Significance
Acute kidney injury (AKI) is a common and significant clinical problem for which no specific therapy has been developed. There is controversy about the origin of the regenerating tubular cells after AKI. Attention has recently focused on “scattered tubular cells” (STCs), which are by far the best candidate cells for the postulated fixed progenitor population of kidney tubular cells. In the present study, we clarify this question by genetic cell fate labeling using a unique transgenic mouse. We show that STCs may arise from any tubular cell and that these cells do not represent fixed progenitor cells. Rather, upon different injuries, proximal tubular cells transiently acquire the STC phenotype, which we show to have reparative characteristics.
Keywords: regeneration, lineage tracing, cell fate tracking, stem cells
Abstract
Acute kidney injury (AKI) is associated with high morbidity and mortality. Recent genetic fate mapping studies demonstrated that recovery from AKI occurs from intrinsic tubular cells. It is unresolved whether these intrinsic cells (so-called “scattered tubular cells”) represent fixed progenitor cells or whether recovery involves any surviving tubular cell. Here, we show that the doxycycline-inducible parietal epithelial cell (PEC)–specific PEC–reverse-tetracycline transactivator (rtTA) transgenic mouse also efficiently labels the scattered tubular cell population. Proximal tubular cells labeled by the PEC–rtTA mouse coexpressed markers for scattered tubular cells (kidney injury molecule 1, annexin A3, src-suppressed C-kinase substrate, and CD44) and showed a higher proliferative index. The PEC–rtTA mouse labeled more tubular cells upon different tubular injuries but was independent of cellular proliferation as determined in physiological growth of the kidney. To resolve whether scattered tubular cells are fixed progenitors, cells were irreversibly labeled before ischemia reperfusion injury (genetic cell fate mapping). During recovery, the frequency of labeled tubular cells remained constant, arguing against a fixed progenitor population. In contrast, when genetic labeling was induced during ischemic injury and subsequent recovery, the number of labeled cells increased significantly, indicating that scattered tubular cells arise from any surviving tubular cell. In summary, scattered tubular cells do not represent a fixed progenitor population but rather a phenotype that can be adopted by almost any proximal tubular cell upon injury. Understanding and modulating these phenotypic changes using the PEC–rtTA mouse may lead to more specific therapies in AKI.
Acute kidney injury (AKI) is common, is associated with a significantly increased morbidity and mortality, and predisposes to chronic kidney disease and vice versa. AKI occurs in response to a variety of renal insults, most commonly transient ischemia.
The renal tubule has an extraordinary capacity to undergo regeneration within a few days after AKI. The source of the regenerating cells is still not known, and this hampers the design of specific strategies to boost recovery. Different hypotheses have been proposed regarding the cellular source (1–4). In early descriptive studies, it has been proposed that regeneration of proximal tubular cells occurred from any surviving tubular cell following mild to moderate injury (5–7). In addition, stainings for cyclin D1, Ki-67, and BrdU suggested that fully differentiated tubular cells were either growth arrested or had progressed to the G1 phase, potentially acting as a reserve to rapidly reenter the cell cycle upon injury (6).
However, definite experimental evidence can only be obtained using genetic cell fate tracing. So far, it could only be excluded that extrinsic cells such as bone marrow or mesenchymal stem cells are the origin of tubular recovery using this unique method (8–10). Presently, only two major hypotheses remain, namely that tubules regenerate from any surviving tubular cell or that a specific tubular cell subpopulation with high regenerative potential (so-called “scattered tubular cells,” STCs, as outlined in the following paragraph) exists—or both.
An STC population has been proposed as a candidate for a progenitor cell population within the proximal tubule (11). They can be found throughout the mammalian tubule in a scattered fashion. Phenotypically, these cells show signs of dedifferentiation and express marker proteins, which are also expressed by other stem or progenitor cells or in renal development (11, 12). In contrast to stem cells, progenitor cells show a limited proliferative capacity, but both terms are often used synonymously. However, many of the initial studies on potential tubular progenitor cells relied on surface markers and subsequent characterization of the cells in vitro with all of the caveats relevant for in vitro studies (e.g., cell-culture–induced changes in morphology, behavior, and marker expression) (13).
Others used sequential labeling using thymidine analogs to trace the fate of proliferating cells after ischemic AKI; cell proliferation occurred preferentially in tubular epithelia, which expressed injury/dedifferentiation markers or lost differentiation markers (9). However, such studies predominantly label rapidly proliferating cells so that a presumptive slow-cycling fixed progenitor population might have been missed. In additional studies, improved recovery after AKI after injection of putative renal stem or progenitor cells could be largely attributed to paracrine actions (14).
Romagnani first pointed out the striking similarity of the protein expression pattern of STCs and parietal epithelial cells (PECs) (15). We later confirmed that nearly all marker proteins (45 of 49) that we found to be expressed by STCs were also expressed by PECs (12). This finding triggered us to use our recently developed transgenic PEC–reverse-tetracycline transactivator (rtTA) mouse line (16) for the present study on the origin and functional role of STCs in experimental AKI.
Results
PEC–rtTA Transgenic Mouse Labels STCs.
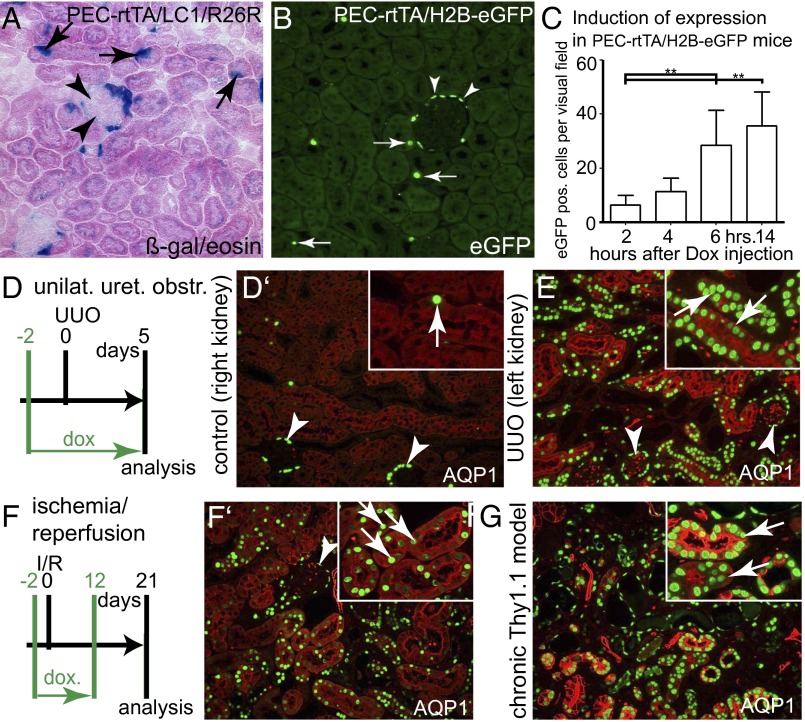
First it was tested whether transcriptional activity is induced not only in PECs but also in STCs upon administration of doxycycline (Dox) in normal adult transgenic PEC–rtTA mice. For this purpose, two alternative reporter lines were used: (i) LC1/R26R, mediating irreversible labeling via constitutive expression of the cytosolic reporter gene beta-galactosidase (beta-gal), and (ii) H2B–EGFP, mediating nuclear accumulation of EGFP-tagged histone in labeled cells. As shown in Fig. 1 A and B, individual tubular cells scattered throughout the renal cortex were genetically labeled in addition to PECs by the PEC–rtTA transgenic mouse line. This observation was consistent using both reporter lines. Renal cells were efficiently labeled within 6 h (Fig. 1C). For all subsequent pulse labeling experiments, Dox was therefore administered 8 h before analysis.
Fig. 1.
Transcriptional activity of PEC–rtTA mice in tubular cells. (A and B) Using two alternative reporter mice, PEC–rtTA mice are transcriptionally active in PECs (arrowheads) and also in individual STCs (arrows) in the renal cortex of 20-wk-old normal mice (200× magnification). (C) In a time course experiment, tubular cells could be efficiently labeled with EGFP–histone within 6 h after induction using a single i.p. injection of Dox (absolute numbers of EGFP-positive tubular cells in one visual field at 200× magnification, five fields per time point; **P < 0.01, one-way ANOVA with Bonferroni’s posttest, BPT). (D–G) Up-regulation of PEC–rtTA transcriptional activity in tubular cells upon injury. Three different models of tublar injury were induced in PEC–rtTA/H2B–EGFP mice: UUO (D and E), I/R (F and F’), and the chronic Thy1.1 model (resulting in glomerular proteinuria, G). All different modes of tubular injury resulted in significant up-regulation of PEC–rtTA transcriptional activity primarily in proximal tubular cells (marked by AQP1). Arrowheads, EGFP-positive PECs; arrows, EGFP-positive tubular cells.
Tubular Injury Up-Regulates Tubular PEC–rtTA Transcriptional Activity.
In healthy nonmanipulated adult mice, the PEC–rtTA mouse is transcriptionally active primarily in glomerular PECs and very little activity can be observed throughout the tubular system of the renal cortex (Fig. 1 A–C and Fig. S1 A–A’’’). Three different tubular injuries were induced: unilateral ureteral obstruction (UUO), causing synchronized loss of tubular cells; ischemia reperfusion (I/R) injury, causing ischemic loss primarily of proximal tubular cells; and the chronic cluster of differentiation 90 (CD90) model, characterized by progressive glomerulosclerosis and secondary tubulointerstitial damage (Fig. 1 D–G and Fig. S1 A–D’’’). In all three models, transcriptional activity was up-regulated within tubular cells of PEC–rtTA mice. Costaining for the proximal tubular cell marker aquaporin 1 (AQP1), collecting duct marker AQP2 or Tamm–Horsfall protein (THP, marker for the ascending loop of Henle and distal tubule), showed that the increased transcriptional activity primarily localized to proximal tubular cells. Similar numbers of tubular cells were labeled after I/R injury within the outer and inner cortex, indicating that the PEC–rtTA mouse is transcriptionally active in the S1+2 as well as the S3 segments of the proximal tubule (Fig. S1 E–E’’). Endogenous podocalyxin was not up-regulated in tubule cells after I/R injury, confirming that the PEC–rtTA mouse does not recapitulate the endogenous expression pattern of podocalyxin in podocytes (Fig. S1F).
PEC–rtTA-Positive Tubular Cells Proliferate After I/R Injury.
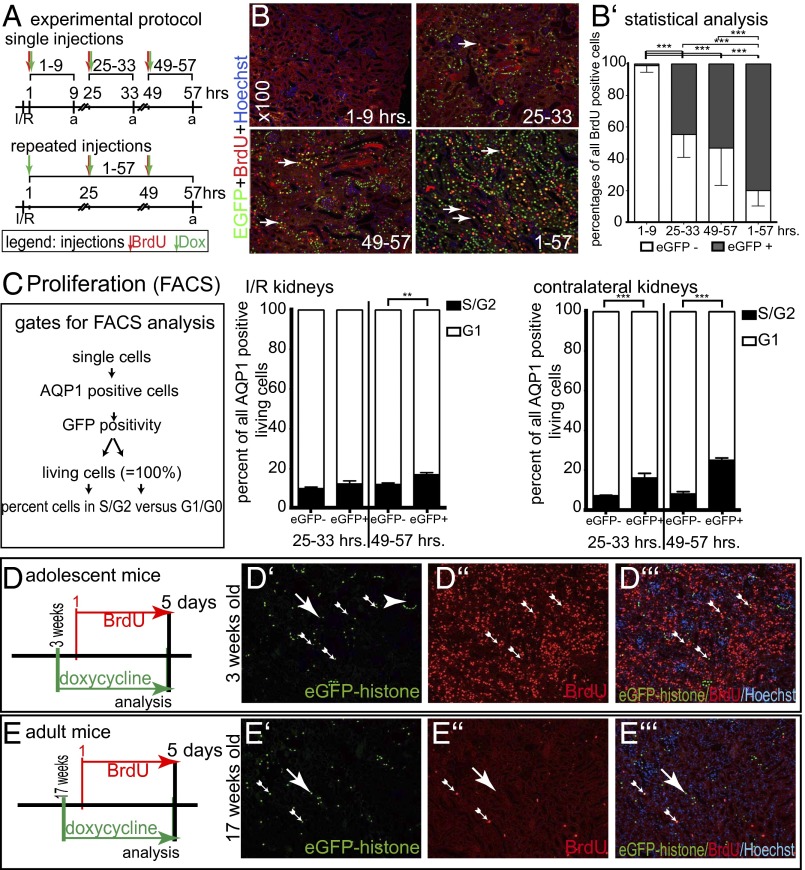
Cell proliferation was analyzed within three different time intervals during the first 3 d after I/R injury. Single or multiple injections of Dox and BrdU were applied at the time points indicated in Fig. 2A. At least about 50% of the BrdU-incorporating cells (indicating transition through S phase) also expressed the PEC–rtTA transgene (i.e., were EGFP positive) at 25–33 or 49–57 h after I/R (Fig. 2 B and B’). When repetitive injections of Dox and BrdU were administered, more than 80% of BrdU-labeled tubular cells were EGFP positive.
Fig. 2.
Increased proliferation of PEC–rtTA-labeled proximal tubular cells. (A) Experimental setup. Four groups of PEC–rtTA/H2B–EGFP mice received I/R surgery and Dox and/or BrdU injections as indicated. To evaluate the cumulative effects, the 1–57 h group received repeated injections. a, analysis. (B) Representative stainings for EGFP, BrdU, and Hoechst on paraffin sections of each experimental group (100× magnification). Arrows, tubular epithelial cells with nuclear EGFP and BrdU colabeling. (B’) Percentage of EGFP–histone-positive cells costaining for BrdU (n = 3 for each group, four visual fields of 100× magnification were analyzed for each mouse; ***P < 0.001, one-way ANOVA with BPT). (C) Pooled single cell suspensions of the renal cortex of two PEC–rtTA/H2B–EGFP mice described in A were stained for AQP1 and Hoechst and subjected to flow cytometric analysis using the indicated gating protocol. EGFP-positive proximal tubular cells were more likely to be proliferating (i.e., in S or G2 phase) compared with EGFP-negative proximal tubular cells. The difference was even more obvious in the contralateral control kidneys of the same animals (which contained less cellular debris). n = 3 for each time point; error bars mark SD (**P < 0.01; ***P < 0.001, one-way ANOVA BPT). (D–E’’’) No increased labeling by PEC–rtTA in developmental renal hypertrophy of mice at 3 wk of age (adolescent mice). In a costaining for EGFP–histone, BrdU and Hoechst significantly increased BrdU incorporation, but no EGFP labeling was detected in tubular cells (D–D’’’). Seventeen-week-old adult mice served as controls (E–E’’’).
To verify increased proliferation of PEC–rtTA-labeled cells, single cell suspensions of the renal cortex were prepared and stained for AQP1 and Hoechst (to quantify DNA content and evaluate cell viability). Using FACS analysis, the percentage of proliferating cells (i.e., cells in S or G2 phase) was significantly increased in EGFP-positive versus -negative proximal tubular cells in kidneys with I/R injury at 57 h after induction (Fig. 2C). Of note, in these experiments, the percentage of proliferating PEC–rtTA-labeled cells was likely underestimated because single cell suspensions of I/R kidneys contained cellular debris of necrotic cells and EGFP–histone fluorescence in the labeled cells was not enhanced by an additional anti-GFP immunostaining.
In contralateral control kidneys, EGFP-positive proximal tubular cells were even more likely to be in S or G2 phase compared with EGFP-negative cells at 33 and 57 h after I/R (increased proliferation was probably due to the absence of cellular debris compared with the I/R kidney) (Fig. 2C, contralateral kidneys).
No Increased Labeling by the PEC–rtTA Transgene of Tubular Cells in Adolescent Mice.
To rule out that labeling of tubular cells occurs primarily as a consequence of cellular proliferation, 3-wk-old transgenic PEC–rtTA/H2B–EGFP mice received Dox and BrdU via the drinking water for 5 d before sacrifice (Fig. 2 D–E’’’). Compared with 17-wk-old, adult transgenic mice, significantly more renal cells were BrdU positive in the growing mice, confirming that the kidneys of these mice were still growing (Fig. 2 D’’ vs. E’’). In contrast, transcriptional activity of the PEC–rtTA transgene was not increased (Fig. 2 D’ vs. E’). Similarly, after uninephrectomy (UNx), no increase in transcriptional activity was observed within the remaining kidneys (Fig. S1 G–G’’’). Only a minor increase in BrdU labeling was observed, consistent with the fact that tubule cells undergo both cellular hypertrophy and hyperplasia after UNx. These data suggest that transcriptional activity of PEC–rtTA mice in tubular cells does not depend on cellular proliferation per se but is rather a common response to tubular injury (i.e., proteinuria, ischemia, etc.).
PEC–rtTA Mouse Labels KIM-1–Positive Proximal Tubular Cells After I/R.
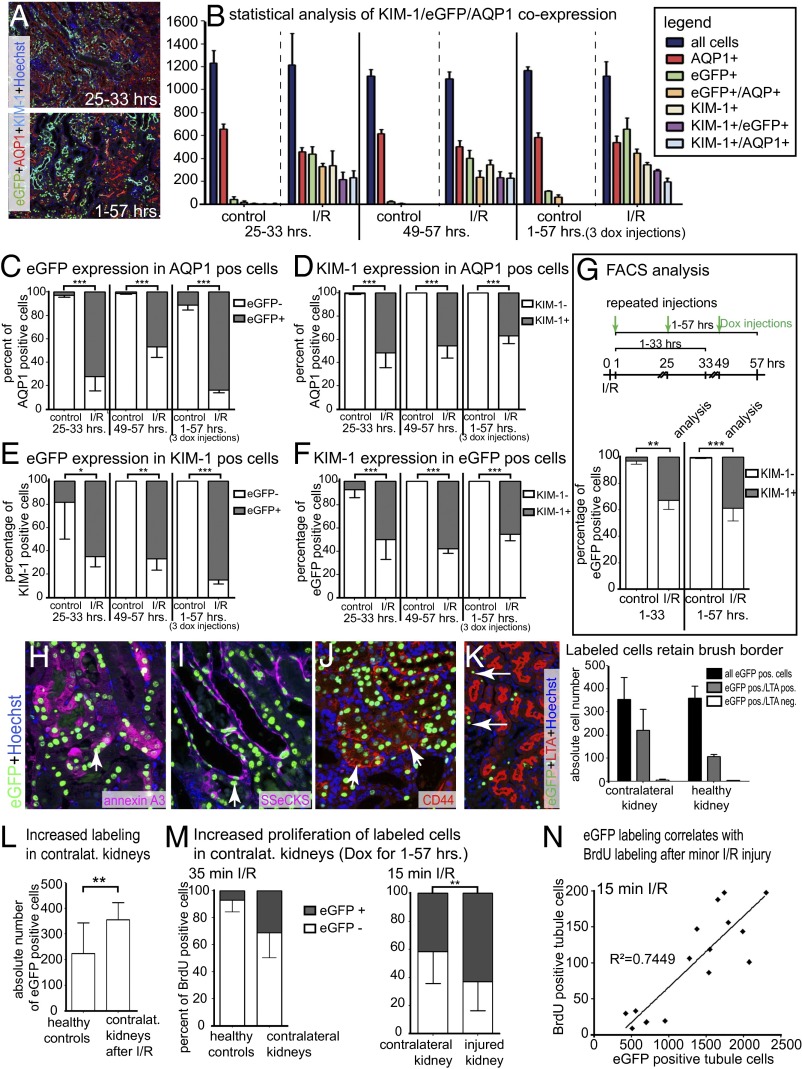
Kidney injury molecule 1 (KIM-1) is expressed by proximal tubular cells showing the STC phenotype (12, 17). To determine whether the PEC–rtTA transgenic mouse indeed preferentially labels STCs, kidneys were quadruple stained for Hoechst (labels all nuclei), AQP1 (proximal tubular cells), KIM-1 (marker of STCs), and EGFP–histone (labeled by the PEC–rtTA mouse) after I/R injury (Fig. 3 A and B). Minor down-regulation of AQP1 was noted in ischemic kidneys (Fig. 3B, red columns). More than 70% of proximal tubular cells were labeled by the PEC–rtTA mouse in I/R kidneys (Fig. 3B, green/orange columns and Fig. 3C). KIM-1 was up-regulated in only 35–50% of proximal tubular cells (Fig. 3B, white/blue columns and Fig. 3D), and more than 85% of these cells coexpressed EGFP–histone (Fig. 3E). At time points 25–33 and 49–57 h, about 50% of the EGFP–histone-labeled cells coexpressed KIM-1, indicating that the PEC–rtTA mouse is transcriptionally active in more tubular cells than can be detected by expression of marker proteins (Fig. 3F).
Fig. 3.
PEC–rtTA-labeled cells coexpress STC markers. For experimental setup, see Fig. 2A. (A) Representative images of EGFP/KIM-1/AQP1/Hoechst stainings of I/R injured kidneys. (B) Absolute numbers of EGFP/KIM-1/AQP1–expressing cortical cells (evaluated in a 200× visual field in three experimental mice at each time point). (C–F) Analysis of marker expression in AQP1- (C and D), KIM-1– (E), or EGFP-positive (F) cells (*P < 0.05; **P < 0.01; ***P < 0.001, one-way ANOVA BPT). (G) FACS analysis of cortical single cell preparations confirmed coexpression of KIM-1 in >30% of EGFP-positive AQP1 proximal tubular cells after I/R. PEC–rtTA/H2B–EGFP mice underwent I/R surgery and received Dox injections after 1, 25, and 49 h after surgery, and all mice were analyzed after 33 or 57 h. For both groups, three independent measurements were made of pooled kidneys of two mice each (total of six mice per group). Error bars mark SD (**P < 0.01, ***P < 0.001, one-way ANOVA). (H and I) Coexpression of EGFP–histone, Hoechst, and annexin A3 (H) or SSeCKS (I) 5 d after I/R (arrows). (J) Coexpression of CD44 with EGFP–histone 5 d after UUO (arrows). (K) No evidence for loss of brush border in PEC–rtTA-labeled cells in healthy and contralateral kidneys. As shown in the triple staining in the Left panel, labeled cells without brush border [i.e., lotus tetragonolobus agglutinin (LTA) negative] were almost always a consequence of the plane of the section (arrows). For statistical analysis, four visual fields at 100× magnification were evaluated in n = 3 animals; error bars mark SD. (L) Absolute numbers of EGFP-positive cells in four visual fields show increased labeling in contralateral control kidneys of animals subjected to I/R and receiving repeated Dox/BrdU injections (1–57 h) versus healthy controls (P < 0.01, unpaired t test). (M) PEC–rtTA-labeled EGFP-positive cells also showed increased BrdU incorporation in contralateral kidneys after I/R injury (n = 3, unpaired t test P = 0.0698). (Right) Similarly labeled cells proliferated more even after only 15 min of ischemia (n = 4). Error bars mark SD; **P < 0.01. (N) Absolute numbers of EGFP-positive tubular cells correlate with BrdU-positive cells in kidneys with different degrees of tubular damage after only 15 min of ischemia; R2 = 0.7449, P < 0.0001.
Preferential coexpression of KIM-1 in EGFP–histone-positive tubular cells was confirmed in single cell suspensions of renal cortices by FACS analysis (Fig. 3G). Out of all EGFP-positive cells, about 30–35% coexpressed KIM-1 (Fig. 3G). Again, the percentage of KIM-1 coexpression will likely be underestimated using FACS analysis (ca. 33% vs. ca. 43%) (Fig. 3E) because of cellular debris as outlined above.
EGFP–histone-positive cells also coexpressed other markers of STCs, namely annexin A3 and the PEC marker src-suppressed C-kinase substrate (SSeCKS) after I/R (Fig. 3 H and I) and CD44 after UUO (Fig. 3J). In summary, these experiments establish coexpression of STC markers in EGFP–histone-positive proximal tubular cells after injury.
PEC–rtTA Mouse Marks More STCs than Can Be Detected by Immunostainings.
In the PEC–rtTA mice subjected to I/R injury, more EGFP-labeled proximal tubular cells were detected compared with cells stained for the STC markers KIM-1 (Fig. 3 A and B), annexin A3, SSeCKS, and CD44 (Fig. 3 H–K). The expression of the latter STC markers was associated with a visible injury of the tubular cells (i.e., cell flattening and loss of brush border). Similarly, in contralateral control kidneys of mice subjected to I/R injury, more tubular cells were labeled by the PEC–rtTA mouse compared with healthy controls as determined by immunohistological analysis (Fig. 3L and Fig. S2 A, 2 vs. A, 5). These cells did not show a marked injury as they retained their brush border (Fig. 3K and Fig. S2 A, 6). When staining these contralateral control kidneys of mice subjected to I/R injury for markers of STCs, KIM-1 was expressed in less than 10 tubular cells per entire renal cross-section (Fig. S2B). Of note, about 50% of these few cells were also labeled by the PEC–rtTA mouse (Fig. S2 B, 3). In addition, the PEC–rtTA mouse detected significantly more STCs in contralateral control kidneys than could be detected by KIM-1 expression (Fig. S2 B, 1–3). Other conventional markers—that is, annexin A3, SSeCKS, and CD44—were not expressed by any tubular cell in contralateral control kidneys (Fig. S2 B, 4–6) or in normal healthy mouse kidneys. Labeled cells also showed an increased proliferation, indicating that the PEC–rtTA mouse also detects the STC population in these control kidneys (Fig. 3M) even though most of these cells did not express the classical markers of STCs (Fig. S2B). These results were confirmed in kidneys subjected to only minor I/R injury (i.e., 15 min ischemia), where again BrdU incorporation into tubular cells correlated with labeling by the PEC–rtTA mouse (Fig. 2 M, Right, and N). Fifteen minutes of ischemia induced transgenic labeling by the PEC–rtTA mouse much more significantly than up-regulation of KIM-1 (Fig. S3). These results indicate that STCs are undetectable using these immunohistological markers in the absence of marked tubular injury. The most sensitive method to detect STCs was the PEC–rtTA mouse, which labels low numbers of tubular cells also in (presumptively uninjured) contralateral control kidneys of mice subjected to I/R injury and also under physiological conditions in healthy kidneys.
PEC–rtTA Mouse Does Not Label a Fixed Progenitor Cell Population.
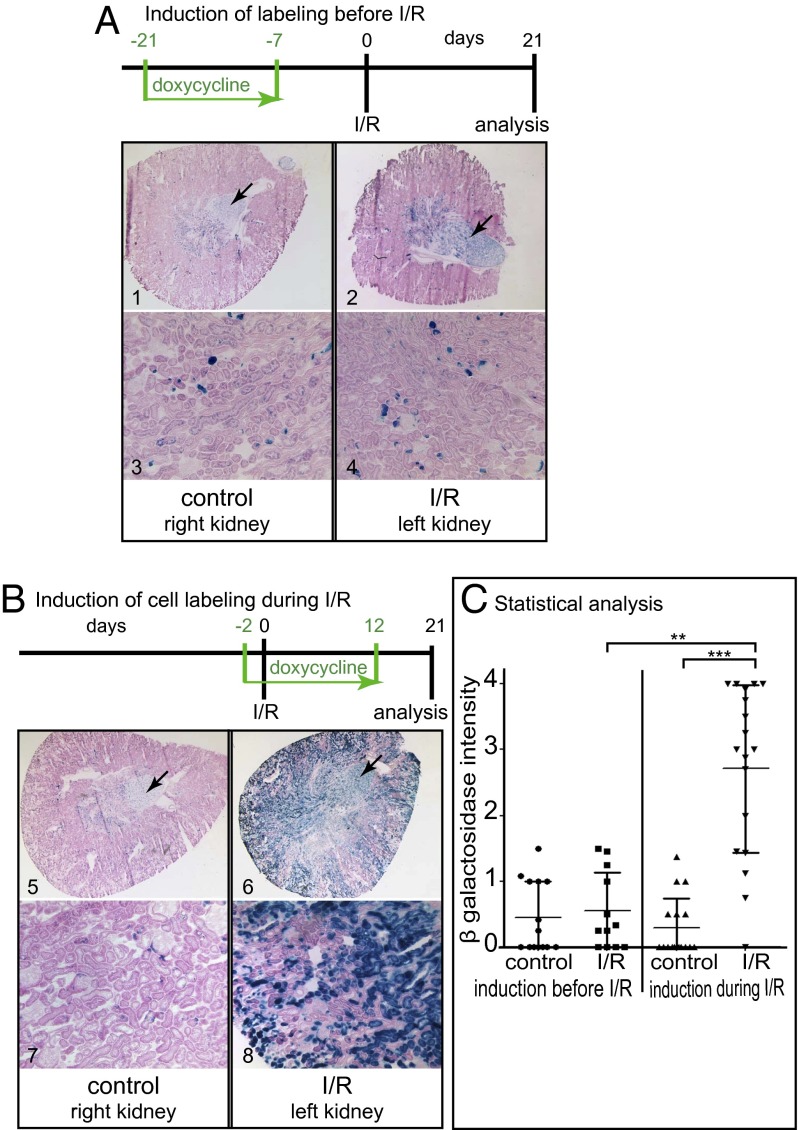
To resolve the question of whether the PEC–rtTA mouse labels a fixed intratubular progenitor population, healthy PEC–rtTA/LC1/R26R mice received Dox (Fig. 4A). This induces irreversible labeling of the STC population and forces them to constitutively express beta-gal. After a washout phase of 7 d, the mice were subjected to I/R injury. After 21 d, the number of labeled cells within the renal cortex was evaluated. A blinded semiquantitative scoring system was used because enzymatic beta-gal staining on cryosections does not allow distinguishing of individual cells. Compared with contralateral control kidneys, no significant increase in labeled cells was observed (Fig. 4C). This result indicates that the labeled STC population does not participate to a major degree in tubular regeneration after I/R.
Fig. 4.
PEC–rtTA-labeled cells are not a fixed progenitor population. (A) To test whether the PEC–rtTA mouse labels a fixed tubular progenitor population, irreversible genetic tagging was induced before I/R injury in PEC–rtTA/LC1/R26R mice. There was no increase of labeled tubular cells 21 d after I/R in the injured kidney (beta-gal/eosin–stained cryosections at a magnification of 25× or 100×). Constitutively labeled cells in the medulla serve as positive controls for beta-gal staining (arrows, n = 14). (B) To test whether the PEC–rtTA mouse labels injured and regenerating tubular cells, irreversible genetic labeling was induced during I/R. Compared with the contralateral control kidneys, significantly more tubular cells were genetically labeled in the injured kidney (n = 18). (C) Beta-gal staining intensity was evaluated in a blinded semiquantitative fashion (***P < 0.0001; **P < 0.01, Kruskal Wallis test with Dunn’s posttest). A significant increase in PEC–rtTA-labeled cells was observed only when genetic labeling was induced during I/R and recovery.
To test whether STCs increase in numbers during I/R injury and recovery, genetic labeling was activated during the I/R injury and subsequent regeneration phase (Fig. 4B). After 21 d, significantly more tubular cells were genetically labeled by the PEC–rtTA transgenic mouse (Fig. 4C). This result indicates that during I/R injury and subsequent regeneration, the PEC–rtTA transgene was induced to express in previously unlabeled “normal” tubular cells (i.e., labeled de novo by the PEC–rtTA mouse).
Discussion
Transgenic PEC–rtTA Mouse Efficiently Labels STCs.
Our first major finding is that the inducible PEC–rtTA transgenic mouse labels STCs besides PECs but not other adjacent epithelial cells (i.e., podocytes or all remaining proximal tubular cells). This is consistent with previous proposals and data (12, 15, 17) that STCs express almost the same marker proteins as PECs, which are not shared by normal tubular cells in human kidney, suggesting a common transcriptional program in STCs and PECs. More than 80% of KIM-1–positive proximal tubular cells were also labeled by the PEC–rtTA mouse, and these cells consistently showed a higher proliferative index in vivo, which are characteristic features of STCs (11, 12, 17).
PEC–rtTA Mouse Is a Useful Tool to Identify and Manipulate STCs at an Early Time Point.
Our results indicate that the PEC–rtTA transgenic mouse becomes transcriptionally active as soon as 24 h after tubular cell injury. In addition, the transgenic PEC–rtTA mouse was even more sensitive than classical markers for STCs. Because labeled cells showed increased proliferation, they could still be identified as STCs (despite being negative for “classical” STC markers). These findings also suggest that tubular cells respond to injury in a graded fashion. Transcriptional activity of PEC–rtTA mice mimics the expression of KIM-1, an injury marker of the proximal tubule, and thus differs from the neutrophil gelatinase-associated lipocalin (NGAL) reporter mouse (18), where transcriptional activity is more specific for the distal tubule and collecting duct system.
The PEC–rtTA mouse did not label increased numbers of STCs in physiological growth or after UNx. This is consistent with findings of Le Hir and coworkers, who showed that in growing adolescent normal rats, tubular cells undergo cellular divisions while remaining fully differentiated (19, 20). In contrast, in human kidneys, we noted that STCs showed a dedifferentiated phenotype with loss of brush border and of the basolateral labyrinth (12). Therefore, the common STC transcriptional program in response to different tubular injuries is different from physiological growth of tubular cells.
STCs Are Not a Fixed Intratubular Progenitor Cell Population.
The present study clarifies the functional significance of STCs in vivo. Previously, an up-regulation of “stem cell” or “progenitor” marker proteins has been described in tubular cells upon injury (11, 14, 21, 22). However, these markers can also be associated with “dedifferentiation,” and so far, there is no evidence that any of them can identify stem or progenitor properties with sufficient certainty. When irreversibly labeling the STC population in healthy mice, their frequency did not change after recovery from I/R injury compared with controls. This strongly argues against regeneration occurring preferentially from this cell population. As we did not observe an increase of labeled cells after I/R, transformation into the STC phenotype must occur only transiently under physiological conditions.
When genetic labeling was induced during ischemia and subsequent recovery, significantly more tubular cells were genetically labeled. In time course experiments, a sharp increase in PEC–rtTA-labeled (i.e., EGFP-positive) tubular cells was observed 25–33 h after labeling but not at 1–9 h. This rapid increase between 9 and 25 h is unlikely to result from proliferation of a small pool of fixed progenitors. In addition, one would expect EGFP-positive cells to be arranged in clusters (surrounding the putative scattered progenitor cells), which was not the case. The increase in STCs within only 24 h after injury is incompatible with the notion of a fixed progenitor cell population. Rather, STCs must have been recruited from any surviving tubular cells during the recovery phase. The absence of a fixed tubular progenitor population is also suggested by the fact that virtually no cells express STC markers in healthy rat (12) or mouse kidneys (this study).
In summary, this is a cell fate tracking experiment of regenerating proximal tubular cells. Our results establish that regeneration of tubular cells after I/R occurs from any surviving tubular cell. Tubular cells appear to switch to a common injury response program characterized by graded expression of a set of specific markers (“STC phenotype”). This common injury program is presumptively associated with tubular regeneration because STCs have a higher proliferative index. Clarifying this injury response further may open new approaches to booster renal tubular regeneration after AKI.
Materials and Methods
For a detailed description, see SI Materials and Methods. All animal procedures were approved by the Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen. Animals received Dox via drinking water (1 mg/mL) or as i.p. injections (50 µg/g body weight). We administered 2 mg BrdU dissolved as bolus or via the drinking water at 0.8 mg/mL.
Regarding the I/R model, mice were anesthetized and the left kidney was occluded for 35 or 15 min in male mice. For UUO or UNx, the ureter of the left kidney was occluded by two electrocoagulations. For UNx, the hilus of the left kidney was ligated, the capsule removed, and the kidney removed close to the hilum. Mice always received analgesia for 24 h. Tissues were recovered after perfusion with normal saline 0.9% under anesthesia for 3 min.
For enzymatic beta-gal staining, 6 µm cryosections were incubated for 5 min in 2% glutaraldehyde, 0.01% sodium deoxycholate, 0.02% octylphenoxypolyethoxyethanol (IGEPAL CA-630) (Sigma Chemical Co.), 1 mM MgCl2, in PBS (pH 7.8), and incubated overnight at 33 °C in 1 mg/mL X-Gal, 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, and 2 mM MgCl2 in PBS (pH 7.8). EGFP- and BrdU-positive cells were counted using Keyence BZ II Analyzer software.
For flow cytometric analysis, small pieces of the renal cortex were incubated in 1 mg/mL collagenase type IV (Worthington) for 30 min at 37 °C. Single cell suspensions were generated by sieving with 100 µm and 40 µm sieves and fixed in 4% (wt/vol) formaldehyde for 10 min and 0.1% Triton X-100 for 15 min. For a list of antibodies used, see Table S1.
Supplementary Material
Acknowledgments
We apologize for not having cited all the excellent studies due to size limitations. C.L., J.F., and M.J.M. are members of the SFB/Transregio 57 DFG consortium “Mechanisms of Organ Fibrosis.” This work was supported by TP04, TP09, TP25, and TP17 SFB/Transregio 57 of the Deutsche Forschungsgemeinschaft (DFG) (to J.-M.B., C.L., L.H., J.F., and M.J.M.), a grant by the DFG (BO 3755/1-1 to B.S.), and the E-Rare (European Research Projects on Rare Diseases) Project Rare-G 01 GM 1208A (to M.J.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1316177111/-/DCSupplemental.
References
- 1.McCampbell KK, Wingert RA. Renal stem cells: Fact or science fiction? Biochem J. 2012;444(2):153–168. doi: 10.1042/BJ20120176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duffield JS, Humphreys BD. Origin of new cells in the adult kidney: Results from genetic labeling techniques. Kidney Int. 2011;79(5):494–501. doi: 10.1038/ki.2010.338. [DOI] [PubMed] [Google Scholar]
- 3.Little MH, Bertram JF. Is there such a thing as a renal stem cell? J Am Soc Nephrol. 2009;20(10):2112–2117. doi: 10.1681/ASN.2009010066. [DOI] [PubMed] [Google Scholar]
- 4.Reule S, Gupta S. Kidney regeneration and resident stem cells. Organogenesis. 2011;7(2):135–139. doi: 10.4161/org.7.2.16285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glaumann B, Glaumann H, Trump BF. Studies of cellular recovery from injury. III. Ultrastructural studies on the recovery of the pars recta of the proximal tubule (P3 segment) of the rat kidney from temporary ischemia. Virchows Arch B Cell Pathol Incl Mol Pathol. 1977;25(4):281–308. [PubMed] [Google Scholar]
- 6.Vogetseder A, et al. Proliferation capacity of the renal proximal tubule involves the bulk of differentiated epithelial cells. Am J Physiol Cell Physiol. 2008;294(1):C22–C28. doi: 10.1152/ajpcell.00227.2007. [DOI] [PubMed] [Google Scholar]
- 7.Witzgall R, Brown D, Schwarz C, Bonventre JV. Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest. 1994;93(5):2175–2188. doi: 10.1172/JCI117214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duffield JS, Bonventre JV. Kidney tubular epithelium is restored without replacement with bone marrow-derived cells during repair after ischemic injury. Kidney Int. 2005;68(5):1956–1961. doi: 10.1111/j.1523-1755.2005.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Humphreys BD, et al. Repair of injured proximal tubule does not involve specialized progenitors. Proc Natl Acad Sci USA. 2011;108(22):9226–9231. doi: 10.1073/pnas.1100629108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin F, Moran A, Igarashi P. Intrarenal cells, not bone marrow-derived cells, are the major source for regeneration in postischemic kidney. J Clin Invest. 2005;115(7):1756–1764. doi: 10.1172/JCI23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindgren D, et al. Isolation and characterization of progenitor-like cells from human renal proximal tubules. Am J Pathol. 2011;178(2):828–837. doi: 10.1016/j.ajpath.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smeets B, et al. Proximal tubular cells contain a phenotypically distinct, scattered cell population involved in tubular regeneration. J Pathol. 2013;229(5):645–659. doi: 10.1002/path.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chittiprol S, Chen P, Petrovic-Djergovic D, Eichler T, Ransom RF. Marker expression, behaviors, and responses vary in different lines of conditionally immortalized cultured podocytes. Am J Physiol Renal Physiol. 2011;301(3):F660–F671. doi: 10.1152/ajprenal.00234.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langworthy M, Zhou B, de Caestecker M, Moeckel G, Baldwin HS. NFATc1 identifies a population of proximal tubule cell progenitors. J Am Soc Nephrol. 2009;20(2):311–321. doi: 10.1681/ASN.2008010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romagnani P. Family portrait: Renal progenitor of Bowman’s capsule and its tubular brothers. Am J Pathol. 2011;178(2):490–493. doi: 10.1016/j.ajpath.2010.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Appel D, et al. Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol. 2009;20(2):333–343. doi: 10.1681/ASN.2008070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ichimura T, et al. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273(7):4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 18.Paragas N, et al. The Ngal reporter mouse detects the response of the kidney to injury in real time. Nat Med. 2011;17(2):216–222. doi: 10.1038/nm.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vogetseder A, Palan T, Bacic D, Kaissling B, Le Hir M. Proximal tubular epithelial cells are generated by division of differentiated cells in the healthy kidney. Am J Physiol Cell Physiol. 2007;292(2):C807–C813. doi: 10.1152/ajpcell.00301.2006. [DOI] [PubMed] [Google Scholar]
- 20.Vogetseder A, Karadeniz A, Kaissling B, Le Hir M. Tubular cell proliferation in the healthy rat kidney. Histochem Cell Biol. 2005;124(2):97–104. doi: 10.1007/s00418-005-0023-y. [DOI] [PubMed] [Google Scholar]
- 21.Gupta S, et al. Isolation and characterization of kidney-derived stem cells. J Am Soc Nephrol. 2006;17(11):3028–3040. doi: 10.1681/ASN.2006030275. [DOI] [PubMed] [Google Scholar]
- 22.Kitamura S, et al. Establishment and characterization of renal progenitor like cells from S3 segment of nephron in rat adult kidney. FASEB J. 2005;19(13):1789–1797. doi: 10.1096/fj.05-3942com. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.