Abstract
The Anaphase-Promoting Complex/Cyclosome (APC/C) ubiquitin ligase mediates degradation of cell cycle proteins during mitosis and G1. Cdc20/Fzy and Cdh1/Fzr are substrate-specific APC/C activators. The level of mammalian Cdh1 is high in mitosis, but it is inactive and does not bind the APC/C. We show that when Cdh1 is active in G1 and G0, its levels are considerably lower and almost all of it is APC/C associated. We demonstrate that Cdh1 is subject to APC/C-specific degradation in G1 and G0, and that this degradation depends upon two RXXL-type destruction boxes. We further demonstrate that addition of Cdh1 to Xenopus interphase extracts, which have an inactive APC/C, activates it to degrade Cdh1. These observations indicate that Cdh1 mediates its own degradation by activating the APC/C to degrade itself. Elevated levels of Cdh1 are deleterious for cell cycle progression in various organisms. This auto-regulation of Cdh1 could thus play a role in ensuring that the level of Cdh1 is reduced during G1 and G0, allowing it to be switched off at the correct time.
Keywords: APC/C, cyclosome, destruction box, fizzy, fizzy- related
Introduction
Ubiquitin-mediated proteolysis plays a key role in the regulation of the cell cycle. The anaphase-promoting complex/cyclosome (APC/C) has been identified as the ubiquitin ligase that mediates the degradation of mitotic cyclins (King et al, 1995; Sudakin et al, 1995). About 20 different cell cycle proteins have been identified so far as APC/C degradation substrates (Harper, 2002; Peters, 2002).
The APC/C is active in a cell cycle-specific manner from metaphase until the end of G1. APC/C activity is regulated by phosphorylation of several of its subunits (Shteinberg et al, 1999; Kramer et al, 2000; Rudner and Murray, 2000; Golan et al, 2002), as well as by the association with Cdc20/fizzy or Cdh1/Hct1/fizzy related. Cdc20 is an essential protein, which is required for the activation of the APC/C in mitosis (Sigrist et al, 1995; Visintin et al, 1997). Cdc20 associates with the APC/C in its phosphorylated form (Shteinberg et al, 1999; Kramer et al, 2000; Rudner and Murray, 2000) and is required for the degradation of mitotic APC/C substrates like cyclins A and B, securin and Xkid. Cdh1 (Schwab et al, 1997; Sigrist and Lehner, 1997; Visintin et al, 1997) is required for the activation of the APC/C during G1 (Hagting et al, 2002; Zur and Brandeis, 2002). The APC/CCdh1 can target all the substrates of the APC/CCdc20, and in addition targets several other specific substrates that are not targeted by the APC/CCdc20. The observation that Cdc20 and Cdh1 determine the substrate specificity of the APC/C has suggested that they serve as adaptor proteins that bind the different degradation substrates. This hypothesis has been supported by several observations of direct interaction between Cdc20 and Cdh1 and degradation substrates (Burton and Solomon, 2001; Hilioti et al, 2001; Pfleger et al, 2001a; Schwab et al, 2001). Intriguing recent experiments in mitotic Xenopus extracts have, however, shown that substrates can remain in complex with the APC/C without Cdc20 (Yamano et al, 2004). This interaction could possibly be mediated by APC10 (Vodermaier et al, 2003).
The activity of the APC/C is regulated in a very tight and complex manner, as would be expected from such an important element of the cell cycle. Auto-regulation is a central motif in the regulation of the APC/C. Many of the proteins that play a role in the activation and inhibition of the APC/C are themselves subsequently degraded by it. Both cyclin B1 and Plk1 (Shteinberg et al, 1999; Golan et al, 2002; Kraft et al, 2003), which phosphorylate the APC/C so that it can bind Cdc20 in prometaphase, are subsequently degraded by the APC/C. Cdc20 itself is degraded by the APC/CCdh1 (Shirayama et al, 1998; Pfleger and Kirschner, 2000), as is also E2-C, one of the ubiquitin-conjugating enzymes that interacts with the APC/C (Yamanaka et al, 2000).
Cdh1 is a potent APC/C activator. In contrast to Cdc20, which can bind the APC/C only when it is phosphorylated by mitotic kinases, Cdh1 can activate the APC/C any time. This makes Cdh1 a potentially dangerous and toxic cell cycle protein that must be tightly regulated. Cdh1 is indeed regulated at multiple levels. Its transcription and mRNA stability are both cell cycle specific (Inbal et al, 1999). During the S phase, as well as during G2 and the early stages of mitosis, Cdh1 is inactivated by phosphorylation by cyclin A2-cdk2 and cyclin B1-Cdk1 (Lukas et al, 1999; Listovsky et al, 2000). The inactivation of the APC/CCdh1 at the end of G1 is mediated by Emi1, which binds Cdh1 and turns off APC/CCdh1 activity (Hsu et al, 2002). Phosphorylation of Cdh1 by cyclin A2-cdk2 in S phase prevents the re-association of Cdh1 with the APC/C. Relatively moderate overexpression of Cdh1 is sufficient for preventing its inactivation at G1/S, leading to severe cell cycle effects both in yeast, Drosophila and in mammalian cells (Schwab et al, 1997; Sigrist and Lehner, 1997; Visintin et al, 1997; Sorensen et al, 2000).
Cdh1 levels have been shown to decline during G1 (Kramer et al, 2000), but the nature of this regulation is unknown. We show here that mammalian Cdh1 is degraded in G1 and G0 and that this degradation is mediated by Cdh1-activated APC/C.
Results
Cdh1 levels oscillate during the cell cycle
To characterize the changes of Cdh1 levels during the mammalian cell cycle, we synchronized mouse NIH3t3 fibroblasts in prometaphase by nocodazole treatment for 16 h. The arrested cells were obtained by mitotic shake-off and either harvested immediately or released into fresh medium to proceed with the cell cycle. Cells were harvested at different times throughout the cell cycle. Cell synchronization was verified by probing the extracts with an antibody for cyclin B1, which is known to be degraded upon exit from mitosis and to re-accumulate upon entry into the S phase 8–10 h later (Brandeis and Hunt, 1996). These extracts were then probed with a Cdh1-specific antibody. Figure 1A shows that Cdh1 levels declined gradually during early G1 and reached minimal levels in late G1 about 8 h after release from nocodazole. This result was somewhat puzzling given the fact that Cdh1 is active during this phase of the cell cycle (Zur and Brandeis, 2002). The slightly shifted bands of Cdh1 in nocodazole-arrested and S-phase extracts show that Cdh1 is phosphorylated from S phase to mitosis. This phosphorylation is known to prevent its association with, and activation of, the APC/C (Lukas et al, 1999; Listovsky et al, 2000).
Figure 1.
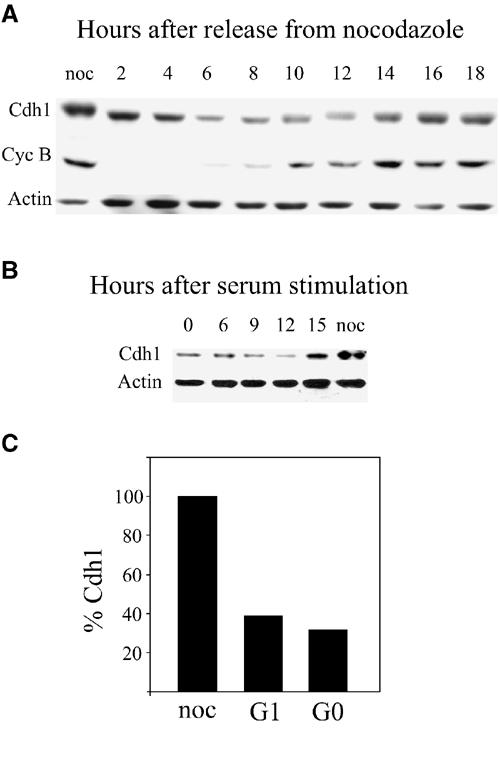
Levels of Cdh1 oscillate during the cell cycle. (A) Cells synchronized in prometaphase by nocodazole arrest and shake-off were released and harvested at the indicated time points. Cell extracts were immunoblotted with Cdh1, cyclin B1 and actin antibodies. (B) Cells were synchronized by serum deprivation in G0, released and harvested at the indicated times for immunoblotting with Cdh1 and actin antibodies. (C) The ratio between Cdh1 and actin in nocodazole-arrested, mid-G1 (6 h after release from nocodazole) and serum-starved (G0) cells was calculated from Cdh1 and actin signals quantified on a FUJIFILM LAS-1000 intelligent dark box II. The amount of Cdh1 in nocodazole-arrested cells was set arbitrarily to 100%.
We next studied the levels of Cdh1 in serum-starved G0 arrested cells. Cells arrested in G0 have an active APC/CCdh1 (Brandeis and Hunt, 1996). Figure 1B shows that in G0, levels of Cdh1 are low and they rise about 15 h after stimulation with serum, a time point that coincides with late G1.
Figure 1C shows the quantiation of Cdh1 versus actin levels at different times of the cell cycle. The levels of Cdh1 in G1 and G0 cells is only 40 and 30%, respectively, of that of nocodazole-arrested cells. The absolute levels of Cdh1 are probably even lower, as actin also declines in serum-starved cells.
We have previously shown that Cdh1 binds the APC/C throughout G1, until the entry into S phase (Zur and Brandeis, 2001). We therefore used gel filtration to study the amount of free and APC/C-associated Cdh1 in the cell at different phases of the cell cycle. Cells were either arrested by nocodazole in prometaphase, released from nocodazole into G1, or arrested in G0 by serum starvation. Cell extracts were loaded onto a Sephacryl S200 gel filtration column, and the eluted fractions were immunoblotted with antibodies for Cdh1 and for the APC/C subunit Cdc27. Figure 2A shows that in G1 and G0 most of the Cdh1 co-eluted from the column with the large APC/C complex. In prometaphase, in contrast, Cdh1 did not co-elute with the APC/C but in lower molecular weight fractions, suggesting that it is monomeric and not bound to the APC/C. A side-by-side comparison of Cdc27 from the different phases shows the mitotic phosphorylation of Cdc27 (Figure 2B).
Figure 2.
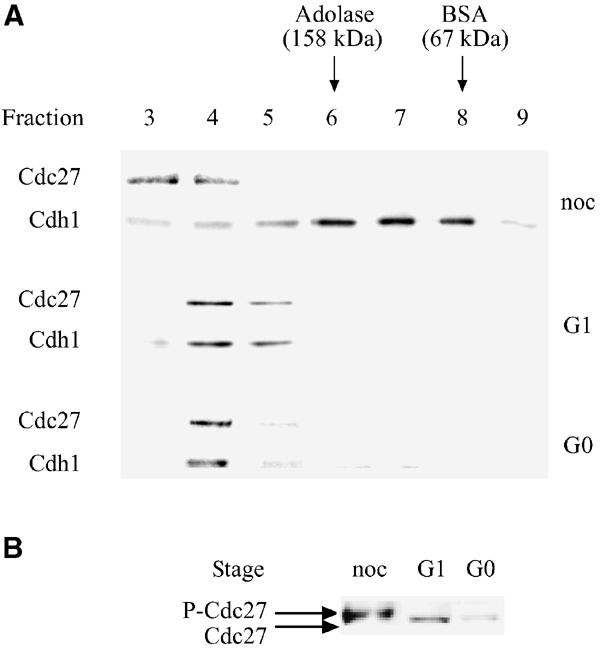
During G1 and G0, most of the Cdh1 in the cell is associated with the APC/C. (A) Extracts of nocodazole (noc)-arrested G1 prepared by releasing nocodazole-arrested cells for 6 h and G0 cells prepared by 48 h serum deprivation were fractionated by gel filtration. Samples of each fraction were blotted with Cdc27 and Cdh1 antibodies. (B) Fraction 4 of gel filtrates of cell extracts of the different phases were resolved side by side to show that Cdc27 was phosphorylated in prometaphase, but not in G1 or G0.
Cdh1 is degraded in G1 in G0
The significant reduction of Cdh1 levels during G1 suggested that it is degraded. To address this question, we synchronized cells with nocodazole in prometaphase and released them into G1. Cells were treated with the protein synthesis inhibitor cyclohexamide (100 μg/ml), 1 h after release from nocodazole. Treated and untreated cells were harvested after an additional 2 and 5 h. Figure 3 shows that by 3 h the amount of Cdh1 was reduced by two-thirds in the presence of the inhibitor and only by a third in its absence. By 6 h, most of the Cdh1 was degraded also in the absence of the inhibitor, probably because it was no longer transcribed (Inbal et al, 1999). These observations suggest that Cdh1 is indeed degraded with an approximate half-life of about 1 h.
Figure 3.
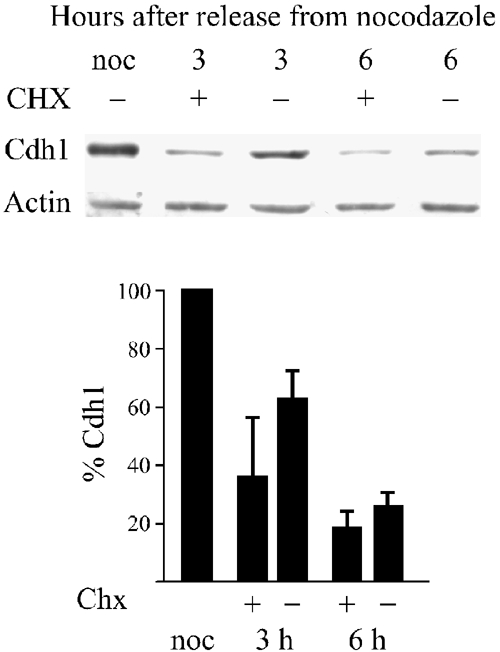
Cdh1 is degraded in G1. Cells were synchronized with nocodazole and prometaphase-arrested cells were obtained by mitotic shake-off. Cells were released into fresh medium and after 1 h cells were either treated (+) or not treated (−) with 100 μg/ml cyclohexamide. Cells were harvested and immunoblotted at the indicated time points with Cdh1 and actin antibodies. The histogram shows the quantitative data from three different experiments.
In order to examine Cdh1 degradation in more detail, we fused different parts of Cdh1 to the Chloramphenicol Acetyl Transferase (CAT) reporter gene. We stably expressed expression vectors for Cdh-CAT in NIH3t3 cells and studied their cell cycle-specific CAT activity. We used this CAT-based assay to study the degradation of various proteins in the past (Zur and Brandeis, 2002). Figure 4A shows that a fusion protein comprising the N-terminal 180 amino acids of Cdh1 and CAT was stable in mitosis and degraded in G1. Wild-type CAT, in contrast, did not change at all during the cell cycle in accordance with the high stability of this protein. Cells expressing the Cdh-CAT and wt CAT expression vectors were also arrested in G0 by serum starvation and their CAT activity was monitored as they were stimulated with serum to re-enter the cell cycle. Cdh-CAT activity was very low in G0 and rose only after 15 h when cells approached the S phase. Wt CAT was again stable throughout the experiment (Figure 4B). Fusion constructs with less than 180 amino acids were also tested but were not degraded (data not shown).
Figure 4.
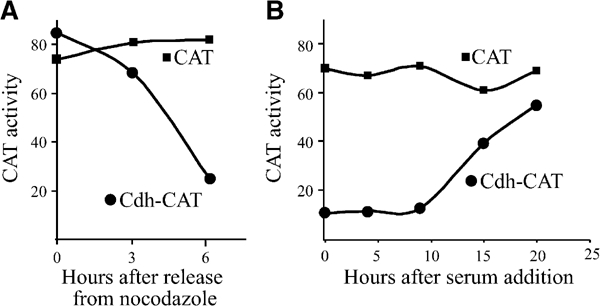
Cdh-CAT fusion proteins are degraded in G1 and G0. (A) A vector expressing a fusion between the N-terminal 180 amino acids of Cdh1 and CAT, and a vector expressing CAT alone, were stably expressed in cells. Cells were synchronized by nocodazole arrest and mitotic shake-off, released into fresh medium, harvested at the indicated times and assayed for CAT activity. The plotted CAT activity represents the proportion of di-acetylated [14C]chloramphenicol of the total amount of [14C]chloramphenicol. (B) The same cell lines were arrested in G0 by serum deprivation and released by addition of serum. Cells were harvested at the indicated times and assayed for CAT activity.
Cdh1 has two RXXL destruction boxes and is degraded by the APC/C
The only known cell cycle-specific proteolysis mechanism in G1 and G0 is the APC/C. We wondered therefore whether Cdh1 could be degraded by the APC/C. This might have been a somewhat paradoxical hypothesis as Cdh1 is also the sole known activator of the APC/C during G1 and G0. We examined the sequence of Cdh1 and identified two motifs RRLLRQIVI and RRTLTPASS (single-letter amino-acid code) that have the two essential residues (R and L) of the RXXL destruction box. This RXXL box is one of the two known motifs that targets substrates for degradation by the APC/C. We termed these putative RXXL destruction boxes DB1 and DB2.
We used site-directed mutagenesis to change the R and L residues of either or both of DB1 and DB2 to make DM1, DM2 and DM1+2, respectively (Figure 5A), and studied the degradation of the mutated fusion proteins in G1. Figure 5B shows that mutating either or both of these RXXL motifs completely abolished the degradation of the fusion proteins.
Figure 5.
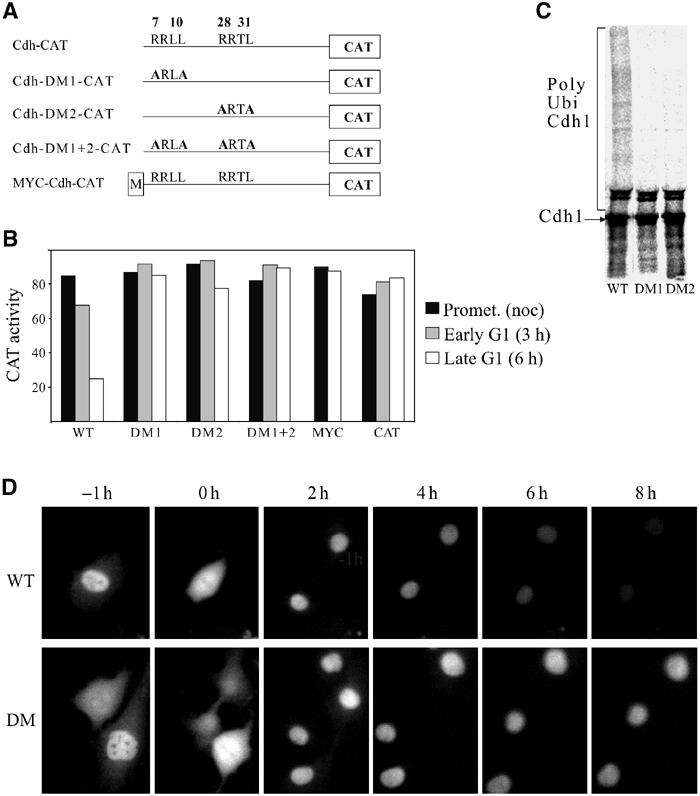
Cdh1 has two RXXL boxes that are required for its degradation in G1. (A) Schematic presentation of the fusion constructs of wild-type and RXXL box mutants, as well as an N-terminally myc-tagged Cdh-CAT construct. The numbers above depict the location of the amino acid in the protein; the constructs are not drawn to scale. (B) Cells stably expressing the various fusion proteins shown in (A), as well as cells expressing CAT, were arrested with nocodazole and obtained by mitotic shake-off. Cells were either harvested immediately (black) or released into fresh medium and harvested after 3 (early G1—gray) or 6 (mid-late G1—white) h for CAT assays. (C) Full-length Cdh1, Cdh1-DM1 and Cdh1-DM2 were transcribed and translated in reticulocyte lysate in the presence of [35S]methionine. They were subsequently incubated with APC/C purified from interphase cells, E2-C, E1, ubiquitin and an energy-regenerating system, and resolved by SDS–PAGE. (D) Cells were injected with expression vectors for Cdh-YFP (top) and Cdh-DM1+2-YFP (bottom). Expressing cells were followed by time-lapse photography and photographed every 5 min. Representative images showing the same cells 1 h before metaphase (−1 h) at metaphase (0 h), and every 2 h for 8 h after metaphase are presented.
The addition of sequences upstream of destruction boxes also often interferes with degradation. We therefore tested the degradation of Cdh-CAT N-terminally tagged with a single myc tag. Figure 5B shows that this construct was completely stable. This result is of particular interest as overexpression of moderate levels of myc-Cdh1 lead to a particularly severs phenotype (Sorensen et al, 2000).
We used an in vitro ubiquitilation assay with purified APC/C and in vitro transcribed and translated full-length wild-type or destruction box mutant Cdh1 as substrates. Figure 5C shows that Cdh1 was highly ubiquitylated and that mutations of either DB1, or DB2, almost completely abolished this ubiquitylation.
To follow degradation of Cdh1 in real time, we generated expression vectors encoding the 180 N-terminal amino acids of Cdh1 and the double destruction box mutant Cdh1-DM1+2 to Yellow Fluorescent Protein (YFP). We injected these vectors into cells and followed expressing cells by time-lapse photography. Figure 5D shows that the fluorescence of cells that express Cdh-YFP gradually declines after mitosis throughout G1. Cells expressing Cdh-DM1+2-YFP, in contrast, remain fluorescent throughout the cell cycle.
Cdh1 is degraded by the APC/CCdh1 and not by the APC/CCdc20
The APC/C in mammalian cells can be activated either by Cdc20 or by Cdh1. Activation of the APC/C by Cdc20 takes place in mitosis. Cdc20 is dissociated from the APC/C upon exit from mitosis and is degraded by Cdh1-activated APC/C. The APC/CCdh1 is capable of targeting all the substrates of the APC/CCdc20 as well as several additional substrates.
We used several assays to examine whether Cdh1 is degraded by both the APC/CCdc20 and the APC/CCdh1, or only by the APC/CCdh1. We transfected cells stably expressing fusion reporter vectors with an expression vector of nondegradable cyclin B1. Cyclin B1-Cdk1 inactivation is essential for cells to exit mitosis and nondegradable cyclin B1 arrests cells in mitosis (Wheatley et al, 1997; Stemmann et al, 2001). Cyclin B1-Cdk1 phosphorylates Cdh1 and prevents it from binding and activating the APC/C (Listovsky et al, 2000). Cells are thus arrested in mitosis with a highly active APC/CCdc20. Several APC/CCdc20 and APC/CCdh1 substrates tested by this method have shown that only APC/CCdc20 substrates are degraded under these conditions (Hagting et al, 2002; Zur and Brandeis, 2002). This is so far the only in vivo test in mammalian cells for APC/CCdc20-specific degradation. Figure 6A shows that while the cyclin B1-CAT fusion reporter is degraded in these cells, Cdh-CAT remains stable. Both reporters are degraded in G1. This assay suggests that Cdh1 is not an APC/CCdc20 substrate.
Figure 6.
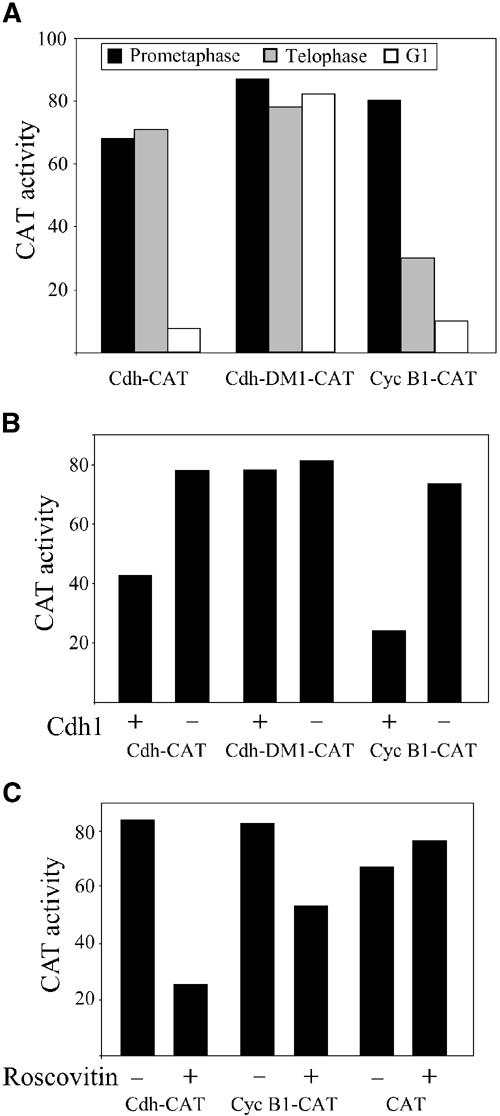
Cdh1 degradation is mediated by the APC/CCdh1 and not by the APC/CCdc20. (A) Cells stably expressing Cdh-CAT, Cdh-DM1-CAT and cyclin B1-CAT were synchronized in prometaphase with nocodazole (black), in telophase by expression of nondegradable cyclin B1 (gray) and in G1 by release from nocodazole arrest (white), and assayed for CAT activity. (B) Cells were transiently co-transfected with a full-length Cdh1 expression vector (+) or an empty vector (−), and with Cdh-CAT, Cdh-DM1-CAT or cyclin B1-CAT as indicated. The transfected cells were arrested with nocodazole, harvested by shake-off and assayed for CAT activity. (C) Cells stably expressing Cdh-CAT, cyclin B1-CAT and CAT were arrested with nocodazole overnight and harvested by shake-off. They were then incubated in medium with both nocodazole and roscovitin for 3 h, harvested and assayed for CAT activity. A representative experiment of at least three repeats is shown for each of the experiments.
The only other APC/C activator known so far in mammals is Cdh1 itself, so that it is possible to assume that the APC/CCdh1 is degrading Cdh1. To test this hypothesis in a more direct manner, we co-transfected cells with a Cdh1 expression vector together with fusion reporters. Cells were then arrested with nocodazole in prometaphase. We have previously shown that the checkpoint-arrested APC/C in nocodazole-arrested cells can be activated by Cdh1 overexpression (Listovsky et al, 2000). Figure 6B shows that Cdh-CAT, as well as cyclin B1-CAT, was effectively degraded in these cells. The Cdh1-DM1 mutant, in contrast, was not degraded. The APC/CCdh1 can be activated in prometaphase-arrested cells also by chemical inhibition of Cdk1 kinase activity (Listovsky et al, 2000). We arrested cells stably expressing fusion reporters in prometaphase with nocodazole and treated them with the Cdk1 inhibitor roscovitin. Figure 6C shows that roscovitin induced the degradation of both Cdh-CAT and cyclin B1-CAT, but not of CAT.
We addressed Cdh1 degradation also in Xenopus egg interphase extracts. Xenopus eggs lack a G1 phase and do not express Cdh1 (Lorca et al, 1998). We prepared interphase extracts by standard methods (Murray, 1991) and used them for degradation assays. As a substrate, we used Schizosaccharomyces pombe cyclin B (cdc13) in parallel with its nondegradable N-terminal deletion mutant Δ67, which served as a loading control and a control for nonspecific degradation. Figure 7A shows that, as expected, the interphase extract did not degrade cyclin B. When we added Cdh1, or its destruction box mutant, to the interphase extracts, cyclin B was degraded. The C-terminal arginine residue of Cdh1 is required for its binding and activating of the APC/C (Vodermaier et al, 2003). We therefore generated a Cdh1 mutant with a deleted c-terminal arginine (Cdh1-R). Adding Cdh1-R to the interphase extract failed, as expected, to activate the APC/C to degrade cyclin B. Figure 7B shows that when Cdh1 was added to the interphase extracts it was degraded. A destruction box mutant of fzr was considerably stabilized and the Cdh1-R was completely stable. The observation that Cdh1, which activates the APC/C, is degraded, while Cdh1-R, which does not activate the APC/C, is not degraded, suggests that Cdh1 degradation is indeed auto-regulated.
Figure 7.

Cdh1 activates Xenopus interphase extracts to degrade itself. (A) Xenopus interphase extracts were used for a destruction assay of [35S]methionine-labeled S. pombe cyclin B (cdc13) and its destruction box deletion cdc13Δ67, which served as a loading control and control for nonspecific degradation. Extracts were supplemented with Cdh1, Cdh1-R and Cdh1DM as indicated. Aliquots were collected at the indicated times (in hours), and resolved by SDS–PAGE. (B) [35S]methionine-labeled Cdh1, Cdh1-R and Cdh1DM were added to Xenopus interphase extracts and analyzed like in (A). (C) Xenopus interphase extracts pre-activated with Cdh1 mRNA were used for a destruction assay of [35S]methionine-labeled S. pombe cyclin B (cdc13), cdc13Δ67, wild-type and mutant Cdh1 and human securin.
In a further set of experiments, we prepared interphase extracts, treated them with RNAse and subsequently inhibited RNAse activity (Murray, 1991). To these extracts, we added Cdh1 mRNA and allowed it to be translated for 1 h. [35S]methionine added to an aliquot of these extracts showed that Cdh1 was efficiently translated (not shown). Figure 7C shows that Cdh1-activated interphase extracts efficiently degrade cyclin B and securin but, as expected, not cyclin B-Δ67. Surprisingly, neither Cdh1 nor Cdh1-R was degraded in these extracts, which were pre-activated with Cdh1. It is thus possible that the Cdh1 bound already to the APC/C competitively inhibited the interaction and degradation of the labeled Cdh1 that we added to the reaction.
Discussion
Many cellular pathways are regulated by ubiquitin-mediated proteolysis. An essential characteristic of such a regulation is its high substrate specificity. Cell cycle regulation by proteolysis is further characterized by high temporal specificity. The APC/C has three different states—it is inactive from S phase until metaphase, it has a Cdc20-specific activity from metaphase until telophase and a Cdh1-specific activity during G1. The APC/C is a large complex of about a dozen different subunits; however, almost all the mechanisms that regulate its activity, discovered so far, focus on the availability and binding of Cdc20 and Cdh1. The binding of Cdc20 requires the phosphorylation of several subunits, which takes place only in mitosis (Kramer et al, 1998; Shteinberg et al, 1999; Rudner and Murray, 2000; Kraft et al, 2003). Cdc20 is negatively regulated by phosphorylation (Yudkovsky et al, 2000), by binding of checkpoint proteins (Fang et al, 1998; Sudakin et al, 2001; Tang et al, 2001), by Emi1 (Reimann et al, 2001) and by APC/CCdh1-specific degradation (Shirayama et al, 1998; Pfleger and Kirschner, 2000). Cdh1 is regulated by transcriptional regulation and mRNA stability (Inbal et al, 1999), by phosphorylation (Zachariae et al, 1998; Lukas et al, 1999; Listovsky et al, 2000), by Emi1 (Hsu et al, 2002) and by Mad2l2/Mad2B (Chen and Fang, 2001; Pfleger et al, 2001b). We show here that Cdh1 is further negatively regulated by its own degradation by the APC/CCdh1 (Figures 5, 6 and 7). These different inhibitory mechanisms play different roles during different phases of the cell cycle. Cdh1 is negatively regulated by phosphorylation during S, G2 and early mitosis, by Mad2l2 during mitosis, by proteolysis during G1 and by Emi1 at the transit from G1 to S.
The regulation of Cdh1 levels is of great importance and its overexpression is toxic to the cell cycle. Drosophila fzr overexpression downregulates mitotic cyclins in epidermal cells, inhibits mitosis and leads to endoreplication cycles (Sigrist and Lehner, 1997). Moderate overexpression of Cdh1 in mammalian cells leads to constitutive degradation of APC/C substrates, failure to enter mitosis and over-replication of DNA (Sorensen et al, 2000). We show here that the myc-tagged version used in these experiments might be no longer degraded by the APC/C (Figure 5B). This result suggests that degradation of Cdh1 could play a role in regulating the levels of Cdh1 and switching off the APC/CCdh1.
In addition to the RXXL boxes, at least 180 N-terminal amino acids are required for Cdh1 degradation; additional deletions completely stabilized the protein (data not shown). Other APC/C substrates that we tested always required less than 100 residues. It has been shown that a 173 residue long N-terminal fragment of Cdh1 binds to the APC/C but that shorter fragments do not bind (Pfleger et al, 2001a). It is thus possible that Cdh1 must bind the APC/C in order to be degraded. This possibility is further supported by the observation that a surplus of unlabeled Cdh1 inhibits the degradation of labeled Cdh1, but not that of other APC/C substrates (Figure 7C). This competitive inhibition does not depend on an intact RXXL box of the inhibitor (data not shown). In other reported cases of competitive inhibition of APC/C by a surplus of substrate (Holloway et al, 1993; Yamano et al, 1998), inhibition depended on an intact RXXL box and inhibited the degradation of different substrates. The possible requirement for Cdh1 binding to the APC/C for it to be degraded would be similar to what has been observed for the F-box protein Skp2, which is degraded by auto-ubiquitylation (Wirbelauer et al, 2000).
An interesting aspect of Cdh1 degradation is the fact that it requires both RXXL boxes. Several APC/C substrates carry more than one functional d-box. Human securin has both a KEN and an RXXL box, but each box can drive degradation on its own (Zur and Brandeis, 2001). Drosophila securin (pim1) also has both a KEN and an RXXL box, and both are required for degradation (Leismann and Lehner, 2003). Complex degradation signals have also been reported for Drosophila cyclin A (Jacobs et al, 2001). To the best of our knowledge, however, Cdh1 is the first mammalian APC/C substrate that has two functional RXXL boxes that are both required for degradation. Unfortunately, this adds to the prevailing mystery of the role and function of RXXL boxes.
The F-box protein Skp2, which confers substrate specificity to the SCF (Skp2, Cul1, F box) E3 ubiquitin ligase complex, also mediates its own degradation (Wirbelauer et al, 2000). Cdh1 is a substrate specificity factor of the APC/C and therefore plays a role somewhat analogous to that F-box proteins. A mechanism of regulating protein levels by self-degradation might sound somewhat paradoxical, but it is in principle the most reliable and foolproof negative feedback method imaginable. If all what is required for keeping Cdh1 levels low is Cdh1 activity, then no failure of any other protein can lead to a rise in Cdh1 activity. In case any component of the APC/CCdh1 pathway fails, Cdh1 levels might rise, but as the APC/CCdh1 is inactive this will cause no problems. Indeed, the level of Cdh1 is higher when it is inactive in mitosis, but as it is inactive this can cause no harm. The auto-regulatory mechanism of Cdh1 is thus the ultimate way of keeping APC/CCdh1 activity at exactly the desired level. This level will be high enough to activate the APC/C in G1, and low enough to enable it to be switched off at the end of G1. It is further possible that this mode of regulation enables high levels of APC/CCdh1 activity during exit from mitosis and early G1 and reduced activity later in G1. This might reflect the requirement of degrading a large number of cdh1 substrates after cell division, compared to a lower APC/C activity required for G1 maintenance.
Materials and methods
Expression vectors
The sequence coding for the N-terminal 180 amino acids of human Cdh1 was amplified by PCR and cloned into pSV-CAT (Brandeis and Hunt, 1996) upstream of and in frame with the CAT gene. Cdh-YFP was made by cloning the same sequence into pEYFP-C1 (Clontech) upstream of and in frame with the EYFP gene. KS-Cdh1 was made by cloning the full-length Cdh1 gene into Bluescript-KSII downstream of the T7 promoter. All point mutations were performed by the quickchange method (Stratagene). Vectors expressing Cdh1, nondegradable cyclin B1 and human securin have previously been described (Listovsky et al, 2000; Zur and Brandeis, 2001). Cdc13 and its stable N-terminal deletion mutant cdc13Δ67 were a kind gift from Hiro Yamano (Yamano et al, 1998).
Cell culture, transfections, synchronization and CAT assays
All the experiments were performed in NIH3t3 mouse fibroblasts. Cells were maintained in DMEM supplemented with glutamine, pens/strep and 10% fetal calf serum (FCS, Beit Haemek) and transfected by the CaPO4 co-precipitation method (Ausubel et al, 1994). The various CAT fusion expression vectors were co-transfected with a G418 resistance vector, and resistant colonies were pooled. Prometaphase-arrested cells were obtained by shake-off and were processed for CAT assay or immunoblotting. Stable transfectants were either synchronized by nocodazole and shake-off or by serum deprivation in 0.5% FCS for 48 h. The former were washed and released into fresh medium without nocodazole and the latter were released with 10% FCS.
All the experiments, except those in Figure 6B, were performed with stably transfected lines to prevent fluctuations due to transfection efficiency. For the experiment shown in Figure 6B, cells were co-transfected with the various plasmids, as described under Results. They were incubated overnight with the precipitate, washed the following morning, were allowed to recover for several hours and were treated with nocodazole for about 16 h.
Nocodazole (Sigma—2 μM) and roscovitin (Biomol—28 μM) were dissolved in DMSO. CAT assays were performed by standard methods (Ausubel et al, 1994) using [14C]chloramphenicol (Amersham) and acetyl CoA (Roche). The plotted CAT activity represents the proportion of di-acetylated [14C]chloramphenicol of the total amount of [14C]chloramphenicol, as quantified by a Fuji phosphorimager.
Antibodies
Monoclonal antibodies to Cdh1 were a kind gift from Julian Gannon (ICRF, London). Hybridoma supernatant was used for Western blotting. Cdc27 monoclonal antibodies were purchased from transduction laboratories (C40920) and goat anti-actin antibodies from Santa Cruz Biotechnology (#SC-1616); both were used at a 1:1000 dilution.
Gel filtration
Cell extracts were prepared from synchronized cells in 20 mM Tris–HCl (pH 7.5), 100 mM NaCl, 20 mM β-glycerophosphate, 1 mM NaF, 0.5 mM DTT, 10% glycerol and 0.2%NP-40 (Vodermaier et al, 2003). The samples were fractionated on a 300 × 10 mm Sephacryl S200 gel filtration column with extraction buffer, on an AKTA Explorer system (Amersham Biosciences) at 1 ml/min. Fractions (1 ml) were collected after 0.3 column volumes. In all, 20 μl of each sample was resolved by SDS–PAGE and immunoblotted with Cdc27 and Cdh1 antibodies.
Injection and time lapse
NIH3t3 cells were plated on 35 mm plates and injected with an AIS2 (cell biology trading) computer-assisted microinjection setup. Cells were followed on an IX70 inverted Olympus microscope fitted with a heated stage, two filter wheels (Sutter) and a Magnafire CCD camera (Optronics). The cells were viewed using a YFP filter (Chroma) and the Magnafire was used in the monochrome mode. The entire setup was controlled using a macro-program developed in-house and running under ImagePro version 4.5 and ScopePro version 4 (Media Cybernetics).
In vitro ubiquitylation assays
The in vitro ubiquitylation assays were performed as previously described (Yudkovsky et al, 2000). The substrates of ubiquitylation were prepared by in vitro transcription and translation of KS-Cdh1 and its mutant derivatives, KS-Cdh1-DM1 and KS-Cdh1-DM2, in a coupled reticulocyte lysate system (Promega) in the presence of [35S]methionine. Each reaction contained partially purified APC/C, E1, E2-C and an energy-regenerating system.
Degradation assays in Xenopus interphase extracts
Xenopus egg interphase extracts were prepared as previously described (Murray, 1991). Fresh extracts were treated with RNAse (1 ng/μl) for 15 min at 10°C. RNAse was inactivated by adding the RNAsin RNAse inhibitor (3 units/μl, Amersham) for 10 min at 10°C. Cdh1 mRNA, transcribed from a T7 promoter with an mMassage mMachine kit (Ambion), was added to the extract (approximately 20 ng/μl) and allowed to translate for 1 h at 23°C. The extract was then aliquoted and snap frozen in liquid nitrogen. To follow Cdh1 synthesis, a 5 μl aliquot was removed immediately after adding the mRNA and mixed with 1 μl of [35S]methionine+cystein (Promix, Amersham). The labeled Cdh1 was analyzed by gel electrophoresis.
Substrates for destruction assays were synthesized in a coupled in vitro transcription–translation reaction mix (TNT, Promega) in the presence of [35S]methionine+cystein. For destruction assays, extracts were first treated with cyclohexamide (0.1 mM) for 10 min at 23°C. Subsequently, 0.5 μl of substrate was added to 10 μl of interphase extract (either with or without Cdh1) and incubated at 23°C. Aliquots of this reaction were removed at the indicated time points and resolved by PAGE.
Acknowledgments
We thank Avram Hershko and Tim Hunt for their generous help, Julian Gannon for Cdh1 antibodies, Miriam Broit for technical help and Christian Lehner and Jamie Simpson for fruitful discussions. This work was funded by the German Israeli Foundation (GIF 658/2000) and the Association for International Cancer Research (AICR). MB is the recipient of an ASCBI fellowship of the International Union Against Cancer (UICC).
References
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (1994) Current Protocols in Molecular Biology. New York: John Wiley & Sons Inc [Google Scholar]
- Brandeis M, Hunt T (1996) The proteolysis of mitotic cyclins in mammalian cells persists from the end of mitosis until the onset of S phase. EMBO J 15: 5280–5289 [PMC free article] [PubMed] [Google Scholar]
- Burton JL, Solomon MJ (2001) D box and KEN box motifs in budding yeast Hsl1p are required for APC-mediated degradation and direct binding to Cdc20p and Cdh1p. Genes Dev 15: 2381–2395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Fang G (2001) MAD2B is an inhibitor of the anaphase-promoting complex. Genes Dev 15: 1765–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang G, Yu H, Kirschner MW (1998) The checkpoint protein MAD2 and the mitotic regulator CDC20 form a ternary complex with the anaphase-promoting complex to control anaphase initiation. Genes Dev 12: 1871–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan A, Yudkovsky Y, Hershko A (2002) The cyclin-ubiquitin ligase activity of cyclosome/APC is jointly activated by protein kinases Cdk1-cyclin B and Plk. J Biol Chem 277: 15552–15557 [DOI] [PubMed] [Google Scholar]
- Hagting A, Den Elzen N, Vodermaier HC, Waizenegger IC, Peters JM, Pines J (2002) Human securin proteolysis is controlled by the spindle checkpoint and reveals when the APC/C switches from activation by Cdc20 to Cdh1. J Cell Biol 157: 1125–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JW (2002) A phosphorylation-driven ubiquitination switch for cell-cycle control. Trends Cell Biol 12: 104–107 [DOI] [PubMed] [Google Scholar]
- Hilioti Z, Chung Y, Mochizuki Y, Hardy CF, Cohen-Fix O (2001) The anaphase inhibitor Pds1 binds to the APC/C-associated protein Cdc20 in a destruction box-dependent manner. Curr Biol 11: 1347–1352 [DOI] [PubMed] [Google Scholar]
- Holloway SL, Glotzer M, King RW, Murray AW (1993) Anaphase is initiated by proteolysis rather than by the inactivation of maturation-promoting factor. Cell 73: 1393–1402 [DOI] [PubMed] [Google Scholar]
- Hsu JY, Reimann JD, Sorensen CS, Lukas J, Jackson PK (2002) E2F-dependent accumulation of hEmi1 regulates S phase entry by inhibiting APC(Cdh1). Nat Cell Biol 4: 358–366 [DOI] [PubMed] [Google Scholar]
- Inbal N, Listovsky T, Brandeis M (1999) The mammalian Fizzy and Fizzy-related genes are regulated at the transcriptional and post-transcriptional levels. FEBS Lett 463: 350–354 [DOI] [PubMed] [Google Scholar]
- Jacobs HW, Keidel E, Lehner CF (2001) A complex degradation signal in Cyclin A required for G(1) arrest, and a C-terminal region for mitosis. EMBO J 20: 2376–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King RW, Peters JM, Tugendreich S, Rolfe M, Hieter P, Kirschner MW (1995) A 20S complex containing CDC27 and CDC16 catalyzes the mitosis-specific conjugation of ubiquitin to cyclin B. Cell 81: 279–288 [DOI] [PubMed] [Google Scholar]
- Kraft C, Herzog F, Gieffers C, Mechtler K, Hagting A, Pines J, Peters JM (2003) Mitotic regulation of the human anaphase-promoting complex by phosphorylation. EMBO J 22: 6598–6609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer ER, Gieffers C, Holzl G, Hengstschlager M, Peters JM (1998) Activation of the human anaphase-promoting complex by proteins of the CDC20/Fizzy family. Curr Biol 8: 1207–1210 [DOI] [PubMed] [Google Scholar]
- Kramer ER, Scheuringer N, Podtelejnikov AV, Mann M, Peters JM (2000) Mitotic regulation of the APC activator proteins CDC20 and CDH1. Mol Biol Cell 11: 1555–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leismann O, Lehner CF (2003) Drosophila securin destruction involves a D-box and a KEN-box and promotes anaphase in parallel with Cyclin A degradation. J Cell Sci 116: 2453–2460 [DOI] [PubMed] [Google Scholar]
- Listovsky T, Zor A, Laronne A, Brandeis M (2000) Cdk1 is essential for mammalian cyclosome/APC regulation. Exp Cell Res 255: 184–191 [DOI] [PubMed] [Google Scholar]
- Lorca T, Castro A, Martinez A, Vigneron S, Morin N, Sigrist S, Lehner C, Doree M, Labbe J (1998) Fizzy is required for activation of the APC/cyclosome in Xenopus egg extracts. EMBO J 17: 3565–3575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas C, Sorensen CS, Kramer E, Santoni-Rugiu E, Lindeneg C, Peters JM, Bartek J, Lukas J (1999) Accumulation of cyclin B1 requires E2F and cyclin-A-dependent rearrangement of the anaphase-promoting complex. Nature 401: 815–818 [DOI] [PubMed] [Google Scholar]
- Murray AW (1991) Cell cycle extracts. Methods Cell Biol 36: 581–605 [PubMed] [Google Scholar]
- Peters JM (2002) The anaphase-promoting complex. Proteolysis in mitosis and beyond. Mol. Cell 9: 931–943 [DOI] [PubMed] [Google Scholar]
- Pfleger CM, Kirschner MW (2000) The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev 14: 655–665 [PMC free article] [PubMed] [Google Scholar]
- Pfleger CM, Lee E, Kirschner MW (2001a) Substrate recognition by the Cdc20 and Cdh1 components of the anaphase-promoting complex. Genes Dev 15: 2396–2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfleger CM, Salic A, Lee E, Kirschner MW (2001b) Inhibition of Cdh1-APC by the MAD2-related protein MAD2L2: a novel mechanism for regulating Cdh1. Genes Dev 15: 1759–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann JD, Freed E, Hsu JY, Kramer ER, Peters JM, Jackson PK (2001) Emi1 is a mitotic regulator that interacts with Cdc20 and inhibits the anaphase promoting complex. Cell 105: 645–655 [DOI] [PubMed] [Google Scholar]
- Rudner AD, Murray AW (2000) Phosphorylation by Cdc28 activates the Cdc20-dependent activity of the anaphase-promoting complex. J. Cell Biol 149: 1377–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab M, Lutum A, Seufert W (1997) Yeast Hct1 is a regulator of Clb2 cyclin proteolysis. Cell 90: 683–693 [DOI] [PubMed] [Google Scholar]
- Schwab M, Neutzner M, Mocker D, Seufert W (2001) Yeast Hct1 recognizes the mitotic cyclin Clb2 and other substrates of the ubiquitin ligase APC. EMBO J 20: 5165–5175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama M, Zachariae W, Ciosk R, Nasmyth K (1998) The Polo-like kinase Cdc5p and the WD-repeat protein Cdc20p/fizzy are regulators and substrates of the anaphase promoting complex in Saccharomyces cerevisiae. EMBO J 17: 1336–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shteinberg M, Protopopov Y, Ganoth D, Listovsky T, Brandeis M, Hershko A (1999) Phosphorylation of the cyclosome is required for its stimulation by Fizzy/Cdc20. Biochem Biophys Res Commun 260: 193–198 [DOI] [PubMed] [Google Scholar]
- Sigrist SJ, Jacobs H, Stratmann R, Lehner CF (1995) Exit from mitosis is regulated by Drosophila fizzy and the sequential destruction of cyclins A, B and B3. EMBO J 14: 4827–4838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigrist SJ, Lehner CF (1997) Drosophila fizzy-related down-regulates mitotic cyclins and is required for cell proliferation arrest and entry into endocycles. Cell 90: 671–681 [DOI] [PubMed] [Google Scholar]
- Sorensen CS, Lukas C, Kramer ER, Peters JM, Bartek J, Lukas J (2000) Nonperiodic activity of the human anaphase-promoting complex-Cdh1 ubiquitin ligase results in continuous DNA synthesis uncoupled from mitosis. Mol Cell Biol 20: 7613–7623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemmann O, Zou H, Gerber SA, Gygi SP, Kirschner MW (2001) Dual inhibition of sister chromatid separation at metaphase. Cell 107: 715–726 [DOI] [PubMed] [Google Scholar]
- Sudakin V, Chan GK, Yen TJ (2001) Checkpoint inhibition of the APC/C in HeLa cells is mediated by a complex of BUBR1, BUB3, CDC20, and MAD2. J Cell Biol 154: 925–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudakin V, Ganoth D, Dahan A, Heller H, Hershko J, Luca FC, Ruderman JV, Hershko A (1995) The cyclosome, a large complex containing cyclin-selective ubiquitin ligase activity, targets cyclins for destruction at the end of mitosis. Mol Biol Cell 6: 185–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z, Bharadwaj R, Li B, Yu H (2001) Mad2-independent inhibition of APCCdc20 by the mitotic checkpoint protein BubR1. Dev Cell 1: 227–237 [DOI] [PubMed] [Google Scholar]
- Visintin R, Prinz S, Amon A (1997) CDC20 and CDH1: a family of substrate-specific activators of APC-dependent proteolysis. Science 278: 460–463 [DOI] [PubMed] [Google Scholar]
- Vodermaier HC, Gieffers C, Maurer-Stroh S, Eisenhaber F, Peters JM (2003) TPR subunits of the anaphase-promoting complex mediate binding to the activator protein CDH1. Curr Biol 13: 1459–1468 [DOI] [PubMed] [Google Scholar]
- Wheatley SP, Hinchcliffe EH, Glotzer M, Hyman AA, Sluder G, Wang Y (1997) CDK1 inactivation regulates anaphase spindle dynamics and cytokinesis in vivo. J Cell Biol 138: 385–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirbelauer C, Sutterluty H, Blondel M, Gstaiger M, Peter M, Reymond F, Krek W (2000) The F-box protein Skp2 is a ubiquitylation target of a Cul1-based core ubiquitin ligase complex: evidence for a role of Cul1 in the suppression of Skp2 expression in quiescent fibroblasts. EMBO J 19: 5362–5375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka A, Hatakeyama S, Kominami K, Kitagawa M, Matsumoto M, Nakayama K (2000) Cell cycle-dependent expression of mammalian E2-C regulated by the anaphase-promoting complex/cyclosome. Mol Biol Cell 11: 2821–2831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano H, Gannon J, Mahbubani H, Hunt T (2004) Cell cycle-regulated recognition of the destruction box of cyclin B by the APC/C in Xenopus egg extracts. Mol Cell 13: 137–147 [DOI] [PubMed] [Google Scholar]
- Yamano H, Tsurumi C, Gannon J, Hunt T (1998) The role of the destruction box and its neighbouring lysine residues in cyclin B for anaphase ubiquitin-dependent proteolysis in fission yeast: defining the D-box receptor. EMBO J 17: 5670–5678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkovsky Y, Shteinberg M, Listovsky T, Brandeis M, Hershko A (2000) Phosphorylation of Cdc20/fizzy negatively regulates the mammalian cyclosome/APC in the mitotic checkpoint. Biochem Biophys Res Commun 271: 299–304 [DOI] [PubMed] [Google Scholar]
- Zachariae W, Schwab M, Nasmyth K, Seufert W (1998) Control of cyclin ubiquitination by CDK-regulated binding of Hct1 to the anaphase promoting complex. Science 282: 1721–1724 [DOI] [PubMed] [Google Scholar]
- Zur A, Brandeis M (2001) Securin degradation is mediated by fzy and by fzr and is required for complete chromatid separation but not for cytokinesis. EMBO J 20: 792–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zur A, Brandeis M (2002) Timing of APC/C substrate degradation is determined by fzy/fzr specificity of destruction boxes. EMBO J 21: 4500–4510 [DOI] [PMC free article] [PubMed] [Google Scholar]


