Significance
In a social process called “quorum sensing” (QS) bacteria secrete and share signaling molecules that bind to specific receptors and induce adaptive responses within the population. We use the ComQXP QS system of Bacillus subtilis to study the intracellular codependence of two essential QS functions: signal production and signal response. We demonstrate that the QS signal-deficient mutants have an overly responsive QS system, a disturbed balance between primary and secondary metabolism, and an overproduction of the secondary metabolite surfactin in the presence of exogenously provided signal. Such mutants fail to compete with the socially active signal producers due to surfactin-related mechanisms that discriminate the two populations. We believe that a constraint on signal production preserves QS functionality in the natural microbial populations.
Keywords: social interactions, social evolution
Abstract
Bacteria coordinate their behavior using quorum sensing (QS), whereby cells secrete diffusible signals that generate phenotypic responses associated with group living. The canonical model of QS is one of extracellular signaling, where signal molecules bind to cognate receptors and cause a coordinated response across many cells. Here we study the link between QS input (signaling) and QS output (response) in the ComQXPA QS system of Bacillus subtilis by characterizing the phenotype and fitness of comQ null mutants. These lack the enzyme to produce the ComX signal and do not activate the ComQXPA QS system in other cells. In addition to the activation effect of the signal, however, we find evidence of a second, repressive effect of signal production on the QS system. Unlike activation, which can affect other cells, repression acts privately: the de-repression of QS in comQ cells is intracellular and only affects mutant cells lacking ComQ. As a result, the QS signal mutants have an overly responsive QS system and overproduce the secondary metabolite surfactin in the presence of the signal. This surfactin overproduction is associated with a strong fitness cost, as resources are diverted away from primary metabolism. Therefore, by acting as a private QS repressor, ComQ may be protected against evolutionary competition from loss-of-function mutations. Additionally, we find that surfactin participates in a social selection mechanism that targets signal null mutants in coculture with signal producers. Our study shows that by pleiotropically combining intracellular and extracellular signaling, bacteria may generate evolutionarily stable QS systems.
Bacteria secrete and share quorum-sensing (QS) signaling molecules that bind to specific receptors, and upon reaching critical concentration induce cell density-dependent adaptive responses within the population (1). In Gram-positive bacteria small peptide QS signals are typically produced from oligopeptide precursors that are modified by a signal-processing enzyme before they are secreted from the cell. It is assumed that these bacteria secrete QS signals during growth, and then in the stationary phase when the signal threshold concentration is reached, the signals activate specific histidine kinase receptors. These activate specific response regulators through phosphotransfer, which then initiate a QS response (2). The QS response often involves expression of adaptive extracellular factors (such as food-degrading enzymes, virulence factors, antibiotics, or biosurfactants) that are considered public goods (3, 4), as they can be shared within the population. Recently, however, it was shown that the QS of Gram-negative bacteria may also regulate adaptive metabolic pathways that produce molecules that remain private as they are not secreted by the responsive cells (5).
The ComQXPA QS system of Bacillus subtilis is a typical QS system of Gram-positive bacteria that controls expression of nearly 200 genes, including both extracellular and private cellular factors (6). This QS system involves four proteins: the ComQ isoprenyl transferase, the ComX signal peptide, the ComP histidine kinase, and the ComA response regulator. The signaling peptide ComX is initially synthesized as a 55-residue propeptide and then processed and modified by the isoprenyl transferase ComQ (7, 8). The isoprenylated ComX is then secreted (7, 9) and upon reaching the critical concentration, it activates autophosphorylation of the membrane-bound ComP, which then phosphorylates the transcriptional activator ComA (10). Phosphorylated ComA directly modulates the expression of various genes, including the srfA operon (6, 11) needed for nonribosomal synthesis of the major lipopeptide antibiotic surfactin (12). Surfactin is also one of the most effective biosurfactants discovered so far (13–15); it can penetrate cell membranes of various bacteria (16), inhibit surface adhesion of different pathogens, and act as an antiviral (17, 18) or hemolytic agent (18). As surfactin is secreted and affects nonsecretors (19), it can be considered a public good (3, 4).
In this study we use a model ComQXPA system of B. subtilis to test the coregulation of the QS input (signaling) and QS output (response). We do this by coculturing signal-deficient mutants with signal-producing wild-type cells or by culturing the strains in the presence of purified ComX, and by monitoring the expression of surfactin we evaluate the QS response. We provide the first evidence that QS signal-deficient mutants have an overactive QS response in the presence of the exogenously provided signal that results in overproduction of public goods and a dramatic loss of fitness. In addition, we show that these mutants have a disturbed balance between primary and secondary metabolism indicating that QS regulates the well being of growing bacteria and of those entering the stationary phase. Finally, we reveal that both signal and surfactin production contribute to QS stability in B. subtilis.
Results
The QS Wild Type and QS Signal-Deficient Mutant Have Different QS Response Dynamics.
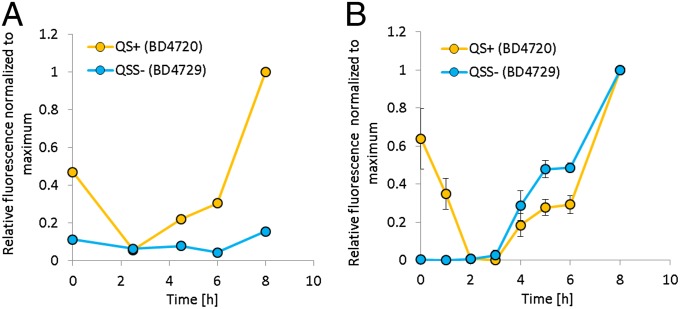
The ComX-dependent QS response (measured as srfA expression) is induced at the beginning of the stationary phase (20, 21). We investigated the dynamics of srfA expression in the QS proficient (QS+) B. subtilis BD4720 (srfA-yfp) and QS signal-deficient (QSS−) B. subtilis BD4729 (srfA-cfp) strains. The latter strain carries a mutation in comQ, which is responsible for signal processing and modification. Both strains, QS proficient and signal deficient, showed very similar growth kinetics (Fig. S1A) but differed significantly in their QS response (Fig. 1A). The QS response of the signal-producing population was already induced during the overnight growth. After inoculation into fresh medium, expression decreased transiently and was induced again at 4 h, reaching a maximum after incubation for 8 h (during the late stationary phase). The isogenic strain with a different fluorescent fusion, B. subtilis BD4726 (srfA-cfp), gave a comparable result (Fig. S2A), showing that the difference between the QS-proficient B. subtilis BD4720 (srfA-yfp) and QS signal-deficient B. subtilis BD4729 (srfA-cfp) is not due to the different fluorophore. The QS response of the QS signal-deficient mutant, however, remained very low throughout the same incubation period, confirming the ComX-dependent expression of srfA (Fig. 1A).
Fig. 1.
The relative QS response was monitored by single-cell fluorescent microscopy during growth of (A) domesticated QS-proficient (QS+) B. subtilis BD4720 (srfA-yfp) and QS signal-deficient (QSS−) B. subtilis BD4729 (srfA-cfp) grown alone and (B) in 1:1 cocultures of B. subtilis BD4720 and B. subtilis BD4729. The fluorescence of the srfA-yfp and srfA-cfp reporter fusions was normalized to maximal fluorescence for each fluorophore, and presented as relative fluorescence (Methods and SI Methods). Data are presented as mean values of at least three biological replicates and the SE is indicated for every time point.
Next we monitored growth and the QS response in cocultures of ComX signal-producing and signal-deficient cells mixed in a 1:1 ratio. The growth kinetics of the two strains in coculture were similar (Fig. S1B). Signal producers showed very similar relative QS response dynamics in monoculture and in coculture, but after incubation for 5 h the signal-deficient population showed a twofold higher relative QS response in terms of srfA expression than the signal producer (P < 0.0001). This difference in the relative QS response was lost after incubation for 8 h (Fig. 1B). These results were confirmed in cocultures containing two strains with switched fluorescent markers (Fig. S2B). Additionally, when two QS-proficient strains were labeled with different fluorescent fusions [B. subtilis BD4720 (srfA-yfp) and BD4726 (srfA-cfp)] and cocultured in a 1:1 ratio, the QS response dynamics of both strains were highly comparable (Fig. S2C), suggesting that the observed responses are not due to the intrinsic properties of the fluorescent proteins.
The Signal-Deficient Mutant Is Overly Sensitive to Exogenous ComX.
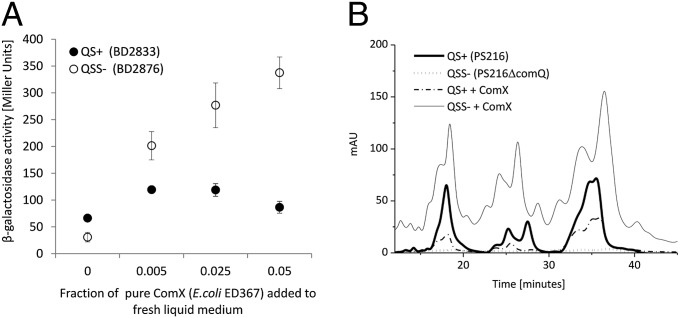
The results described above for 1:1 cocultures indicated a difference in the QS response dynamics of the QS signal-producing and signal-deficient populations. We speculated that signal-producing and signal-deficient strains may differ in their sensitivity to ComX. Therefore, the responsiveness of the ComX signal-producing (BD2833) and signal-deficient (BD2876) strains tagged with the srfA-lacZ reporter fusion was assessed during exposure to different concentrations of the purified ComX. Indeed, the signal-deficient strain was more responsive to exogenous ComX than the QS signal-producing strain at all fractions tested (0.005–0.05) and was fourfold (P < 0.000001) more responsive with the 0.05 fraction, estimated to contain ∼10 nM ComX (Fig. 2A). This concentration corresponds to the saturating levels of ComX produced by the QS-proficient strains (7). In a complementary approach, the 0.05 fraction of exogenous ComX was added to the monocultures of the fluorescently labeled signal-producing strain BD4726 (srfA-cfp) and signal-deficient strain BD4729 (srfA-cfp) and the QS response was measured during growth. At all time points the fluorescence of the QS signal-deficient mutant grown with exogenous ComX was higher than that of the signal producer, which even showed a small decrease in fluorescence in the presence of exogenous ComX (Fig. S3A). Exchange of fluorescent reporters did not influence the results (Fig. S3B) and confirmed the greater sensitivity of the signal-deficient mutant to ComX. Despite the pronounced difference in the ComX-induced srfA response, the specific growth rates of the signal-producing and signal-deficient strains were not significantly different (Fig. S3C).
Fig. 2.
(A) The QS response of the domesticated and QS+ B. subtilis BD2833 (srfA-lacZ) and QS signal-deficient (QSS−) B. subtilis BD2876 (srfA-lacZ) was monitored by β-galactosidase activity. Cultures were incubated until the stationary phase with a range of concentrations of the ComX pheromone purified from recombinant E. coli ED367. Error bars represent the SE indicated for every time point. (B) Chromatograms were obtained after HPLC analysis of surfactin isolated from the spent media of the B. subtilis PS216 and PS 216ΔcomQ control cultures (no ComX) and cultures supplemented with 0.05 fraction purified ComX.
QS Signal Induces Dramatically Higher Surfactin Secretion in Undomesticated Signal-Deficient B. subtilis.
We next hypothesized that the increased srfA expression observed in the domesticated signal-deficient mutant would translate into increased secretion of surfactin in the mutant derived from the undomesticated isolate B. subtilis PS-216 (22) that, unlike the domesticated strain, synthesizes and secretes surfactin in response to ComX. To compare surfactin secretion in the PS216 (srfA-cfp) and signal-deficient PS216ΔcomQ (srfA-cfp) mutants, we took advantage of the hemolytic property of this lipopeptide antibiotic (18) and measured its activity in spent medium of the four experimental variants described above: PS216 signal-producer alone, signal-producer with added ComX, signal-deficient mutant B. subtilis PS216ΔcomQ, and signal-deficient mutant with added ComX. Indeed, the spent medium taken from the signal-deficient mutant grown in the presence of ComX produced at least a twofold (P < 0.0006) stronger hemolytic activity at several time points during the growth than the signal-producing B. subtilis PS216 grown in the presence or absence of ComX (Fig. S4A).
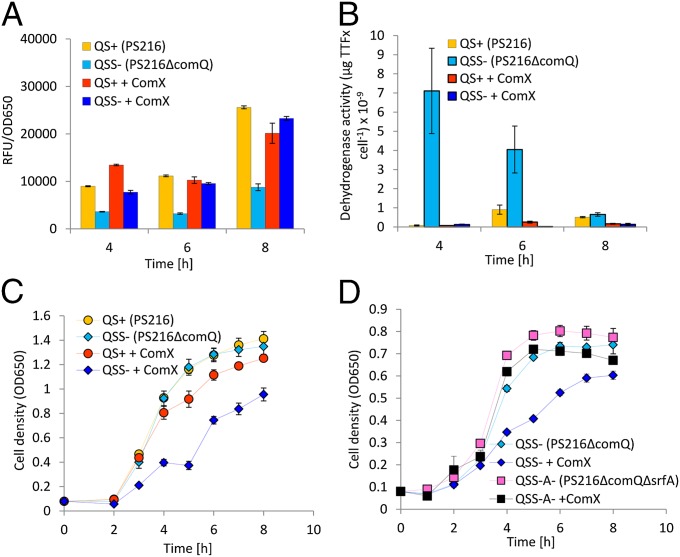
To further link the hemolytic activity to surfactin itself, the concentration of this lipopeptide antibiotic was determined by reverse-phase HPLC in the supernatant of all four experimental variants. Chromatographic peaks corresponding to peaks of commercially available surfactin standard were evident in all experimental variants except the B. subtilis PS216ΔcomQ culture without added ComX. Again, concentration of surfactin was highest in the supernatant of the PS216ΔcomQ strain with added ComX, reaching almost 80 μM after 8 h (Fig. 2B and Fig. S4B). In contrast, the signal-producer PS216 with and without ComX produced only up to 10 μM of surfactin at the same time (P < 0.02) (Fig. 2B and Fig. S4B). Similarly, addition of ComX to the signal-deficient PS216ΔcomX mutant resulted in increased surfactin production (Fig. S4 C and D). This mutant has the crucial tryptophan residue changed to alanine (8) and is unable to produce an active ComX, but has an intact ComQ protein. This result indicates that the synthesis or/and secretion of active ComX is important for modulation of the QS response. Surprisingly, total fluorescence of srfA-cfp in the PS216ΔcomQ background with exogenous ComX (Fig. 3A) was comparable with that of the QS-proficient PS216, which is different from that of domesticated strains. Only PS216 produces surfactin and it was proposed recently that surfactin negatively regulates its own expression (19), which may explain the observed difference in the srfA-expression pattern between domesticated and PS216 derivatives. Interestingly, single-cell microscopy analysis revealed that, despite similar cumulative fluorescence of PS216 and the signal-deficient derivative PS216ΔcomQ in the presence of ComX, the distributions of srfA expression differed between the two strains: in PS216 30 ± 7% of cells did not express srfA-cfp regardless of ComX exposure, but only 14 ± 1% of the mutant population remained uninduced in the presence of ComX (Fig. S5 A–C).
Fig. 3.
(A) Expression of srfA was measured spectro-fluorimeterically at three different time points in srfA-cfp–labeled strains: QS-proficient (QS+) B. subtilis PS216 and signal-deficient (QSS−) PS216ΔcomQ incubated with or without purified ComX. (B) The dehydrogenase activity of B. subtilis PS216 and signal-deficient PS216ΔcomQ grown with or without ComX (0.05 fraction) was determined after 4, 6, and 8 h of incubation. (C) Growth of QS+ B. subtilis PS216 and signal-deficient (QSS−) PS216ΔcomQ in competence medium without and with purified ComX (0.05 fraction) was monitored spectro-photometrically at 650 nm. (D) Growth of QSS− B. subtilis PS216ΔcomQ and double-mutant QSS−A− PS216ΔcomQΔsrfA deficient in ComX and surfactin production in the absence (control) and presence of ComX (0.05 fraction) was monitored spectro-photometrically at 650 nm. Data are presented as the mean of biological triplicates with SE indicated for every time point.
Increased Synthesis of Surfactin Is Associated with Loss of Fitness of Undomesticated, Signal-Deficient B. subtilis.
We further investigated whether the increased surfactin production observed for the undomesticated PS216ΔcomQ strain in the presence of ComX is associated with the negative fitness effects. We hypothesized that these effects might be either due to autotoxicity or metabolic burden. To test both hypotheses, we first monitored the growth of the signal-producing B. subtilis PS216 and the signal-deficient PS216ΔcomQ mutant in the presence and absence of exogenously added ComX. The growth curves of the signal-producing and signal-deficient monocultures were very similar in the absence of exogenously added ComX, despite the fact that the former produced ComX and surfactin in response to it (Fig. 3C). In contrast, growth of the signal-deficient mutant PS216ΔcomQ was negatively affected by the presence of exogenous ComX with a 20% (P < 0.002) decrease in the growth rate (Fig. 3C). Similarly, negative fitness effects were observed for PS216ΔcomX, which also showed increased surfactin production (Fig. S4E).
To further confirm the role of srfA in the ComX-mediated growth breakdown of the mutant, we constructed the PS216ΔcomQΔsrfA double mutant, which was not able to synthesize ComX or surfactin, and monitored its growth in the presence of exogenous ComX (Fig. 3D). In contrast to PS216ΔcomQ, no dramatic fitness effect was observed for the PS216ΔcomQΔsrfA double mutant, strongly supporting the hypothesis that increased synthesis of surfactin in the undomesticated signal-deficient mutant exerts a negative fitness effect (Fig. 3D).
Additionally we tested the influence of the exogenous ComX on the PS216ΔcomQXP mutant, which was both signal deficient (due to ΔcomQ or ΔcomX) and receptor deficient (due to ΔcomP). No ComX-dependent negative fitness effect was observed in the absence of the ComP receptor, confirming that the srfA expression in the signal-deficient mutant was induced by a specific ComX–ComP interaction and that overproduction of surfactin is responsible for the negative fitness effect in monoculture (Fig. S4F).
Primary/Secondary Metabolic Imbalance as a Cost of Being Signal Deficient.
Next we investigated the mechanism of surfactin-dependent growth inhibition in signal-deficient mutants overproducing surfactin. This effect might be due to membrane damage induced by surfactin, as it was shown that high concentrations of this lipopeptide antibiotic may induce death of B. subtilis cells (23). To examine this hypothesis we used a commercially available staining kit, LIVE/DEAD BacLight (Molecular Probes Europe), and determined the number of damaged cells in signal-producing and signal-deficient B. subtilis cultures in the presence and absence of exogenous ComX at three time points. The PS216ΔcomQ population, which did not produce surfactin, contained the lowest number of damaged cells, ∼10%. The highest proportion of damaged cells (33 ± 17% to 49 ± 12%) was detected at 4 and 6 h for both the signal-producing and signal-deficient cultures grown in the presence of exogenous ComX (Fig. S6A). Interestingly, after 8 h only 10% of damaged cells remained in all cultures, indicating decreased sensitivity to surfactin in the late stationary phase (8 h) (Fig. S6A). The percent of damaged cells was similar for the QS+ and the signal-deficient (QSS−) populations supplemented by ComX, indicating that antimicrobial effects of surfactin are not responsible for the surfactin-dependent growth deceleration observed only in the signal-deficient population. Therefore, the negative fitness associated with high production of surfactin might be associated with metabolic overinvestment of the mutant in the stationary phase and possibly decreased investment in primary, growth-supporting metabolism. To look closer into this prediction, we evaluated the role of QS in primary metabolism using a dehydrogenase activity assay, which is routinely used for this purpose (24).
Indeed, in the absence of ComX the signal-deficient PS216ΔcomQ mutant showed a strong increase in dehydrogenase activity, which dropped dramatically and below the levels observed for the QS+ PS216 strains, if the mutant was exposed to ComX (Fig. 3B). The dehydrogenase assay performed for other mutants (lacking the ComP receptor, the receptor and the signal, or just the srfA gene) revealed that signal production and surfactin production are both required to preserve moderate primary metabolic activity. ComX could decrease this activity only in the signal-deficient strain with an intact ComP receptor, but not in strains where either the comP or the srfA genes were missing (Fig. S6B). This strongly supports the role of the ComQXP QS system in coupling primary and secondary metabolism.
The Signal-Deficient Mutant Fails in Competition with the Wild Type.
On the basis of the results described above we hypothesized that the signal-deficient mutant PS216∆comQ will fail in competition with the QS wild-type PS216, because it would suffer from negative fitness effects when facing the QS signal produced by the wild type. Consistent with this hypothesis, the proportion of the signal-deficient derivative PS216ΔcomQ in a 1:1 coculture with the signal-producing B. subtilis PS216 was reduced to 10% (P < 0.002) after incubation for 8 h, indicating a significant advantage of the wild-type signal producer (Fig. 4A). We next examined the fitness of the double-mutant PS216∆comQ∆srfA that did not show fitness loss when exposed to ComX in monoculture (Fig. 3C) and surprisingly we discovered that it was also outcompeted by the wild type, comprising less than 10% (P < 0.001) of the community after incubation for 8 h (Fig. 4B). To explore this phenomenon further we excluded surfactin expression from the system by challenging the mutant in surfactin production (PS216∆srfA) against the double mutant (PS216∆comQ∆srfA). PS216∆srfA did not show any changes in fitness against parental strains with the intact srfA gene (Fig. S7 A and B) and the ratio of PS216∆srfA mutant and the signal-deficient double-mutant PS216∆comQ∆srfA did not change dramatically, with 44 ± 3% of the double mutant remaining in the coculture after incubation for 8 h (Fig. 4D). This confirmed the role of surfactin in dominance of the wild-type population over the double mutant, which was further confirmed when the coculture was supplemented with exogenous surfactin (final concentration 20 µg/mL), again reducing the ratio of PS216∆comQ∆srfA from 52 ± 4% to 19 ± 3% (Fig. 4E). Interestingly, despite surfactin being associated with the disadvantage of the signal-deficient double mutant (PS216∆comQ∆srfA) in coculture, this lipopeptide antibiotic did not strongly influence growth of the double mutant in monocultures. A slight decrease in optical density was only observed in surfactin-treated double mutant after 6 and 8 h of incubation, but not during logarithmic growth (Fig. S7D). This suggests that surfactin, produced by the wild type or added to the coculture predominantly indirectly contributes to fitness loss of the double mutant and that the presence of the QS-proficient population is required to observe a strong negative fitness effect on the signal-deficient double mutant (PS216∆comQ∆srfA). However, surfactin itself also shows a minor direct negative effect on the double mutant during stationary phase, which may at least in part account for the difference in fitness during competition between the surfactin producer and the double mutant.
Fig. 4.
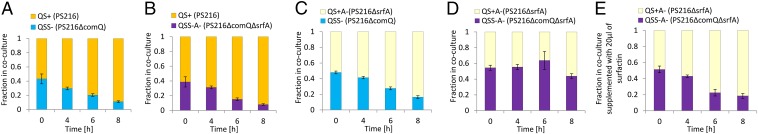
Fractions of (A) PS216 and PS216ΔcomQ, (B) PS216 and PS216ΔcomQ∆srfA, (C) PS216∆srfA and PS216ΔcomQ, (D) PS216∆srfA and PS216ΔcomQ∆srfA cells in coculture, and (E) PS216∆srfA and PS216ΔcomQ∆srfA cells supplemented with surfactin (20 µg/mL) in coculture were determined over time by viable cell counts (CFU) using antibiotic selection. Data are presented as the mean of biological triplicates with SE indicated.
The question that remains is whether the fitness loss of the signal-deficient mutant PS216∆comQ is linked to surfactin overproduction, observed in monocultures grown in the presence of exogenous ComX. We believe this mechanism does contribute to the fitness loss of PS216∆comQ when challenged with QS-proficient populations. For example, in the coculture containing the surfactin-deficient PS216∆srfA that produces ComX and the signaling mutant PS216∆comQ, the ratio of the latter decreased from 48 ± 2% to 16 ± 2% after 8 h (Fig. 4C). This is consistent with the hypothesis that surfactin overproduction adds to fitness loss of the signaling mutant. Therefore, two surfactin-dependent mechanisms may exist: a direct mechanism related to surfactin overproduction and an indirect mechanism when the competitor produces surfactin, reducing the fitness of the signaling mutant through an as-yet-unknown mechanism. Both mechanisms may contribute to the social selection that preserves the functionality of the QS in the natural populations of B. subtilis (Fig. 5).
Fig. 5.
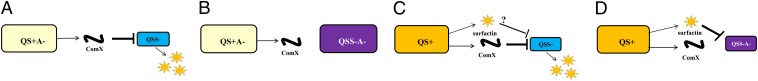
Proposed strategies that discriminate signal-deficient mutants in cocultures with QS-proficient (QS+) and QS-proficient-but-surfactin-deficient (QS+A−) B. subtilis. The size of the cell indicates the relative fitness loss of the population. (A) Signal-deficient mutant QSS− facing the QS signal ComX secreted by QS+A− suffers from fitness loss associated with overproduction of surfactin. (B) The signal-deficient surfactin-deficient double-mutant QSS−A−, which does not overproduce surfactin, is released from the ComX-mediated fitness loss when competed with the signal-producing-but-surfactin-deficient QS+A− strain. (C) Signal-deficient mutant suffers from fitness loss when cocultured with the QS+ strain due to a ComX-mediated mechanism and possibly due to another mechanism that depends on surfactin. (D) The surfactin-dependent mechanism leads to the fitness loss of the QSS−A− double mutant when it is cocultured with the surfactin-producing QS+ strain.
Discussion
In this study we use the ComQXPA model system of B. subtilis to evaluate the intracellular codependence of two essential QS functions: signaling and response. We show that when the responder does not produce its own signal, the QS response is significantly increased in comparison with the response of the signal-producing strain. The accurate QS response therefore requires both sufficient ComX, which is secreted from the cell, and an intracellular signal that is ComQ dependent. Our work provides unique evidence that fine tuning of the QS response is linked to signal production and its ComQ-dependent modification at the posttranslational level. This regulation may depend on physical interactions between ComQ and ComX or even ComQ, ComX, and ComP. However, a precise understanding of these interactions awaits further study.
In domesticated comQ strains the increased QS response was visible at the level of srfA transcription, which dramatically increased in the presence of ComX. A small decrease in srfA expression in the presence of exogenous ComX observed for the domesticated signal-proficient strain suggests negative feedback regulation as an intrinsic property of the ComQXPA system. It additionally indicates that the structural integrity of the ComQXPA system is sufficient to prevent overresponse. In undomesticated PS216ΔcomQ mutants the effect was also detectable at the level of surfactin synthesis, which was significantly higher than in QS wild-type controls. In addition, we observed that a higher fraction of comQ cells activated srfA gene transcription in the presence of ComX than in the undomesticated QS+ strains. Bimodal expression of srfA in undomesticated strains was reported previously (19) and was suggested to lead to phenotypic differentiation of the Bacillus population and a division of labor that provide fitness benefits (25). Our results show that the private link between signal production and QS response also has consequences for bimodal expression of srfA in the B. subtilis population. Recently it was reported that in Salmonella, which shows bimodal expression of virulence, the existence of an avirulent subpopulation is essential for the fitness and evolutionary stability of virulence (26). A similar mechanism may decrease the cost of surfactin production in B. subtilis populations where only a fraction of cells express surfactin. In fact, under our experimental conditions, synthesis of surfactin was not charged with any obvious fitness costs as the growth of the QS+ wild type was very similar to that of the signaling mutant when no exogenous signal was added. However, this changed dramatically when signal was added to the comQ mutant, decreasing the fitness of the mutant. Therefore, generating a higher proportion of surfactin producers may have significantly contributed to the fitness loss of the mutant. Another mechanism that may decrease the fitness cost is related to prudent regulation of public goods in Pseudomonas aeruginosa (27). Production of rhamnolipid biosurfactant is limited to the stationary phase and thus free of fitness charges, but release of biosurfactant production from QS control (using an inducible promoter for its induction) resulted in its imprudent production and fitness loss (27). In B. subtilis, surfactin is also produced during the stationary phase, but in signal-deficient mutants this prudency is lost due to overproduction of surfactin.
Whereas signal-deficient mutants are common among certain bacterial species such as P. aeruginosa (28–30), they have not been detected among more than 60 strains of B. subtilis isolated from various environments (9, 22, 31, 32). Our data offer an explanation for this observation. The private repressive QS effect of signal production identified in our system means that signal-null mutants effectively behave as hyper-cooperators with respect to surfactin production. The result is that mutants fare poorly in cocultures with the wild type and their ratio significantly decreases within only a few hours. The private regulatory link between signaling and response may therefore mean that signal-deficient mutants are rapidly lost from native populations of B. subtilis.
Moreover our results show that surfactin itself, when produced by the competitor, is indirectly involved in the fitness loss of the signaling mutant. This mechanism negatively affects the signal-deficient mutants even if they are released from the ComX-dependent disadvantage of surfactin overproduction (e.g., in the double-mutant PS216∆comQ∆srfA). This is consistent with current knowledge that surfactin serves as a signaling molecule that triggers cannibalism in the B. subtilis population (33, 34). The advantage of the surfactin-deficient mutant (PS216∆srfA) over the double-mutant PS216∆comQ∆srfA in the presence of exogenously provided surfactin suggests that the QS+A− and QSS−A− populations differ in response to surfactin. This may translate, for example, into generation of higher numbers of cannibals in the QS+ population than in the QSS− population and provide a testable hypothesis that, however, awaits further study. The idea that pleiotropic links between public and private effects can help to stabilize social traits has been raised for other systems (35). Most notably, Dandekar et al. (5) recently argued that private effects in the QS response can limit the evolution of QS receptor null mutants in P. aeruginosa. Here we show that similar pleiotropic constraints exist at the level of signal production.
How did the pleiotropic constraint on signal loss arise? It could be that the private effect of the signal evolved specifically because it limits the potential for loss-of-function mutations. More likely, the private effect evolved to improve the regulatory performance of the QS system, which then serendipitously helped ensure its evolutionary stability. Whatever the situation, it is clear that signal production now plays an important role in regulating secondary metabolism and other traits related to the response of the population to surfactin. We found that the fitness loss of the signal-deficient mutant is due to the metabolic burden linked to production of a secondary metabolite, surfactin, rather than due to the autotoxicity of surfactin. In addition, these mutants showed increased primary metabolic activity in the absence of the signal but decreased primary metabolism in its presence, emphasizing the link between QS and primary metabolism. Finally, fitness loss of the signal-deficient mutant is caused by the second, surfactin-linked mechanism that acts even in the absence of metabolic constraint. The signal-null, double-mutant PS216∆comQ∆srfA, which does not bear the metabolic burden of surfactin overproduction, still fails to compete with the QS wild type, probably due to as-yet-unknown mechanism governed by the surfactin itself. This surfactin-dependent mechanism, which is able to differentiate between the ComX signal producers and nonproducers, again represents the very strong evolutionary pressure for preservation of QS signaling in B. subtilis.
This work shows that the intracellular production of the QS signal can act as a private good that acts to limit cooperation of expressing cells. Inactivation of this switch results in overinvestment in secondary metabolism, and increased sensitivity to surfactin-mediated punishment, making the signaling mutant less fit to compete with the socially active wild-type populations. We believe that such constraints on signal production promote QS functionality in natural populations and account for a unique mechanism that preserves cell–cell communication.
Methods
Bacterial Strains and Growth Conditions.
Strains used in this study and construction of their mutant derivatives are described in Tables S1 and S2.
Growth Conditions.
Growth experiments were performed in competence medium supplemented with l-histidine, l-leucine, and l-methionine (50 μg⋅mL–1) as described previously (36). Overnight cultures were grown at 37 °C in 50-mL tubes (Duran) containing 5 mL LB broth with vigorous shaking (200 rpm) and were supplemented with chloramphenicol (5 μg⋅mL–1), kanamycin (5 μg⋅mL–1), spectinomycin (100 μg⋅mL–1), or tetracycline (20 μg⋅mL–1) as appropriate. For experiments in cocultures the cell numbers were estimated by OD650 and wild-type and signal-deficient overnight cultures (or control QS-proficient culture) were mixed in Eppendorf tubes in a 1:1 ratio in fresh medium. Cultures were prepared in 10 mL fresh competence liquid medium with a 2% (vol/vol) inoculum and were incubated at 37 °C for 8 h with shaking at 200 rpm.
Fluorescence Microscopy.
Fluorescence images were taken using a Zeiss Axio Observer Z1 with 100×/1.40 oil Plan apochromat objective and equipped with an AxioCam MRm Rev.3 camera. The fluorescent light source was an HBO 100 Illuminator using 47HE and 48HE filters for excitation and emission, respectively. Flat-field correction and calibration was performed using sodium fluorescein (0.75 g⋅mL–1) as a standard (37). The captured images were analyzed with ImageJ (Version 1.43u) software (38) and artifact objects were removed manually before calculating fluorescence. The weighted average of the mean normalized intensities of objects was calculated for individual images with the fluorescence of each sample determined in five technical replicates obtained by imaging five different fields of 50- to 700-cell samples. Histograms were obtained using the OriginPro (OriginLab Corporation) program. Slide preparation is briefly described in SI Methods.
Spectro-Fluorimetry.
The cells were centrifuged and resuspended in equal volumes of 0.9% NaCl. Fluorescence was measured using a Safire II microplate reader (Tecan). The excitation/emission wavelengths for CFP and YFP dyes were 455/500 nm and 513/527 nm respectively. The data are expressed as relative fluorescence units and then normalized to OD650.
Purification of QS Signal and Surfactin.
Expression and purification of ComX from the Escherichia coli ED367 producer strain was carried out according to procedure of Ansaldi et al. (9) (SI Methods). Surfactin was isolated according to the protocol described by Cooper et al. (39) (SI Methods).
β-Galactosidase Assay.
β-Galactosidase was assayed using a Multiscan Spectrum Microplate Reader (Thermo Scientific). The absorbance at 420 nm was measured at 30°C immediately after the addition of ortho-nitrophenyl-β-galactoside substrate (SI Methods).
Hemolytic Assay.
The assay was performed using bovine red blood cells (40) as described in SI Methods.
Live/Dead Staining.
The LIVE/DEAD BacLight bacterial viability kit was used for microscopy and quantitative analysis.
Dehydrogenase Activity.
The reduction of 2,3,5-triphenyltetrazolium chloride to the 2,3,5-triphenyltetrazolium formazan was measured according to the modified Maness et al. procedure (24) (SI Methods).
Supplementary Material
Acknowledgments
We thank Prof. D. Dubnau for providing laboratory space and advice on strain constructions; J. I. Prosser for encouragement and for proofreading the manuscript; and Prof. K. R. Foster for encouragement, constructive discussions, and advice regarding manuscript preparation. This work was supported by Slovenian Research Agency Grant J4-3631 (to I.M.M.), ARRS Program Grant JP4-116, and an ARRS Young Investigator grant.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1316283111/-/DCSupplemental.
References
- 1.Miller MB, Bassler BL. Quorum sensing in bacteria. Annu Rev Microbiol. 2001;55:165–199. doi: 10.1146/annurev.micro.55.1.165. [DOI] [PubMed] [Google Scholar]
- 2.Kleerebezem M, Quadri LE, Kuipers OP, de Vos WM. Quorum sensing by peptide pheromones and two-component signal-transduction systems in Gram-positive bacteria. Mol Microbiol. 1997;24(5):895–904. doi: 10.1046/j.1365-2958.1997.4251782.x. [DOI] [PubMed] [Google Scholar]
- 3.Crespi BJ. The evolution of social behavior in microorganisms. Trends Ecol Evol. 2001;16(4):178–183. doi: 10.1016/s0169-5347(01)02115-2. [DOI] [PubMed] [Google Scholar]
- 4.West SA, Griffin AS, Gardner A, Diggle SP. Social semantics: Altruism, cooperation, mutualism, strong reciprocity and group selection. Nat Rev Microbiol. 2006;8:597–607. doi: 10.1111/j.1420-9101.2006.01258.x. [DOI] [PubMed] [Google Scholar]
- 5.Dandekar AA, Chugani S, Greenberg EP. Bacterial quorum sensing and metabolic incentives to cooperate. Science. 2012;338(6104):264–266. doi: 10.1126/science.1227289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Comella N, Grossman AD. Conservation of genes and processes controlled by the quorum response in bacteria: Characterization of genes controlled by the quorum-sensing transcription factor ComA in Bacillus subtilis. Mol Microbiol. 2005;57(4):1159–1174. doi: 10.1111/j.1365-2958.2005.04749.x. [DOI] [PubMed] [Google Scholar]
- 7.Magnuson R, Solomon J, Grossman AD. Biochemical and genetic characterization of a competence pheromone from B. subtilis. Cell. 1994;77(2):207–216. doi: 10.1016/0092-8674(94)90313-1. [DOI] [PubMed] [Google Scholar]
- 8.Bacon Schneider K, Palmer TM, Grossman AD. Characterization of comQ and comX, two genes required for production of ComX pheromone in Bacillus subtilis. J Bacteriol. 2002;184(2):410–419. doi: 10.1128/JB.184.2.410-419.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ansaldi M, Marolt D, Stebe T, Mandic-Mulec I, Dubnau D. Specific activation of the Bacillus quorum-sensing systems by isoprenylated pheromone variants. Mol Microbiol. 2002;44(6):1561–1573. doi: 10.1046/j.1365-2958.2002.02977.x. [DOI] [PubMed] [Google Scholar]
- 10.Weinrauch Y, Penchev R, Dubnau E, Smith I, Dubnau D. A Bacillus subtilis regulatory gene product for genetic competence and sporulation resembles sensor protein members of the bacterial two-component signal-transduction systems. Genes Dev. 1990;4(5):860–872. doi: 10.1101/gad.4.5.860. [DOI] [PubMed] [Google Scholar]
- 11.Roggiani M, Dubnau D. ComA, a phosphorylated response regulator protein of Bacillus subtilis, binds to the promoter region of srfA. J Bacteriol. 1993;175(10):3182–3187. doi: 10.1128/jb.175.10.3182-3187.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakano MM, Marahiel MA, Zuber P. Identification of a genetic locus required for biosynthesis of the lipopeptide antibiotic surfactin in Bacillus subtilis. J Bacteriol. 1988;170(12):5662–5668. doi: 10.1128/jb.170.12.5662-5668.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishigami Y, et al. Significance of β-sheet formation for micellization and surface adsorption of surfactin. Colloids and Surfaces B. 1995;4(6):341–348. [Google Scholar]
- 14.Peypoux F, Bonmatin JM, Wallach J. Recent trends in the biochemistry of surfactin. Appl Microbiol Biotechnol. 1999;51(5):553–563. doi: 10.1007/s002530051432. [DOI] [PubMed] [Google Scholar]
- 15.Heerklotz H, Seelig J. Detergent-like action of the antibiotic peptide surfactin on lipid membranes. Biophys J. 2001;81(3):1547–1554. doi: 10.1016/S0006-3495(01)75808-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergey JGH, Krieg NR, Sneath PHA. Bergey’s Manual of Determinative Bacteriology. 9th Ed. Baltimore: Lippincott Williams & Wilkins; 1994. [Google Scholar]
- 17.Vollenbroich D, Ozel M, Vater J, Kamp RM, Pauli G. Mechanism of inactivation of enveloped viruses by the biosurfactant surfactin from Bacillus subtilis. Biologicals. 1997;25(3):289–297. doi: 10.1006/biol.1997.0099. [DOI] [PubMed] [Google Scholar]
- 18.Kracht M, et al. Antiviral and hemolytic activities of surfactin isoforms and their methyl ester derivatives. J Antibiot (Tokyo) 1999;52(7):613–619. doi: 10.7164/antibiotics.52.613. [DOI] [PubMed] [Google Scholar]
- 19.López D, Vlamakis H, Losick R, Kolter R. Paracrine signaling in a bacterium. Genes Dev. 2009;23(14):1631–1638. doi: 10.1101/gad.1813709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakano MM, et al. srfA is an operon required for surfactin production, competence development, and efficient sporulation in Bacillus subtilis. J Bacteriol. 1991;173(5):1770–1778. doi: 10.1128/jb.173.5.1770-1778.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hahn J, Dubnau D. Growth stage signal transduction and the requirements for srfA induction in development of competence. J Bacteriol. 1991;173(22):7275–7282. doi: 10.1128/jb.173.22.7275-7282.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stefanic P, Mandic-Mulec I. Social interactions and distribution of Bacillus subtilis pherotypes at microscale. J Bacteriol. 2009;191(6):1756–1764. doi: 10.1128/JB.01290-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tsuge K, Ohata Y, Shoda M (2001) Gene yerP, involved in Surfactin self-resistance in Bacillus subtilis. Antimicrob Agents Chemother 45(12):3566–3573. [DOI] [PMC free article] [PubMed]
- 24.Maness PC, et al. Bactericidal activity of photocatalytic TiO(2) reaction: Toward an understanding of its killing mechanism. Appl Environ Microbiol. 1999;65(9):4094–4098. doi: 10.1128/aem.65.9.4094-4098.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.López D, Kolter R. Extracellular signals that define distinct and coexisting cell fates in Bacillus subtilis. FEMS Microbiol Rev. 2010;34(2):134–149. doi: 10.1111/j.1574-6976.2009.00199.x. [DOI] [PubMed] [Google Scholar]
- 26.Diard M, et al. Stabilization of cooperative virulence by the expression of an avirulent phenotype. Nature. 2013;494(7437):353–356. doi: 10.1038/nature11913. [DOI] [PubMed] [Google Scholar]
- 27.Xavier JB, Kim W, Foster KR. A molecular mechanism that stabilizes cooperative secretions in Pseudomonas aeruginosa. Mol Microbiol. 2011;79(1):166–179. doi: 10.1111/j.1365-2958.2010.07436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamood AN, Griswold J, Colmer J. Characterization of elastase-deficient clinical isolates of Pseudomonas aeruginosa. Infect Immun. 1996;64(8):3154–3160. doi: 10.1128/iai.64.8.3154-3160.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rumbaugh KP, Griswold JA, Hamood AN. Pseudomonas aeruginosa strains obtained from patients with tracheal, urinary tract and wound infection: Variations in virulence factors and virulence genes. J Hosp Infect. 1999;43(3):211–218. doi: 10.1053/jhin.1999.0252. [DOI] [PubMed] [Google Scholar]
- 30.Dénervaud V, et al. Characterization of cell-to-cell signaling-deficient Pseudomonas aeruginosa strains colonizing intubated patients. J Clin Microbiol. 2004;42(2):554–562. doi: 10.1128/JCM.42.2.554-562.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tortosa P, et al. Specificity and genetic polymorphism of the Bacillus competence quorum-sensing system. J Bacteriol. 2001;183(2):451–460. doi: 10.1128/JB.183.2.451-460.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stefanic P, et al. The quorum sensing diversity within and between ecotypes of Bacillus subtilis. Environ Microbiol. 2012;14(6):1378–1389. doi: 10.1111/j.1462-2920.2012.02717.x. [DOI] [PubMed] [Google Scholar]
- 33.González-Pastor JE, Hobbs EC, Losick R. Cannibalism by sporulating bacteria. Science. 2003;301(5632):510–513. doi: 10.1126/science.1086462. [DOI] [PubMed] [Google Scholar]
- 34.López D, Vlamakis H, Losick R, Kolter R. Cannibalism enhances biofilm development in Bacillus subtilis. Mol Microbiol. 2009;74(3):609–618. doi: 10.1111/j.1365-2958.2009.06882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foster KR, Shaulsky G, Strassmann JE, Queller DC, Thompson CR. Pleiotropy as a mechanism to stabilize cooperation. Nature. 2004;431(7009):693–696. doi: 10.1038/nature02894. [DOI] [PubMed] [Google Scholar]
- 36.Albano M, Hahn J, Dubnau D. Expression of competence genes in Bacillus subtilis. J Bacteriol. 1987;169(7):3110–3117. doi: 10.1128/jb.169.7.3110-3117.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Model MA, Burkhardt JK. A standard for calibration and shading correction of a fluorescence microscope. Cytometry. 2001;44(4):309–316. doi: 10.1002/1097-0320(20010801)44:4<309::aid-cyto1122>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 38.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cooper DG, Macdonald CR, Duff SJ, Kosaric N. Enhanced production of surfactin from Bacillus subtilis by continuous product removal and metal cation additions. Appl Environ Microbiol. 1981;42(3):408–412. doi: 10.1128/aem.42.3.408-412.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morán AC, Martínez MA, Siñeriz F. Quantification of surfactin in culture supernatants by hemolytic activity. Biotechnol Lett. 2002;24(3):177–180. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.