.
It is now well recognized that the complement system, an integral component of innate immunity, also has a prominent influence on adaptive immunity. In addition to lowering the threshold for B-cell stimulation (1, 2), more recent studies have highlighted the contribution of complement to T-cell immunity, suggesting an involvement of complement component 3 (C3) or its activation fragments in T-cell regulation and activation (3, 4). However, the mechanisms by which C3 contributes to antigen-specific T-cell reactivity remain poorly understood. Whether it modulates the response to apoptotic cell-associated antigens is also unclear.
Phagocytosis is an efficient route for delivering antigens into major histocompatibility complex (MHC)-rich compartments (5). Professional antigen-presenting cells (APC), like dendritic cells (DCs), have the extraordinary ability to internalize large particles and induce tolerance or immunity. The activation of naïve T cells and the subsequent immunological outcomes may depend on the endocytic compartment to which the internalized cargo is delivered, and this process may vary in different DC subsets (6). For example, the CD8α+ DC subset is remarkably efficient at capturing material from dying cells (7) and at processing and presenting cell-associated antigens on both MHC class I and II (8). Autoantigens are displayed on the surface of apoptotic cells (9) and an impaired clearance of these cells, as a result of deficiency in opsonic proteins or their receptors, predisposes to a lupus-like disease in humans and mice (10). Recently it has been suggested that apoptotic cell-binding opsonins not only control the rate of their ingestion, but also regulate the intracellular processing preventing excessive T-cell activation (11); this elegant study with milk fat globule EGF factor 8 (MFG-E8)-deficient mice focused on MHC class I cross-presentation and the response of CD8+ T cells to self-antigens. However, lupus is generally associated with abnormal CD4+ T activation (12–14). To what extent apoptotic cell-binding opsonins regulate the MHC class II presentation of apoptotic cell-associated self-antigens and whether other opsonins operate in a similar manner to MFG-E8 remains unknown.
Complement C3 is the point of convergence for the three complement activation pathways. The liver is the primary source of circulating C3 that is critical for the clearance of particulate antigens such as microorganisms, whereas local synthesis of C3 by myeloid-derived cells and parenchymal cells appears to regulate adaptive immune responses (15). Consistent with this notion, the ability to mount an antibody response to an exogenous antigen was restored in C3-deficient mice (C3−/−) reconstituted with wild-type (WT) bone marrow (BM) cells despite the lack of detectable C3 in circulation (16). Moreover, defects in T-cell polarization and T-cell responses have been reported in C3−/− animals in several models including viral infection and transplantation (17–20). The lack of signaling through the C3a receptor (C3aR) has been proposed to explain this impaired T-cell reactivity (21). Furthermore, in B cells C3b fragment covalently bound to tetanus toxin has been shown to protect the antigen from excessive degradation in the endosomal compartment promoting and prolonging the antigen presentation by MHC class II (22, 23). These studies indicate that opsonization with C3 split fragments not only facilitates antigen uptake but might also have a direct intracellular effect on the subcellular localization of peptide/MHC complex formation, a pathway that has never been explored for particulate exogenous antigens like dying cells.
Several reports have shown that C3 deposition on apoptotic cells following the activation of the classical or other complement pathways is required for their efficient ingestion by macrophages (24, 25). However, in contrast to deficiencies in the classical complement components, such as C1q and C4, which are strongly associated with the development of lupus-like disease, in humans, C3 deficiency is mainly associated with recurrent pyogenic infections and does not predispose to the development of autoantibodies (26). This observation raises the possibility that C3 deficiency may compensate for the defective clearance and prevent the presentation of self-antigens displayed on dying cells.
In this study, we explored the role of C3 in immunity to dying cells and found that the presence of C3 activation fragments induces a delayed fusion of the apoptotic cargo with lysosomes resulting in an increased antigen presentation by MHC class II molecules. Consistent with this observation, T-cell proliferation in response to apoptotic cell-associated antigens is impaired in the absence of C3 but can be boosted by preopsonizing the apoptotic cells. Finally, we demonstrate that only C3 binding is required, whereas other complement apoptotic cell-binding opsonins, like C1q, are dispensable. This finding is consistent with the hypothesis that opsonization with C3 activation fragments controls the T-cell response to apoptotic cell-associated antigens by influencing the intracellular processing of the apoptotic cargo.
Results
Reduced Presentation and Impaired T-Cell Response to an Apoptotic Cell-Associated Antigen by C3-Deficient DCs.
Local production of C3 by DCs is required for mounting an alloreactive effector T-cell response to MHC disparate tissues (20). To evaluate the role of C3 in the development of T-cell responses to apoptotic cell-associated antigens, a potential source of autoantigens, we cocultured C3-deficient or C3-sufficient immature BM-DCs from B6 mice (H2-Edeficient) with allogeneic H2-E+ apoptotic LPS-treated B cells (LPS B-cell blasts) from C3-deficient BALB/c mice and assessed the presence of the Eα52–68 peptide, derived from H2-E molecule, on H2-Ab-Edeficient B6 DC by using the Y-Ae monoclonal antibody and by T-cell proliferation using 1H3.1 transgenic T cells (27, 28). Comparison of day 6 immature C3-deficient and C3-sufficient BM-DCs showed no differences in surface expression of H2b, CD86, and CD40 before and after apoptotic exposure (Fig. S1 A and B), indicating that the lack of C3 did not affect the activation status of the DCs. We then loaded the BM-DCs with H2-E+ C3−/− apoptotic LPS B-cell blasts nonopsonized (no serum or using C3-deficient serum) or C3 preopsonized using C5-deficient serum (to avoid the membrane attack complex formation) and allowed them to interact for 48 h. The mean fluorescence intensity (MFI) of the Y-Ae antibody was found to be lower in C3−/− B6 BM-DCs pulsed with nonopsonized apoptotic cells compared with the counterpart WT BM-DCs (Fig. 1A), suggesting impaired presentation. Notably, C3 opsonization of the apoptotic LPS B-cell blasts with C5-deficient serum before loading rectified the defect. Consistent with the Y-Ae staining, 1H3.1 CD4+ T cells stimulated with C3−/− B6 BM-DCs pulsed with nonopsonized apoptotic LPS B-cell blasts proliferated significantly less than those stimulated with the counterpart WT BM-DCs, whereas the proliferation was similar when the apoptotic cells used were preopsonized with C3 (C5-deficient serum) (Fig. 1B). In contrast to their processing of apoptotic cells, C3−/− and WT BM-DCs pulsed with the Eα52–68 peptide did not differ in their ability to present the peptide (Fig. 1C) or to induce antigen-specific T-cell expansion (Fig. 1D), arguing that the reduced responses by C3−/− BM-DCs were not due to differences in BM-DC maturation or costimulatory molecule expression.
Fig. 1.
C3 is required for the efficient processing of apoptotic material by BM-DCs. B6 and C3−/− BM-DCs were cultured with apoptotic BALB/c LPS B-cell blasts (ratio 1:2) preincubated with different mouse sera as indicated (A and B) or with different concentrations of the Eα52–68 peptide (C and D). Presence of peptide/MHC complexes on APC was assessed by measuring the MFI of the Y-Ae antibody (A and C). The antigen-specific T response was quantified by 3H-thymidine incorporation of 1H3.1 T cells (B and D). BM-DCs from B6 and C3−/− B6 mice were fed with BALB/c LPS B-cell blasts (48 h) (B) or Eα52–68 peptide (24 h) (D) and then cultured for 24 h with CD4+ 1H3.1 TCR transgenic T cells (T cells:DCs, 2:1 ratio). B6 LPS B-cell blasts or no peptide was used as controls. (B and D) Data are shown as mean ± SEM, n = 3, t test. These results are representative of three independent experiments with three mice in each group.
Although the contribution of complement to the engulfment of apoptotic cells by macrophages is well established (10), its role in the uptake by DCs remains controversial, with conflicting results reported in the literature (29–31). To explore whether the observed differences could be attributed to a reduced ability of the C3−/− BM-DCs to take up the cells, we labeled the apoptotic LPS B-cell blasts with pHrodo, a dye that becomes fluorescent only in the acidic pH inside endocytic vesicles, and incubated them with day 6 immature C3−/− and WT BM-DCs. As shown in Fig. S1C, the percentage of BM-DCs that had engulfed pHrodo-labeled apoptotic cells was similar regardless of the opsonization, which is consistent with previous reports (30). Analysis of coded cytospins confirmed that the number of apoptotic cells engulfed by each BM-DC was equivalent, indicating that C3−/− BM-DCs were as efficient as the WT BM-DCs in the uptake of the apoptotic cells. Taken together, these results ruled out a defect in phagocytosis as the cause of the poorer priming/induction of T-cell responses to antigens derived from apoptotic cells by C3−/− BM-DCs and suggested that lack of C3 may alter the processing/trafficking of apoptotic cells inside the DCs.
C3 Deficiency Leads to an Accelerated Maturation of the Phagosome Containing an Apoptotic Cargo.
Given the absence of a defect in the phagocytosis of apoptotic LPS B-cell blasts by C3−/− BM-DCs, we next explored whether C3 specifically functions by delivering the apoptotic cargo to a subcellular compartment that favors antigen presentation by MHC class II molecules, as suggested for soluble antigens in B cells (22, 23). BM-DCs from B6 and C3−/− mice were loaded with PKH26-labeled apoptotic LPS B-cell blasts, and their fate within the cells was visualized at 3, 6, and 24 h by confocal microscopy by using the lysosomal marker LAMP-1. We found that at 3 and 6 h after internalization, WT DCs contained apoptotic cells that were still intact, whereas C3-deficient DCs already had digested debris fused with lysosomes (Fig. 2 A and B). Quantification of colocalization efficiency revealed that the colocalization with the lysosome marker was significantly enhanced in C3−/− DCs at 3 and 6 h (Fig. 2D). However, by 24 h, the apoptotic cargo was fused with lysosome and the colocalization efficiency in C3-deficient and C3-sufficient DCs was similar (Fig. 2 C and D). More importantly, as with the expression of Y-Ae on the cell surface, opsonization of the apoptotic LPS B-cell blasts before their coculture with C3-deficient DCs delayed LAMP-1 colocalization. The accumulation of the apoptotic cargo in LAMP-1–positive endosomes was similar to that found in WT DCs (Fig. 2D). Taken together, these findings suggest that C3 fragments bound to apoptotic cells may actively regulate the intracellular route of the cargo and delay phagosome maturation, thereby enhancing antigen presentation (32).
Fig. 2.
C3 bound to an apoptotic cargo delays the phagosome maturation. PKH26-labeled (red) apoptotic C3−/− BALB/c LPS B-cell blasts were incubated with WT or C3−/− BM-DCs at a 5:1 ratio for 3 h (A), 6 h (B), and 24 h (C). Cells were fixed and stained for confocal microscopy with DAPI (blue), LAMP-1-biotin, and avidin-Alexa488 (green). To correct C3 deficiency, C3-deficient BM-DCs were cultured with PKH26-labeled apoptotic cells preopsonized with C5−/− serum. Intact apoptotic cells with low colocalization with LAMP-1 are indicated with an asterisk, and smaller cell debris with high colocalization with LAMP-1 are indicated with arrows. (D) Colocalization of LAMP-1 in WT (black bars) and C3−/− BM-DCs (white bars, nonopsonized apoptotic; hatched bars, opsonized apoptotic cells) was quantified within 20 cells for each culture. (Scale bars: 10 μm.) Error bars represent SEM. Results are representative of two independent experiments. Statistical analysis was performed by using Kruskal–Wallis test with Dunn’s post hoc test for multiple groups and Mann–Whitney u test for comparison between two groups (24 h).
In Vivo Antigen-Specific T-Cell Response to an Apoptotic Cell-Associated Antigen Is Impaired in C3-Deficient Mice Regardless of TLR4 Engagement.
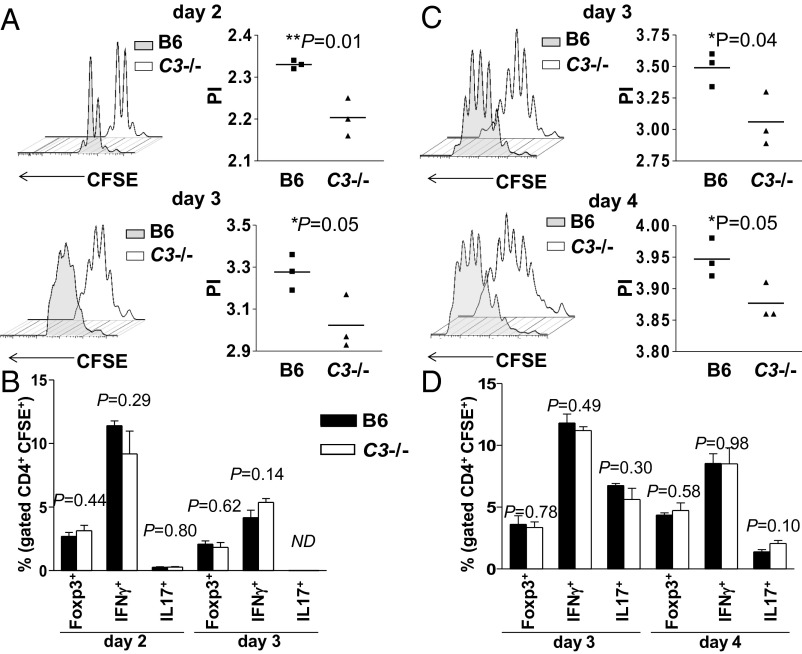
From the above in vitro observations, it appears that C3 is necessary for optimal presentation of apoptotic cell-associated antigens by DCs. To assess the relevance of our findings in vivo, we compared the ability of C3−/− and WT B6 mice to process injected apoptotic BALB/c LPS B-cell blasts and present the Eα52–68 peptide to adoptively transferred 1H3.1 T cells. We initially injected eFluor450-labeled apoptotic C3−/− BALB/c LPS B-cell blasts into C3-deficient and C3-sufficient B6 mice and analyzed by flow cytometry the splenic uptake 24 h later. As reported (7), the engulfment of eFluor450-positive apoptotic cells occurred mainly in the CD8α+ splenic DC subset. Consistent with the in vitro data, a similar uptake was detected between the two groups (Fig. S2A). Notably, we found no phenotypic differences between the C3−/− and WT eFluor450+ CD8α+ splenic DCs, suggesting similar maturation following the ingestion of apoptotic LPS B-cell blasts (Fig. S2B). In subsequent experiments, we injected CFSE-labeled naive CD4+ 1H3.1 T cells into C3−/− and WT mice that had been injected a day earlier with unlabeled H2-E+ apoptotic C3−/− LPS B-cell blasts and then analyzed the expansion and phenotype of the antigen-specific T cells recovered from the spleens at different time points. As illustrated in Fig. 3A, the 1H3.1 T cells proliferated significantly less in vivo on both day 2 and day 3 when primed by C3-deficient DCs, consistent with the notion that C3-deficient DCs are impaired in their ability to drive the expansion of antigen-specific T cells in response to an apoptotic cell-associated antigen. However, the ex vivo analysis of the CFSE+ 1H3.1 T cells recovered from the spleens showed similar frequency of antigen-specific Foxp3+ as well as IFNγ+ and IL-17+ T cells between the two experimental groups (Fig. 3B), indicating that under our experimental conditions, the absence of C3 did not alter 1H3.1 T-cell polarization.
Fig. 3.
C3 deficiency reduces 1H3.1 T-cell expansion in response to Eα protein from apoptotic cells. C3-deficient or C3-sufficient B6 mice received 20 × 106 apoptotic LPS B-cell blasts (A and B) or apoptotic splenocytes (C and D) i.v. followed 1 d later by 2 × 106 CFSE-labeled 1H3.1 CD4+ T cells. 1H3.1 T-cell expansion was measured at different time points as indicated. CFSE dilution (A and C Left) and proliferation index (PI) (A and C Right). The proliferation index was quantified by FlowJo software. Ex vivo splenic CD4+ T cells were restimulated for 4 h with phorbol myristate acetate /ionomycin and intracellular cytokine staining evaluated by flow cytometry (B and D). The percentages of CFSE+ expressing FoxP3 or IFN-γ or IL-17 are shown. Data are shown as mean ± SEM, n = 3, t test. These experiments were carried three times with similar results.
Toll-like receptor (TLR) ligation during recognition of apoptotic cell-associated antigens has been shown to be required for MHC class II presentation and Th17 differentiation (33, 34). Because in our experiments we have used LPS-treated apoptotic B-cell blasts, we considered whether the presence of the TLR ligand within the cargo was required for C3 to regulate the phagosome maturation. To investigate this point, we measured 1H3.1 T proliferation after injection of UV-induced H2-E+ apoptotic C3−/− splenocytes. Compared with the T-cell response induced by the H2-E+ C3−/− LPS B-cell blasts, the kinetics of 1H3.1 T-cell expansion were different and T-cell divisions were detectable only 3 d after the adoptive transfer. More importantly, we found that even in the absence of TLR4 engagement, C3 deficiency significantly decreased the apoptotic-derived H2-E–triggered T-cell expansion (Fig. 3C). However, again, the lack of C3 did not alter the polarization profile of the 1H3.1 T cells (Fig. 3D). Surprisingly, the analysis of CFSE+ 1H3.1 T cells recovered from the spleens showed that the ingestion of UV-induced apoptotic cells by DCs promoted similar, if not even slightly greater, expansion of IL-17+ T cells than the uptake of LPS-treated apoptotic B-cell blasts. Taken together, these results suggest that C3 modulates the efficiency of presentation of apoptotic cell-associated antigens by MHC class II independently from the presence of TLR ligands within the phagocytosed cargo.
C3 Fragments Bound to Apoptotic Cells Modulate the Antigen-Specific T Response.
There is a large body of evidence that C1q and other complement components can act as opsonins and can promote the rate of ingestion of dying cells via direct binding. However, whether they can also play a role in the subsequent intracellular trafficking of the apoptotic cargo and the processing of the apoptotic cell-associated antigens is unknown. Therefore, we extended our in vivo experimental approach to other complement-deficient mice and assessed their ability to induce the expansion of adoptively transferred 1H3.1 T cells. By studying recipient mice lacking C1q (classical pathway) or C4 (classical and lectin pathways), or factor B (alternative pathway) or C5 (terminal pathway), we were able to assess the contribution of the different complement components and activation pathways. As shown in Fig. 4, 1H3.1 T cells injected into none of the above complement-deficient mice displayed a significantly impaired expansion, demonstrating that irrespective of the activation pathway, opsonization of the apoptotic cargo with C3 fragments is critical to promote the MHC class II pathway of antigen presentation. In keeping with this observation, we found that in factor B-deficient animals (Cfb−/−), which lack the amplification loop of the alternative pathway and have impaired C3 opsonization of apoptotic cells despite higher levels of circulating C3 (25, 35), the 1H3.1 T cells showed a consistent trend to expand less than in the WT B6 controls (Fig. 4D). Furthermore, we observed that 1H3.1 CD4+ T cells stimulated in vitro with Cfb−/− BM-DCs pulsed with nonopsonized apoptotic LPS B-cell blasts proliferated markedly less than those stimulated with WT BM-DCs (Fig. S3A). Again, preopsonization of the apoptotic LPS B-cell blasts with C5-deficient serum before loading rectified the defect (Fig. S3A). These findings demonstrate that the alternative pathway is essential for the local C3 activation and subsequent apoptotic cell opsonization by BM-DCs. Consistent with this notion, we found that under our experimental conditions, BM-DCs cultured with apoptotic cells express mRNA transcripts for alternative pathway components (Fig. S3B).
Fig. 4.
T response to an apoptotic cell-associated antigen is impaired only in C3-deficient mice. Mice deficient in C1q (A), C4 (B), C5 (C), factor B (D), factor I (E), and CD11b (F) were injected with 20 × 106 UV-induced apoptotic splenocytes followed by the adoptive transfer of 2 × 106 CFSE-labeled 1H3.1 T cells. Proliferation of the CFSE-labeled 1H3.1 T cells was assessed 3 d later by flow cytometry and expressed as PI. Data are shown as mean ± SEM, n = 3, t test. These experiments were carried three times with similar results.
To characterize the nature of the C3 activation fragment(s) involved in the intracellular processing of the apoptotic cargo, we took advantage of the notion that in the absence of factor I, C3 can only be cleaved into C3b (36). We first confirmed that apoptotic LPS B-cell blasts when incubated in vitro with factor I-deficient serum were coated only with C3b (37), whereas when exposed to wild-type serum, the C3 bound to apoptotic cells is predominantly in the form of iC3b/C3dg (Fig. S3 C and D). Subsequently, we assessed the ability of factor I-deficient mice (Cfi−/−) to present apoptotic cell-associated antigens in vivo and found a significantly impaired expansion of the adoptively transferred 1H3.1 T cells compared with WT B6 mice (Fig. 4E), indicating that the presence of C3b on apoptotic cells cannot rectify the defect in the antigen-specific T-cell expansion. Collectively, these findings demonstrate that the binding of C3 activation fragments, most likely iC3b or its metabolite C3dg, to apoptotic cells allows a more efficient peptide loading on MHC class II molecules and promotes the T-cell proliferation in response to antigens displayed on the engulfed cargo.
Complement receptor 3 (CD11b/CD18) is known to bind iC3b and to mediate the phagocytosis of complement opsonized targets, including apoptotic cells, on macrophages (24). We next explored whether this receptor could also influence the trafficking of the apoptotic cargo. We found that the uptake or presentation of apoptotic cell-associated antigens by BM-DCs was not affected by the absence of complement receptor 3 (CD11b/CD18) (Fig. S4). Furthermore, the T-cell expansion in vivo was not affected by the absence of CD11b (Fig. 4F). Taken together, these data suggest that other receptor(s) or nonreceptor mediated mechanism(s) may operate in BM-DCs.
Discussion
Dying cells can induce tolerance or immunity in different experimental models, and the mechanisms by which they are recognized and processed may dictate the immunological outcome. Until recently, innate opsonic proteins and/or their receptors have been thought to contribute mainly to the engulfment of the apoptotic cells and a defective clearance has been shown to predispose to the development of autoimmunity. However, there is an emerging literature suggesting that some of the innate immune recognition mechanisms may also regulate the intracellular route of the apoptotic cargo (11, 38), resulting in different T-cell responses against apoptotic cell-associated antigens. Herein we demonstrate that opsonization with C3 activation fragments can also influence the intracellular trafficking of the internalized apoptotic cell material by delaying its lysosomal fusion (phagosome maturation), a process that favors the presentation of immunogenic peptides on MHC class II molecules (32). We further show that the altered processing resulted in an impaired antigen-specific T-cell proliferation in vitro and in vivo and that C3 activation fragments covalently bound to the apoptotic cargo specifically mediated these effects. Together these findings suggest an alternative mechanism by which C3 activation may contribute to the control of immune responses to self-antigens expressed by a phagocytosed cargo and prevent the development of autoimmunity.
It has been shown that C3 synthesis by DCs is required for the development of an effective T-cell response (17–20). Consistent with previous observations we found that, in the absence of any source of C3, C3-deficient DCs elicited an impaired CD4+ T-cell proliferation to an apoptotic cell-associated antigen. To explore the mechanisms by which C3 affected DC function, we used apoptotic cells expressing the Eα protein and monitored the presentation of the Eα52–68 peptide by DCs in the context of the H2-Ab–Edeficient complex by using the Y-Ae monoclonal antibody and 1H3.1 TCR transgenic T cells (27, 28). We first excluded the possibility that the reduced presentation/T-cell activation could be the result of an impaired uptake or differences in DC maturation. Indeed, the role of C3 in the clearance of apoptotic cells by DCs remain unclear, with conflicting reports (29–31). The discrepancies in the literature are likely to be due to differences in the experimental conditions applied. The studies showing that C3 facilitates uptake compared cocultures of DCs and apoptotic cells in the presence or absence of serum (29, 31) and did not take into account the contribution of other apoptotic cell-binding proteins present in the serum. In contrast, similarly to Behrens et al. (30), we preopsonized and washed the apoptotic cells before coculturing them with DCs and, more importantly, we used C3-deficient and C5-deficient sera to limit the analysis to C3 without removing the potential contribution of other serum opsonins. In addition, the lack of a detectable phagocytic defect in the absence of CR3 (CD11b-deficient mice), the main phagocytic receptor for iC3b-coated targets, provided further indirect support to the conclusion that C3 opsonization does not affect the engulfment of dying cells by DCs and that, in contrast to macrophages, other mechanisms/receptors operate in these cells. These findings, taken together with the data obtained with Eα52–68 peptide, ruled out a defect in phagocytosis and/or DC maturation as the cause of the reduced T-cell responses elicited by C3−/− DCs and pointed to an abnormal intracellular processing of the antigen derived from the apoptotic cargo.
The mechanisms that regulate the trafficking of an internalized apoptotic cargo are still poorly understood and only recently have the effects of different subcellular locations on the presentation of antigens derived from dying cells been fully appreciated (11, 38). Opsonins and/or receptors such as MFG-E8 (11) or CLEC9A (38) have been shown to direct the trafficking of the dying cargo, changing the subsequent cross-presentation. Because the covalent binding of C3b to a soluble antigen such as tetanus toxin can enhance the antigen presentation by B cells by protecting the antigen from endosomal cathepsin D-mediated proteolysis (22, 23), we next considered whether opsonization of the apoptotic material with C3-split products could divert or prolong the sequestration of the cargo in a compartment that facilitates peptide loading on MHC class II molecules. We found that, despite ingestion of apoptotic cells being similar between C3−/− and WT BM-DCs, in the absence of C3, the fusion of the apoptotic cell-containing phagosomes with lysosomes was accelerated, and cell debris were already detectable within 3 h after ingestion. More importantly, the delay in the phagosome maturation was rectified by feeding the C3−/− DCs with complement preopsonized material, demonstrating these cells had no intrinsic defects in processing engulfed targets. These results, taken together with the reduced intensity of the Eα52–68/H2-Ab-Edeficient complex assessed with the Y-Ae antibody and the impaired 1H3.1 T response observed in the absence of C3 in vitro and in vivo, strongly suggest that C3 fragment opsonization regulates the T-cell response to apoptotic cell-associated antigens by orchestrating the intracellular processing of the apoptotic cargo. Hence, our findings agree with the accepted notion that a faster lysosomal degradation reduces antigen presentation on MHC class II (32). They are also consistent with previous observations that C3-deficient DCs are less potent in stimulating alloreactive CD4 T cells, but not CD8 T cells, indicating that the effect of C3 is predominantly on the MHC class II-dependent pathway of antigen presentation (39).
The same phagocytic machine that controls the uptake of a microorganism also mediates the phagocytosis of an apoptotic cargo, but the cellular responses triggered are very different. Previous reports have shown that one of the determining factors is the presence of Toll-like receptor ligands within the cargos (33) because they can drive a distinct program of phagosome maturation, resulting in an efficient antigen processing and MHC class II presentation (40). We tested this notion in vivo by using our adoptive transfer model by analyzing 1H3.1 T proliferation after administration of UV-induced apoptotic splenocytes or LPS B-cell blasts. Although we found that the LPS-carrying cargos triggered a faster 1H3.1 T-cell expansion than the “sterile” cargos, the engagement of TLR signaling was not necessary for the MHC class II presentation of the Eα peptide. In addition, ex vivo analysis of different antigen-specific T-cell subsets failed to detect any substantial difference in the T-cell response induced by the two types of apoptotic cells and showed a predominant Th1 response, consistent with previous reports (34). More importantly, we found that the C3-mediated effect on apoptotic cell-associated antigen presentation by MHC class II was unaffected by the lack of TLR involvement. Therefore, although our data do not seem to support the model that TLR-mediated recognition tightly controls selection of phagocytosed antigens for presentation, it is plausible that the requirements for antigen presentation might vary in vivo under different experimental conditions.
Deficiencies of one of the early components (C1q, C2, and C4) of the classical pathway are known to predispose to systemic lupus erythematosus. Over the years, several hypotheses, not mutually exclusive, have been proposed (41) to explain why only these proteins have a protective function, whereas the lack of C3 does not trigger an autoimmune response. To explain this paradox, most studies until now have focused on the role of the complement in the clearance of apoptotic cells by macrophages, with less attention being paid to its role in the subsequent processing and fate of the antigen displayed by the dying cells. In this context, our data highlight a unique mechanism. We found that only the lack of C3, and none of the other complement molecules, could reduce the in vivo T-cell response to an apoptotic cell-associated antigen. Notably, factor B-deficient mice that have increased the level of circulating C3 but less complement activation on the apoptotic cells (25, 35) displayed a similar trend, demonstrating that the opsonization with C3 activation fragments was responsible for the effect. Consistent with several recent reports (42), we found that the alternative pathway was essential for local C3 activation and the subsequent apoptotic cell opsonization by BM-DCs. Furthermore, the use of factor I-deficient mice and serum allowed us to demonstrate that most likely iC3b or its metabolite C3dg were the main C3 split products involved in the regulation of the intracellular trafficking of the apoptotic cargo.
In summary, taken together, our data have revealed a novel C3 function: the regulation of the endocytic processing of an apoptotic cargo. By delaying the phagosome maturation and prolonging the sequestration of the cargo in a compartment that facilitates peptide loading on MHC class II molecules, C3 contributes to enhance MHC restricted (T-cell) immune responses to an antigen derived from apoptotic cells. Therefore, based on these observations, we would like to suggest that another potential mechanism by which C3 deficiency protects from the development of autoimmunity is by reducing the T-cell response to self-antigens displayed on dying cells.
Materials and Methods
Generation of C3−/−, C1qa−/−, C4−/−, Cfb−/−, C5−/−, Cfi−/− and Itgam−/− mice used in this study are described in SI Material and Methods. All animals were handled in accordance with institutional guidelines and procedures were approved by the UK Home Office. Proliferation assays, flow cytometry, and confocal microscopy were performed by using standard protocols described in SI Material and Methods. In vitro and in vivo experiments are discussed in SI Material and Methods.
Supplementary Material
Acknowledgments
We thank the staff of the Biological Services Unit at our institution for the care of the animals involved in this study and Jessica Strid for critical reading of the manuscript. This work was supported by Wellcome Trust Grant 088517. L.B. was supported by a Swiss National Science Foundation Fellowship and a Novartis Foundation Fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1316877111/-/DCSupplemental.
References
- 1.Pepys MB. Role of complement in induction of antibody production in vivo. Effect of cobra factor and other C3-reactive agents on thymus-dependent and thymus-independent antibody responses. J Exp Med. 1974;140(1):126–145. doi: 10.1084/jem.140.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dempsey PW, Allison ME, Akkaraju S, Goodnow CC, Fearon DT. C3d of complement as a molecular adjuvant: Bridging innate and acquired immunity. Science. 1996;271(5247):348–350. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 3.Sacks SH. Complement fragments C3a and C5a: The salt and pepper of the immune response. Eur J Immunol. 2010;40(3):668–670. doi: 10.1002/eji.201040355. [DOI] [PubMed] [Google Scholar]
- 4.Kemper C, Atkinson JP. T-cell regulation: With complements from innate immunity. Nat Rev Immunol. 2007;7(1):9–18. doi: 10.1038/nri1994. [DOI] [PubMed] [Google Scholar]
- 5.Jutras I, Desjardins M. Phagocytosis: At the crossroads of innate and adaptive immunity. Annu Rev Cell Dev Biol. 2005;21:511–527. doi: 10.1146/annurev.cellbio.20.010403.102755. [DOI] [PubMed] [Google Scholar]
- 6.Thacker RI, Janssen EM. Cross-presentation of cell-associated antigens by mouse splenic dendritic cell populations. Front Immunol. 2012;3:41. doi: 10.3389/fimmu.2012.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iyoda T, et al. The CD8+ dendritic cell subset selectively endocytoses dying cells in culture and in vivo. J Exp Med. 2002;195(10):1289–1302. doi: 10.1084/jem.20020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 9.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179(4):1317–1330. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagata S, Hanayama R, Kawane K. Autoimmunity and the clearance of dead cells. Cell. 2010;140(5):619–630. doi: 10.1016/j.cell.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 11.Peng Y, Elkon KB. Autoimmunity in MFG-E8-deficient mice is associated with altered trafficking and enhanced cross-presentation of apoptotic cell antigens. J Clin Invest. 2011;121(6):2221–2241. doi: 10.1172/JCI43254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wofsy D, Seaman WE. Successful treatment of autoimmunity in NZB/NZW F1 mice with monoclonal antibody to L3T4. J Exp Med. 1985;161(2):378–391. doi: 10.1084/jem.161.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsokos GC, Nambiar MP, Tenbrock K, Juang YT. Rewiring the T-cell: Signaling defects and novel prospects for the treatment of SLE. Trends Immunol. 2003;24(5):259–263. doi: 10.1016/s1471-4906(03)00100-5. [DOI] [PubMed] [Google Scholar]
- 14.Moulton VR, Tsokos GC. Abnormalities of T cell signaling in systemic lupus erythematosus. Arthritis Res Ther. 2011;13(2):207. doi: 10.1186/ar3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: A key system for immune surveillance and homeostasis. Nat Immunol. 2010;11(9):785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer MB, Ma M, Hsu NC, Carroll MC. Local synthesis of C3 within the splenic lymphoid compartment can reconstitute the impaired immune response in C3-deficient mice. J Immunol. 1998;160(6):2619–2625. [PubMed] [Google Scholar]
- 17.Kopf M, Abel B, Gallimore A, Carroll M, Bachmann MF. Complement component C3 promotes T-cell priming and lung migration to control acute influenza virus infection. Nat Med. 2002;8(4):373–378. doi: 10.1038/nm0402-373. [DOI] [PubMed] [Google Scholar]
- 18.Suresh M, et al. Complement component 3 is required for optimal expansion of CD8 T cells during a systemic viral infection. J Immunol. 2003;170(2):788–794. doi: 10.4049/jimmunol.170.2.788. [DOI] [PubMed] [Google Scholar]
- 19.Kaya Z, et al. Contribution of the innate immune system to autoimmune myocarditis: A role for complement. Nat Immunol. 2001;2(8):739–745. doi: 10.1038/90686. [DOI] [PubMed] [Google Scholar]
- 20.Marsh JE, et al. The allogeneic T and B cell response is strongly dependent on complement components C3 and C4. Transplantation. 2001;72(7):1310–1318. doi: 10.1097/00007890-200110150-00022. [DOI] [PubMed] [Google Scholar]
- 21.Strainic MG, et al. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 2008;28(3):425–435. doi: 10.1016/j.immuni.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serra VA, Cretin F, Pépin E, Gabert FM, Marche PN. Complement C3b fragment covalently linked to tetanus toxin increases lysosomal sodium dodecyl sulfate-stable HLA-DR dimer production. Eur J Immunol. 1997;27(10):2673–2679. doi: 10.1002/eji.1830271029. [DOI] [PubMed] [Google Scholar]
- 23.Jacquier-Sarlin MR, Gabert FM, Villiers MB, Colomb MG. Modulation of antigen processing and presentation by covalently linked complement C3b fragment. Immunology. 1995;84(1):164–170. [PMC free article] [PubMed] [Google Scholar]
- 24.Mevorach D, Mascarenhas JO, Gershov D, Elkon KB. Complement-dependent clearance of apoptotic cells by human macrophages. J Exp Med. 1998;188(12):2313–2320. doi: 10.1084/jem.188.12.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quartier P, Potter PK, Ehrenstein MR, Walport MJ, Botto M. Predominant role of IgM-dependent activation of the classical pathway in the clearance of dying cells by murine bone marrow-derived macrophages in vitro. Eur J Immunol. 2005;35(1):252–260. doi: 10.1002/eji.200425497. [DOI] [PubMed] [Google Scholar]
- 26.Pickering MC, Botto M, Taylor PR, Lachmann PJ, Walport MJ. Systemic lupus erythematosus, complement deficiency, and apoptosis. Adv Immunol. 2000;76:227–324. doi: 10.1016/s0065-2776(01)76021-x. [DOI] [PubMed] [Google Scholar]
- 27.Rudensky AYu, Rath S, Preston-Hurlburt P, Murphy DB, Janeway CA., Jr On the complexity of self. Nature. 1991;353(6345):660–662. doi: 10.1038/353660a0. [DOI] [PubMed] [Google Scholar]
- 28.Viret C, Sant’Angelo DB, He X, Ramaswamy H, Janeway CA., Jr A role for accessibility to self-peptide-self-MHC complexes in intrathymic negative selection. J Immunol. 2001;166(7):4429–4437. doi: 10.4049/jimmunol.166.7.4429. [DOI] [PubMed] [Google Scholar]
- 29.Morelli AE, et al. Internalization of circulating apoptotic cells by splenic marginal zone dendritic cells: Dependence on complement receptors and effect on cytokine production. Blood. 2003;101(2):611–620. doi: 10.1182/blood-2002-06-1769. [DOI] [PubMed] [Google Scholar]
- 30.Behrens EM, et al. Apoptotic cell-mediated immunoregulation of dendritic cells does not require iC3b opsonization. J Immunol. 2008;181(5):3018–3026. doi: 10.4049/jimmunol.181.5.3018. [DOI] [PubMed] [Google Scholar]
- 31.Verbovetski I, et al. Opsonization of apoptotic cells by autologous iC3b facilitates clearance by immature dendritic cells, down-regulates DR and CD86, and up-regulates CC chemokine receptor 7. J Exp Med. 2002;196(12):1553–1561. doi: 10.1084/jem.20020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delamarre L, Pack M, Chang H, Mellman I, Trombetta ES. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science. 2005;307(5715):1630–1634. doi: 10.1126/science.1108003. [DOI] [PubMed] [Google Scholar]
- 33.Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006;440(7085):808–812. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- 34.Torchinsky MB, Garaude J, Martin AP, Blander JM. Innate immune recognition of infected apoptotic cells directs T(H)17 cell differentiation. Nature. 2009;458(7234):78–82. doi: 10.1038/nature07781. [DOI] [PubMed] [Google Scholar]
- 35.Manderson AP, Pickering MC, Botto M, Walport MJ, Parish CR. Continual low-level activation of the classical complement pathway. J Exp Med. 2001;194(6):747–756. doi: 10.1084/jem.194.6.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rose KL, et al. Factor I is required for the development of membranoproliferative glomerulonephritis in factor H-deficient mice. J Clin Invest. 2008;118(2):608–618. doi: 10.1172/JCI32525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leung VW, et al. Decay-accelerating factor suppresses complement C3 activation and retards atherosclerosis in low-density lipoprotein receptor-deficient mice. Am J Pathol. 2009;175(4):1757–1767. doi: 10.2353/ajpath.2009.090183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zelenay S, et al. The dendritic cell receptor DNGR-1 controls endocytic handling of necrotic cell antigens to favor cross-priming of CTLs in virus-infected mice. J Clin Invest. 2012;122(5):1615–1627. doi: 10.1172/JCI60644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peng Q, Li K, Patel H, Sacks SH, Zhou W. Dendritic cell synthesis of C3 is required for full T cell activation and development of a Th1 phenotype. J Immunol. 2006;176(6):3330–3341. doi: 10.4049/jimmunol.176.6.3330. [DOI] [PubMed] [Google Scholar]
- 40.Blander JM, Medzhitov R. Regulation of phagosome maturation by signals from toll-like receptors. Science. 2004;304(5673):1014–1018. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- 41.Elkon KB, Santer DM. Complement, interferon and lupus. Curr Opin Immunol. 2012;24(6):665–670. doi: 10.1016/j.coi.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 42.Heeger PS, Kemper C. Novel roles of complement in T effector cell regulation. Immunobiology. 2012;217(2):216–224. doi: 10.1016/j.imbio.2011.06.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.