Significance
Although the majority of cancer-related deaths are consequences of metastatic dissemination, the molecular and cellular forces that drive tumor cell dispersion are still poorly understood. To help identify new regulators that play critical roles in these processes, we screened for human kinases that are important for continued survival of metastatic cells. One kinase identified from these screens, the G-protein–coupled receptor kinase 3 (GRK3; or β-adrenergic receptor kinase 2), was found to have a key role in promoting prostate tumor growth and metastasis in mouse models through enhancing angiogenesis. Notably, GRK3 is overexpressed in human prostate metastatic tumors. Further studies on GRK3 and its pathways promise to expand our knowledge of cancer metastasis and also yield new cancer therapeutic targets.
Keywords: functional screens, essential kinases, ADRBK2, TSP-1, PAI-2
Abstract
The biochemical mechanisms that regulate the process of cancer metastasis are still poorly understood. Because kinases, and the signaling pathways they comprise, play key roles in regulation of many cellular processes, we used an unbiased RNAi in vitro screen and a focused cDNA in vivo screen against human kinases to identify those with previously undocumented roles in metastasis. We discovered that G-protein–coupled receptor kinase 3 (GRK3; or β-adrenergic receptor kinase 2) was not only necessary for survival and proliferation of metastatic cells, but also sufficient to promote primary prostate tumor growth and metastasis upon exogenous expression in poorly metastatic cells in mouse xenograft models. Mechanistically, we found that GRK3 stimulated angiogenesis, at least in part through down-regulation of thrombospondin-1 and plasminogen activator inhibitor type 2. Furthermore, GRK3 was found to be overexpressed in human prostate cancers, especially in metastatic tumors. Taken together, these data suggest that GRK3 plays an important role in prostate cancer progression and metastasis.
Metastasis, and resultant organ failure, is responsible for more than 90% of cancer deaths (1). However, the biochemical and molecular mechanisms that regulate tumor progression to the metastatic phenotype are still poorly understood, especially the control of survival and proliferation of metastatic cells (2, 3). To form metastases, tumor cells need to complete a multistep process, including escape from primary tumor site, intravasation into circulation, and extravasation into secondary sites, where they must be able to survive and proliferate. Cells at these sites then progress from micrometastases to macrometastases through enhancement of angiogenesis and other processes (4, 5). Recent studies have suggested that cell migration and invasion can occur effectively during early phases of primary tumor development (6–8), and survival and proliferation of metastatic cells may be the rate-limiting step for what we often describe as metastasis (2, 3). As such, the ability to initiate angiogenesis at the metastatic site could serve as a distinguishing functional feature between metastatic and nonmetastatic cells.
Our previous work has shown the value of comparing functional differences between closely related cell lines through RNAi screens (9–12). In this study, we performed RNAi screens to compare essential kinase profiles between paired highly metastatic and weakly metastatic human cancer cell lines, and identified kinases that are essential preferentially for highly metastatic cells. To prioritize these kinases for further studies, an orthotopic prostate metastasis model was then used to test the corresponding cDNAs in poorly metastatic cells. From these studies, we discovered that G-protein–coupled receptor kinase 3 (GRK3) is not only essential for survival and proliferation of metastatic cells in vitro and in vivo, but also is capable of promoting primary tumor growth and metastasis in a prostate tumor model. Identifying and studying essential kinases in this manner expands our understanding of pathways controlling survival and proliferation in metastatic cells and may ultimately lead to discovery of novel drug targets for metastasis.
Results
GRK3 Promotes Orthotopic Prostate Tumor Growth and Metastasis.
We first sought to identify human kinases that were essential for highly metastatic cells compared with matched poorly metastatic cells. As such, we performed primary arrayed RNAi screens by using a lentiviral library expressing ∼2,100 shRNAs against ∼440 human kinases on four pairs of human melanoma and prostate and colon cancer cell lines. After applying appropriate criteria to the results from primary and secondary screens as detailed in the SI Materials and Methods (Figs. S1 and S2), we identified 30 essential kinases whose roles in metastasis have heretofore gone largely unstudied.
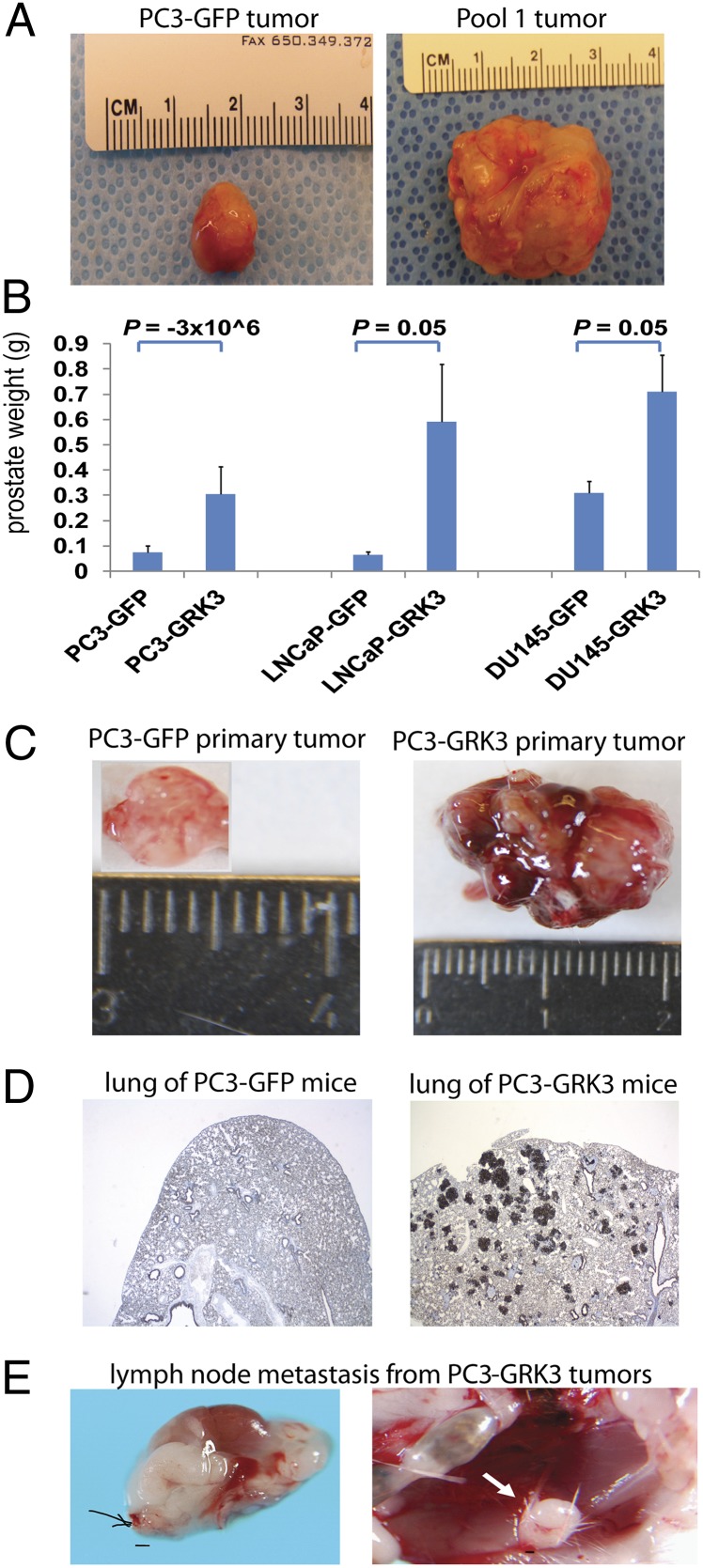
To determine whether any of the 30 kinases had a role in tumorigenesis or metastasis, we performed an in vivo cDNA screen by using a poorly metastatic variant of the American Type Culture Collection PC3 cell line, the PC3M cells (confirmed by DNA fingerprinting; referred as PC3 cells hereafter) (13). We grouped retroviruses for the cDNAs of the 30 kinase candidates randomly into two pools (15 kinases per pool), which were then used to infect PC3 cells. Naïve PC3 and PC3-GFP cells were used as the controls. Four populations of PC3 cells (naïve PC3, PC3-GFP; pool 1 and pool 2) were injected orthotopically into the prostate glands of four cohorts of SCID mice. Pool 1 consistently generated prostate tumors that were >30-fold larger than the naïve PC3 and PC3-GFP controls (Fig. 1A and Fig. S3). Therefore, we chose to focus on pool 1 and divided the 15 kinases in this pool into four subpools, one of which produced larger tumors than the other three subpools in next round. Genomic PCR on the tumors from this subpool identified GRK3 as the dominant gene (Fig. S4). We confirmed the result by testing the four kinases in this subpool individually. Only tumors formed by GRK3 overexpressing cells grew consistently larger than PC3-GFP cells (n = 6–7; P = −3 × 106). Thus, we concluded that GRK3 was the major driver in promoting primary tumor growth in PC3 cells in these experiments.
Fig. 1.
GRK3 promoted prostate tumor growth and metastasis. (A) Representative prostates from mice injected with PC3-GFP or PC3 pool 1 cells. PC3 pool 1 cells were generated from PC3 cells infected with pooled viruses of cDNAs for 15 essential kinases identified from shRNA screens. (B) GRK3 promoted orthotopic tumor growth of three prostate cancer cell lines. The y axis is prostate weight of ICR/SCID mice 8 wk after injection with GFP- or GRK3-expressing cells. P values were calculated by using Student t test. Error bars refer to SEM. (C) GRK3 stimulates prostate tumor growth. Shown are images of representative tumors formed by PC3-GFP or PC3-GRK3 cells. (D) GRK3 stimulates lung metastasis. Representative pan-cytokeratin staining of lungs of mice bearing PC3-GFP or PC3-GRK3 cells are shown (magnification of 25×). Five of seven PC3-GRK3 mice had lung metastases by 12 wk after injection. (E) GRK3 induced lymph node metastasis of PC3 cells. Shown are two examples of enlarged lymph nodes from mice injected with PC3-GRK3 cells. Three of seven mice in the GRK3 group had obviously enlarged lymph nodes. None of 20 mice injected with PC3 cells expressing GFP or three other genes tested simultaneously had obviously enlarged lymph nodes. (Scale bar: 100 µm.)
To extend our observation in PC3 cells to other prostate cancer cell lines, we overexpressed GRK3 in DU145 and LNCaP prostate cancer cells and tested its effect in vivo (n = 3–5 mice). On average, tumors ectopically expressing GRK3 were between 2.4 and 8 times larger than control tumors (Fig. 1 B and C). Notably, when we extended the PC3 in vivo experiment to 12 wk postimplantation, five of seven mice bearing PC3-GRK3 cells developed lung metastases, and three mice also had lymph node metastases. Meanwhile none of the 20 mice bearing PC3 cells expressing GFP or three other genes tested concurrently developed lung or lymph node metastases (P = 0.0003 for lung metastasis and P = 0.01 for lymph node metastasis, Fisher exact test; Fig. 1 D and E).
GRK3 Is Essential for Proliferation of Human Metastatic Cells.
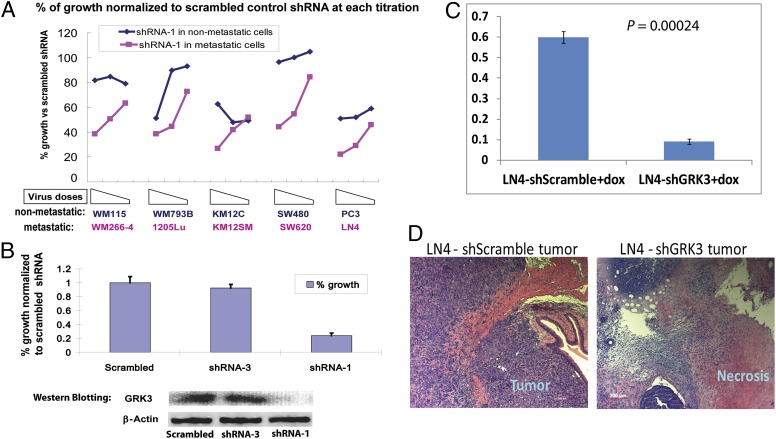
Our previous experience suggested that an shRNA lentiviral titration experiment with several doses of virus is more informative for determining the quantitative differences in sensitivities to shRNA expression between cell lines (12). Therefore, we performed GRK3 shRNA viral titration experiment on five pairs of tumor cell lines and observed that two GRK3 shRNAs (shRNA-1 in Fig. 2A and shRNA-2 in Fig. S5) targeting different regions of GRK3 mRNA clearly had preferential inhibitory effects on metastatic lines (WM266-4, SW620, and LN4 cells in particular). Importantly, although both shRNAs partially inhibited some poorly metastatic lines, these effects were mainly seen at higher viral titers. These findings served as confirmation for the initial shRNA screen and for the cDNA in vivo results described earlier.
Fig. 2.
GRK3 was essential for survival and proliferation of human metastatic cells. (A) GRK3 shRNA-1 preferentially inhibited metastatic cells in culture. Shown on the x axis is three doses of GRK3 shRNA-1 viruses on five pairs of poorly metastatic cell lines (blue) and metastatic lines (pink). Shown on the y axis is the percentage of cell survival and growth normalized to scrambled control shRNA at each titration for each cell line. The three virus titrations were with twofold decrement from left to right. In the absence of puromycin selection, the effects were caused merely by expression of shRNA. (B) GRK3 down-regulation by its shRNAs correlated with inhibition of LN4 cells in vitro. Three LN4 cell lines with Tet-On inducible shRNAs were treated with doxycycline (0.8 µg/mL) for 3 d before being lysed for Western blotting. In a parallel experiment, effects of induced shRNAs on cell survival and proliferation were assessed by Alamar blue at day 5. Shown on the y axis is the average percentage of cell survival and growth from triplicate experiments. Error bars refer to SD. (C) GRK3 is essential for metastatic LN4 cell in vivo. Two LN4 derived lines with doxycycline-inducible shRNAs were injected into prostates of two cohorts of ICR/SCID mice. Two weeks later, shRNAs were induced daily with doxycycline (dox) for an additional 4 wk, at which time the mice were killed. Shown on the y axis are final weights of prostates from LN4 cells expressing doxycycline-induced scrambled control shRNA (Left) or GRK3-shRNA-1 (Right). P values were calculated by using Student t test, and error bars refer to SEM. (D) Representative H&E staining for prostate tumors from LN4 cells expressing doxycycline-inducible shRNAs.
To further confirm that GRK3 played an essential role in vivo, we transduced the highly metastatic LN4 prostate cancer cells with a tetracycline-inducible shRNA lentiviral vector. Induction of shRNA expression by doxycycline treatment in vitro inhibited proliferation in LN4 cells carrying GRK3 shRNA-1, but not in LN4 cells carrying scrambled control shRNA or an ineffective GRK3 shRNA-3 (Fig. 2B). Importantly, inhibitory GRK3 shRNA-1 led to efficient knockdown of GRK3 protein, whereas noninhibitory GRK3 shRNA-3 and scrambled control shRNA did not (Fig. 2B). These results underscore the specificity of the GRK3 shRNA effects observed and mitigate against “off-target” effects of the shRNAs.
We then injected LN4 cells carrying scrambled control shRNA or GRK3 shRNA-1 into the prostate glands of SCID mice. Two weeks later, doxycycline was administrated via i.p. injection daily for 4 wk to induce shRNA expression. Tumor weights in mice injected with LN4 cells expressing doxycycline-induced GRK3 shRNA-1 were significantly smaller than those from mice injected with LN4 cells with doxycycline-induced scrambled shRNA (n = 7–8; P = 0.00024; Fig. 2C). Histological examination revealed that prostate tumors from LN4 cells expressing GRK3 shRNA-1 were largely necrotic (Fig. 2D). This confirmed the essential role of GRK3 for metastatic LN4 cells in vivo.
GRK3 Stimulates Angiogenesis in Vitro and in Vivo.
We next sought to determine the mechanism by which GRK3 promotes primary tumor growth and metastasis. Strikingly, we observed no obvious differences in proliferation rate, migration, or invasion between PC3-GFP or PC3-GRK3 cells (Fig. 3 A and B). To investigate potential mechanisms of GRK3, we performed microarray analysis to examine global changes in gene expression induced by GRK3 and identified 86 genes that were up-regulated or down-regulated by GRK3 by more than 1.5-fold. Interestingly, Gene Ontology analysis on the microarray data using the DAVID tool (The Database for Annotation, Visualization and Integrated Discovery) (14, 15) indicated that wound healing (13 genes; Table S1) was the only biological process enriched among these differentially expressed genes with (P < 0.05 after Bonferroni correction; false discovery rate of 0.01).
Fig. 3.
GRK3 induced angiogenesis in vitro and in vivo. (A) GFP- and GRK3-expressing PC3 cells proliferated at similar rates in vitro. These two cell lines were cultured in media with various concentrations of serum for 3 d. Shown on the y axis are growth rates (expressed in fold change) at different concentrations of serum as normalized to 0% serum (x axis). (B) GFP and GRK3-expressing PC3 cells migrated or invaded at similar rates in vitro. Migration or invasion capabilities of these two cell lines were measured by using Transwell migration or Matrigel invasion assays. Error bars refer to SEM. (C) GRK3-expressing PC3 cells were able to induce migration of human endothelial cell seeded in the upper chamber in Transwell assay. Shown on the y axis are numbers of migrated endothelial cells per high-power field. Error bars refer to SEM. (D) MVD is lower in PC3-GFP (Left) than in PC3-GRK3 primary tumor tissues (Middle). Vessels are stained in red with mouse CD34 (green arrows), and dark blue nuclei stains with mouse Ki-67 indicates proliferating endothelial cells (black arrows). Lung micrometastasis (Right) shows ingrowth of proliferating microvessels by coexpression of the endothelial marker CD34 (red) and endothelial cell proliferation by Ki-67 (blue) (magnification of 400×). (E) Representative CD31 staining in red and DAPI staining in blue for prostate tumors from LN4 cells expressing scrambled shRNA or GRK3-shRNA-1.
Because wound healing is closely linked to angiogenesis, a critical process for tumor growth and progression, we hypothesized that GRK3 promoted tumor growth and metastasis through enhancing angiogenesis (16). We first sought to determine whether GRK3 directly affected endothelial cell migration, an essential step for angiogenesis. We observed that PC3-GRK3 cells stimulated endothelial cell migration fivefold greater than control PC3-GFP cells in vitro (Fig. 3C). This suggests that GRK3 stimulates primary tumor growth and metastasis, at least in part, by stimulating angiogenesis.
We then analyzed the primary tumors generated by PC3-GRK3 and PC3-GFP cells for differences in angiogenesis. To this end, we performed microvessel density (MVD) analysis and examined endothelial cell proliferation. Consistent with the in vitro endothelial migration assays, we observed a significant difference in MVD between GRK3-expressing tumors and GFP control tumors (Fig. 3D). Specifically, PC3-GRK3 tumors had an average MVD of 71.8 per square millimeter (range, 60.2–80.8 per square millimeter; SD, 7.8) compared with 42.8 per square millimeter (range, 28.1–58.9 per square millimeter; SD, 15.4) for PC3-GFP tumors (P = 0.011, Mann–Whitney test; Fig. 3D). We then used another metric for tumor angiogenesis, vascular proliferation by costaining for CD31 and Ki-67 (17). Consistent with the MVD analysis, we observed an increase in proliferating microvessels in the primary tumor and significantly also in the metastases formed by GRK3-expressing tumors (Fig. 3D). Consistently, tumors from LN4 cells expressing GRK3 shRNA had strikingly fewer vessels than those tumors from LN4 cells expressing scramble control shRNA, as shown in Fig. 3E. These findings strongly suggest that GRK3 enhances tumor growth and metastasis by stimulating angiogenesis.
GRK3 Suppresses Expression of Antiangiogenesis Proteins Thrombospondin-1 and Plasminogen Activator Inhibitor 2.
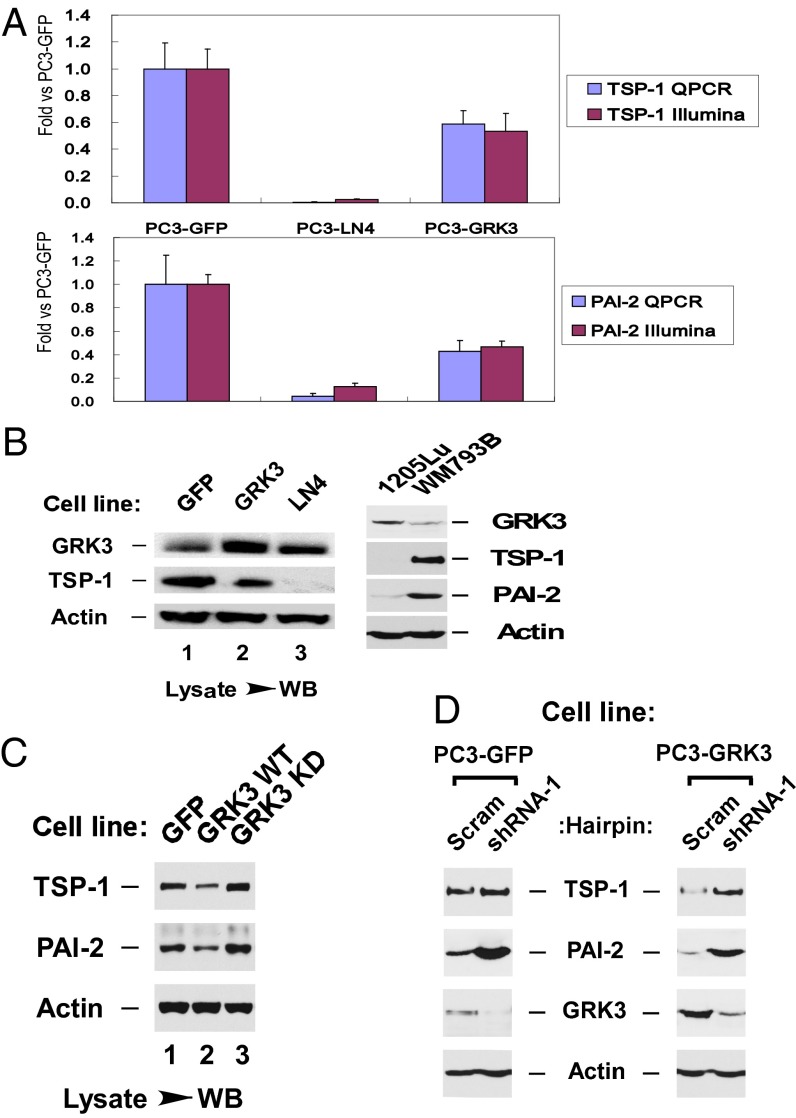
Among the 13 wound healing genes regulated by GRK3, we focused on two genes, thrombospondin-1 (TSP-1; THBS1) and plasminogen activator inhibitor 2 (PAI-2; SERPINB2). Each has been shown to profoundly inhibit angiogenesis in human tumors (18–22). Real-time (RT) QPCR and Western analysis showed that GRK3 is expressed at a higher level in metastatic cell lines than their poorly metastatic parental cell lines, and expression of TSP-1 and PAI-2 were down-regulated by GRK3 (Fig. 4 A and B). We then used a kinase dead mutant of GRK3 (K220R) (23, 24) and observed that GRK3 kinase activity was required for its repression of TSP-1 and PAI-2 (Fig. 4C). Furthermore, shRNA-mediated silencing of GRK3 in PC3-GRK3 cells relieved the repression of TSP-1 and PAI-2 by GRK3 (Fig. 4D). Strikingly, knocking down endogenous levels of GRK3 in PC3-GFP control cells also resulted in an increase in TSP-1 and PAI-2 (Fig. 4D). Furthermore, when GRK3 was knocked down in LN4 cells, PAI-2 expression was also increased, further supporting the suppression of PAI-2 by GRK3 (Fig. S6). These results indicate that GRK3 induces repression of antiangiogenic proteins TSP-1 and PAI-2, which is consistent with our observations that GRK3 stimulates angiogenesis in vitro and in vivo.
Fig. 4.
GRK3 represses TSP-1 and PAI-2 expression. (A) TSP-1 and PAI-2 mRNAs were down-regulated by GRK3. Shown on the x axis are the three cell lines tested. Shown on the y axis are fold changes of mRNA for TSP-1 (Upper) and PAI-2 (Lower) measured by RT-QPCR (blue) or Illumina microarray (red). Error bars refer to SD from replicates. (B–D) TSP-1 and PAI-2 proteins were down-regulated by GRK3, for which its kinase activity is required. (B) Up-regulation of GRK3 in metastatic lines (LN4 and 1205Lu) from parental poorly metastatic lines (PC3-GFP and WM793B), and down-regulation of TSP-1 and PAI-2 by GRK3. (C) Down-regulation of TSP-1 and PAI-2 by GRK3 WT was reversed by expression of GRK3-K220R, a kinase-dead version (KD). (D) Down-regulation of TSP-1 and PAI-2 by GRK3 WT was reversed by GRK3 shRNA silencing in PC3-GFP and PC3-GRK3 cells.
GRK3 Is Overexpressed in Human Malignant Tumors.
The preferential inhibitory effects of GRK3 shRNAs in human metastatic cells, taken together with the ability of GRK3 cDNA to promote xenograft tumor growth and metastasis, suggest that GRK3 may play a physiologically relevant role in promoting human cancer progression. To assess the role of GRK3 in human cancer, we performed immunohistochemistry (IHC) staining of GRK3 protein on a human prostate tumor tissue microarray. GRK3 was significantly stronger in carcinomas compared with benign prostatic hyperplasias (P < 0.0005, Kruskal–Wallis test). Among carcinomas, the strongest expression was observed in nonskeletal metastases (P = 0.001, Kruskal–Wallis test; Fig. 5 A–E and Table 1). The staining results using two different antibodies were equivalent, with a Spearman ρ of 0.65 (rank correlation). These results indicate that GRK3 expression correlates with visceral metastases and supports the experimental xenograft results presented earlier.
Fig. 5.
GRK3 was up-regulated in human metastatic prostate tumors and associated with elevated angiogenesis. IHC staining of GRK3 in benign and malignant prostatic tissues (A–E). Weak and focal staining in benign prostatic hyperplasia and negative luminal cells (A), comparable weak staining in localized prostate cancer (B), stronger staining in advanced castration resistant prostate cancer (C), skeletal metastasis around bone trabeculae demonstrating stronger staining (D), and soft tissue metastasis with the strongest staining observed in these tissue categories (E). This subgroup was significantly stronger than other carcinomas categories (P = 0.001). Magnification of 400×. (Scale bar: 50 µm.) (F) GMP, or vascular nest, by factor VIII staining in a prostate carcinoma (considered to represent VEGF-A–driven angiogenesis; magnification of 400×).
Table 1.
GRK3 IHC staining results on human prostate tissues
| Tissue category | No. | Mean SI* | P value† |
| Prostate hyperplasia | 41 | 4.0 | <0.0005 |
| Localized carcinoma | 104 | 5.3 | — |
| Castration-resistant carcinoma | 33 | 5.1 | — |
| Skeletal metastasis | 13 | 4.6 | — |
| Nonskeletal metastasis | 33 | 6.8 | 0.001 |
| Total | 219 | — | — |
SI, staining index (range, 0–9).
*GRK3 expression.
By Kruskal–Wallis test, all carcinoma groups significantly stronger than hyperplasia. Nonskeletal metastasis strongest among carcinomas in a second Kruskal–Wallis test (hyperplasia excluded).
Furthermore, in the human prostate cancer patient series, there was a strong trend between GRK3 expression and glomeruloid microvascular proliferation (GMP; i.e., vascular nests, a marker of VEGF-A–driven angiogenesis) (25) with a P value of 0.08 by Pearson χ2 test. Specifically, only 6% of patients with low GRK3 expression were positive for GMP, whereas 18% of patients with high GRK3 expression were positive for GMP (Fig. 5F shows a representative GMP+ tumor). These findings further confirm the in vitro and in vivo experimental results and the conclusion that GRK3 stimulates angiogenesis.
Discussion
We report here a previously undescribed role of GRK3 in prostate cancer metastasis. Our findings were the result of a combination of an unbiased shRNA library screen and subsequent validation in vitro and in vivo. Our results indicate that GRK3 is necessary for survival and proliferation of metastatic cells in culture. We confirmed that GRK3 remained essential for metastatic cells in vivo through inducible shRNA expression. Additionally, we demonstrated that GRK3 is also sufficient to promote primary prostate tumor growth and metastasis upon exogenous expression in poorly metastatic cells. Moreover, we have demonstrated that GRK3 is overexpressed in prostate cancers, especially in metastasis, suggesting that it may play physiologic roles in the progression of human prostate cancer. Of note, these findings represent a heretofore undocumented role for GRK3 in human prostate cancer progression.
GRK3 (or β-adrenergic receptor kinase 2) belongs to a subfamily of kinases called GRKs (26–28). GRK3 is best known to phosphorylate the agonist-occupied form of β-adrenergic receptors, leading to desensitization of the receptors to their agonists (29, 30). It has also been shown to regulate signaling and internalization of several other G-protein–coupled receptors (GPCR) (31, 32). Although GRK3 has not been implicated in cancer metastasis, it has been shown to be down-regulated specifically in a subtype of glioblastoma (33), suggesting a subtype- and tissue-specific role of GRK3, which may result from differences in GPCR profiles, tumorigenic pathways, or tumor microenvironment in different organs and cancer types.
We further found that GRK3 enhances angiogenesis through regulation of several genes involved in angiogenesis and microenvironment modulation, including TSP-1 and PAI-2 (or SERPINB2), both of which are down-regulated by GRK3. TSP-1 was the first identified endogenous inhibitor of angiogenesis (21). It is a large glycoprotein (150 kDa) that binds to cell surface receptors CD36 and CD47 and inhibits endothelial proliferation and migration. Reduced expression of TSP-1 is an important component of the angiogenic switch, and decrease in its expression facilitates growth of several tumor types, including bladder, breast, and ovarian cancer, fibrosarcoma, and glioblastoma (18, 19, 22). PAI-2 is one of the two major physiological inhibitors of urokinase plasminogen activator, which has been shown to promote invasion and metastasis in various cancer types (20). Strikingly, a number of studies indicated that higher PAI-2 expression in tumors is linked with prolonged survival, decreased metastasis or decreased tumor size (20). Because access to vasculature is a critical component to metastasis, kinases that increase the angiogenic potential of tumor cells would be expected to increase their metastatic potential as well. It is not clear which signaling pathways underlying GRK3 suppression of TSP-1 and PAI-2 expression, which we are actively investigating.
It is intriguing that GRK3 not only controlled survival and proliferation of metastatic cells, but also promoted primary prostate tumor growth and metastasis by enhancing angiogenesis. Such activity is not without precedent, as several oncogenes, such as Ras and Myc, have been shown to stimulate cellular proliferation as well as angiogenesis (34, 35). Similarly, silencing a key regulator in angiogenesis such as GRK3 could lead to inhibition of cellular survival and proliferation in vitro and in vivo.
Finally, our experimental findings of the role of GRK3 in tumor progression were corroborated by examining human tumors via IHC staining of prostate cancer patient samples. These analyses confirmed that regulation of GRK3 expression was physiologically relevant to human prostate cancer progression. These results may lead to improved disease prognosis using GRK3 as a novel biomarker or part of a panel of biomarkers for prostate cancer progression. Ultimately, as kinases have proven to be successful drug targets, it is our hope that a selective GRK3 inhibitor may be developed as an effective therapy for patients with metastatic cancer.
Materials and Methods
Primary shRNA screens targeting 440 human kinases on four pairs of poorly and highly metastatic human cancer cell lines were done with the optimal viral doses for each pair. For inducible shRNA expression in vivo, LN4 cells carrying inducible scrambled control shRNA or inducible GRK3 shRNA were injected into prostates of two cohorts of male ICR/SCID mice (Taconic). Two weeks later, doxycycline was administrated i.p. daily for 4 wk before the mice were killed and the tumor tissues were weighed. For orthotopic prostate cancer xenograft studies, prostate cancer cells transduced with pooled viruses for multiple kinase cDNAs or singular viruses for individual kinase cDNAs were established by blasticidin selection and then injected into the prostate gland of male ICR/SCID mice. The mice were killed 8 or 12 wk after injection to examine for tumor growth in prostates or in metastatic sites.
Microarray analysis and real time PCR for GRK3-regulating genes were done by using Illumina Ref8-v3 BeadChips and a Roche LightCycler 480 instrument. To further assess the regulation of TSP-1 and PAI-2 protein expression by GRK3 via Western blotting, cell lysates were collected and subjected to SDS/PAGE analysis using antibodies against TSP-1, GRK3, PAI-2 or β-actin.
Migration and invasion of prostate cancer and endothelial were done using Corning Transwell Chambers and BD BioCoat-Matrigel Invasion Chambers, respectively, according to manufacturer guidance. Detection of micrometastases in lungs and livers of mice orthotopically injected with PC3-GFP or PC3-GRK3 cells were done through IHC staining by using anti-human cytokeratin antibody. Staining for microvessels and vascular proliferation was performed by dual IHC using a pan-endothelial marker CD34 rat anti-mouse antibody and Ki-67 rat anti-mouse antibody for endothelial cell proliferation (termed vascular proliferation). The average MVD in tumor tissue was assessed in accordance with the Weidner approach with minor modifications (36).
To determine the protein expression patterns of GRK3 in human prostate cancer tissues, we stained formalin-fixed and paraffin-embedded human prostate tissues with GRK3. A staining index was calculated as a product of staining intensity and proportion of positive tumor cells. Associations between variables were assessed by Pearson χ2 and Kruskal–Wallis tests. The SPSS statistical package (version 19.0; SPSS) was used. GMP, or vascular nests, a marker of VEGF-A–driven angiogenesis, were stained on human prostate tissues and recorded as previously reported (25). Detailed experimental materials and procedures are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
The authors thank all the members of the laboratories of W.L., E.H., and J. LaBaer for their support and help; the members of the RNAi Consortium, including D. Root, N. Hacohen, W. Hahn, E. Lander, D. Sabatini, S. Stewart, and B. Stockwell, for providing their library; Drs. I. Fidler and M. Herlyn for providing human cancer cell lines; and G. L. Hallseth and B. Nordanger for their assistance. This work was supported by a Rising STARS (Science and Technology Acquisition and Retention) Award from the University of Texas System (to W.L.); National Institutes of Health Grants 1R01DK089975 (to S.S.), R01 CA135417 (to R.W.), and P01 CA045548 (to R.W.); an award from Instituto Dermopatico dell’Immacolata (Italy) (to S.S.); and the Elsa U. Pardee Foundation (R.W.).
Footnotes
The authors declare no conflict of interest.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE36022).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1320638111/-/DCSupplemental.
References
- 1.Weigelt B, Peterse JL, van’t Veer LJ. Breast cancer metastasis: Markers and models. Nat Rev Cancer. 2005;5(8):591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- 2.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2(8):563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 3.Steeg PS. Tumor metastasis: Mechanistic insights and clinical challenges. Nat Med. 2006;12(8):895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 4.Fidler IJ. Critical determinants of metastasis. Semin Cancer Biol. 2002;12(2):89–96. doi: 10.1006/scbi.2001.0416. [DOI] [PubMed] [Google Scholar]
- 5.Fidler IJ. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3(6):453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 6.Hüsemann Y, et al. Systemic spread is an early step in breast cancer. Cancer Cell. 2008;13(1):58–68. doi: 10.1016/j.ccr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Podsypanina K, et al. Seeding and propagation of untransformed mouse mammary cells in the lung. Science. 2008;321(5897):1841–1844. doi: 10.1126/science.1161621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinberg RA. Leaving home early: reexamination of the canonical models of tumor progression. Cancer Cell. 2008;14(4):283–284. doi: 10.1016/j.ccr.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Baldwin A, et al. Kinase requirements in human cells: II. Genetic interaction screens identify kinase requirements following HPV16 E7 expression in cancer cells. Proc Natl Acad Sci USA. 2008;105(43):16478–16483. doi: 10.1073/pnas.0806195105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bommi-Reddy A, et al. Kinase requirements in human cells: III. Altered kinase requirements in VHL-/- cancer cells detected in a pilot synthetic lethal screen. Proc Natl Acad Sci USA. 2008;105(43):16484–16489. doi: 10.1073/pnas.0806574105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grueneberg DA, et al. Kinase requirements in human cells: I. Comparing kinase requirements across various cell types. Proc Natl Acad Sci USA. 2008;105(43):16472–16477. doi: 10.1073/pnas.0808019105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grueneberg DA, et al. Kinase requirements in human cells: IV. Differential kinase requirements in cervical and renal human tumor cell lines. Proc Natl Acad Sci USA. 2008;105(43):16490–16495. doi: 10.1073/pnas.0806578105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pettaway CA, et al. Selection of highly metastatic variants of different human prostatic carcinomas using orthotopic implantation in nude mice. Clin Cancer Res. 1996;2(9):1627–1636. [PubMed] [Google Scholar]
- 14.Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 15.Huang W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37(1):1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dvorak HF, et al. Fibrin containing gels induce angiogenesis. Implications for tumor stroma generation and wound healing. Lab Invest. 1987;57(6):673–686. [PubMed] [Google Scholar]
- 17.Gravdal K, Halvorsen OJ, Haukaas SA, Akslen LA. Proliferation of immature tumor vessels is a novel marker of clinical progression in prostate cancer. Cancer Res. 2009;69(11):4708–4715. doi: 10.1158/0008-5472.CAN-08-4417. [DOI] [PubMed] [Google Scholar]
- 18.Alvarez AA, et al. Thrombospondin-1 expression in epithelial ovarian carcinoma: association with p53 status, tumor angiogenesis, and survival in platinum-treated patients. Gynecol Oncol. 2001;82(2):273–278. doi: 10.1006/gyno.2001.6287. [DOI] [PubMed] [Google Scholar]
- 19.Campbell SC, Volpert OV, Ivanovich M, Bouck NP. Molecular mediators of angiogenesis in bladder cancer. Cancer Res. 1998;58(6):1298–1304. [PubMed] [Google Scholar]
- 20.Croucher DR, Saunders DN, Lobov S, Ranson M. Revisiting the biological roles of PAI2 (SERPINB2) in cancer. Nat Rev Cancer. 2008;8(7):535–545. doi: 10.1038/nrc2400. [DOI] [PubMed] [Google Scholar]
- 21.Good DJ, et al. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci USA. 1990;87(17):6624–6628. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu SC, et al. Inhibition of angiogenesis in human glioblastomas by chromosome 10 induction of thrombospondin-1. Cancer Res. 1996;56(24):5684–5691. [PubMed] [Google Scholar]
- 23.Celver J, Sharma M, Kovoor A. RGS9-2 mediates specific inhibition of agonist-induced internalization of D2-dopamine receptors. J Neurochem. 2010;114(3):739–749. doi: 10.1111/j.1471-4159.2010.06805.x. [DOI] [PubMed] [Google Scholar]
- 24.Morris GE, Nelson CP, Standen NB, Challiss RA, Willets JM. Endothelin signalling in arterial smooth muscle is tightly regulated by G protein-coupled receptor kinase 2. Cardiovasc Res. 2010;85(3):424–433. doi: 10.1093/cvr/cvp310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Straume O, et al. Prognostic importance of glomeruloid microvascular proliferation indicates an aggressive angiogenic phenotype in human cancers. Cancer Res. 2002;62(23):6808–6811. [PubMed] [Google Scholar]
- 26.Penela P, Murga C, Ribas C, Lafarga V, Mayor F., Jr The complex G protein-coupled receptor kinase 2 (GRK2) interactome unveils new physiopathological targets. Br J Pharmacol. 2010;160(4):821–832. doi: 10.1111/j.1476-5381.2010.00727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reiter E, Lefkowitz RJ. GRKs and beta-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol Metab. 2006;17(4):159–165. doi: 10.1016/j.tem.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Ribas C, et al. The G protein-coupled receptor kinase (GRK) interactome: Role of GRKs in GPCR regulation and signaling. Biochim Biophys Acta. 2007;1768(4):913–922. doi: 10.1016/j.bbamem.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 29.Benovic JL, et al. Cloning, expression, and chromosomal localization of beta-adrenergic receptor kinase 2. A new member of the receptor kinase family. J Biol Chem. 1991;266(23):14939–14946. [PubMed] [Google Scholar]
- 30.Benovic JL, et al. cDNA cloning and chromosomal localization of the human beta-adrenergic receptor kinase. FEBS Lett. 1991;283(1):122–126. doi: 10.1016/0014-5793(91)80568-n. [DOI] [PubMed] [Google Scholar]
- 31.Vinge LE, et al. Substrate specificities of g protein-coupled receptor kinase-2 and -3 at cardiac myocyte receptors provide basis for distinct roles in regulation of myocardial function. Mol Pharmacol. 2007;72(3):582–591. doi: 10.1124/mol.107.035766. [DOI] [PubMed] [Google Scholar]
- 32.Willets JM, Challiss RA, Nahorski SR. Non-visual GRKs: Are we seeing the whole picture? Trends Pharmacol Sci. 2003;24(12):626–633. doi: 10.1016/j.tips.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Woerner BM, et al. Suppression of G-protein-coupled receptor kinase 3 expression is a feature of classical GBM that is required for maximal growth. Mol Cancer Res. 2012;10(1):156–166. doi: 10.1158/1541-7786.MCR-11-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pelengaris S, Littlewood T, Khan M, Elia G, Evan G. Reversible activation of c-Myc in skin: induction of a complex neoplastic phenotype by a single oncogenic lesion. Mol Cell. 1999;3(5):565–577. doi: 10.1016/s1097-2765(00)80350-0. [DOI] [PubMed] [Google Scholar]
- 35.Chin L, et al. Essential role for oncogenic Ras in tumour maintenance. Nature. 1999;400(6743):468–472. doi: 10.1038/22788. [DOI] [PubMed] [Google Scholar]
- 36.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med. 1991;324(1):1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.