Significance
Infection of pathogenic microbes induces the body to produce cytokines, which are mediators of inflammation. TNFα and IL-1β are two important proinflammatory cytokines that trigger a series of cellular reactions, leading to induction of downstream genes and inflammation. Understanding how the cellular reactions are triggered by the proinflammatory cytokines is important for deciphering the molecular mechanisms of inflammation. In this study, we identified a protein called TRIM38, which negatively regulates TNFα- and IL-1β–triggered cellular reactions by causing degradation of TAB2/3, two cellular components essential for TNFα- and IL-1β–triggered cellular response. This study reveals a mechanism by which cells keep the inflammatory response in check to avoid excessive harmful immune response and may provide clues on treatments of inflammation.
Abstract
TNFα and IL-1β are two proinflammatory cytokines that play critical roles in many diseases, including rheumatoid arthritis and infectious diseases. How TNFα- and IL-1β–mediated signaling is finely tuned is not fully elucidated. Here, we identify tripartite-motif protein 38 (TRIM38) as a critical negative regulator of TNFα- and IL-1β–triggered signaling. Overexpression of TRIM38 inhibited activation of NF-κB and induction of downstream cytokines following TNFα and IL-1β stimulation, whereas knockdown or knockout of TRIM38 had the opposite effects. TRIM38 constitutively interacted with critical components TGF-β–activated kinase 1 (TAK1)-binding protein 2/3 (TAB2/3) and promoted lysosome-dependent degradation of TAB2/3 independent of its E3 ubiquitin ligase activity. Consistently, deficiency of TRIM38 resulted in abolished translocation of TAB2 to the lysosome, increased level of TAB2 in cells, and enhanced activation of TAK1 after TNFα and IL-1β stimulation. We conclude that TRIM38 negatively regulates TNFα- and IL-1β–induced signaling by mediating lysosome-dependent degradation of TAB2/3, two critical components in TNFα- and IL-1β–induced signaling pathways. Our findings reveal a previously undiscovered mechanism by which cells keep the inflammatory response in check to avoid excessive harmful immune response triggered by TNFα and IL-1β.
The proinflammatory cytokines TNFα and IL-1β play central roles in many diseases, including infectious diseases, autoimmunity, and cancers (1–4). One hallmark following TNFα and IL-1β stimulation is the activation of NF-κB and mitogen-activated protein kinases (MAPKs) and the subsequent induction of cytokines and chemokines. TNFα binds to TNF receptor 1, which recruits tumor necrosis factor receptor-associated death domain protein (TRADD) through its death domain. TRADD further recruits TRAF2, TRAF5, cIAP1, cIAP2, and receptor-interacting protein 1 (RIP1) to form a large receptor complex, where RIP1 undergoes K63-linked ubiquitination. The TGF-β–associated kinase 1 (TAK1)-associating chaperones, TAB2 and TAB3 (TAB2/3), bind preferentially to the K63-linked polyubiquitin chains of RIP1, which results in autophosphorylation and activation of TAK1. TAK1 further phosphorylates the MAPK kinases such as MEKK3 and MEKK6 and inhibitor kappa B kinase β (IKKβ). The MEKKs phosphorylate the MAPKs, including JNK and p38, to activate the transcription factor AP-1, whereas IKKβ phosphorylates IκBα, which results in its proteasome-dependent degradation, leading to the release of NF-κB to the nucleus (5, 6). IL-1β binds to IL-1 receptor (IL-1R) and results in recruitment of the IL-1R accessory protein (IL-1RAcP) and the adaptor protein MyD88. MyD88 further recruits IRAK1, IRAK4, and TNF receptor-associated factor 6 (TRAF6) to the receptor complex, where TRAF6 catalyzes K63-linked autoubiquitination and/or the synthesis of free K63-linked polyubiquitin chains. These polyubiquitinchains recruit the TAK1-TAB2/3 complex, leading to TAK1 activation. TAK1 activates NF-κB and MAPKs, leading to induction of various cytokines and chemokines and subsequent inflammatory responses (5, 7). Thus, the TAK1-TAB2/3 complex critically mediates TNFα- and IL-1β–triggered signaling.
TNFα- and IL-1β–triggered signaling is timely terminated or down-regulated to avoid excessive inflammatory responses. About 10 proteins have been reported to attenuate TNFα and IL-1β signaling by targeting various signaling molecules in the pathways. MARCH8, cIAP1, cIAP2, and RBCK1 are E3 ubiquitin ligases that induce K48-linked polyubiquitination and proteasome-dependent degradation of ILRAcP, RIP1, and TAB2/3, respectively (8–10). Tripartite-motif protein 5α (TRIM 5α) interacts with the TAK1-TAB1-TAB2/3 complex and promotes degradation of TAB2 via the lysosomal pathway (11). Several deubiquitinating enzymes have also been reported to negatively regulate TNFα and/or IL-1β signaling. A20, USP2a, USP4, and USP20 deubiquitinate TRAF6 (12–15), whereas CYLD mediates deubiquitination of TRAF6 and IKKγ (16, 17). In addition, A20, CYLD, and USP4 also catalyze deubiquitination of RIP1, TRAF2, and TAK1, respectively (16, 18, 19). Recently, it has been shown that DUSP14 dephosphorylates TAK1, thereby inhibiting TNFα- and IL-1β–triggered signaling (20). Given the diverse mechanisms by which TNFα and IL-1β signaling is down-regulated, it is possible that different enzymes target distinct signaling molecules in different types of cells after TNFα or IL-1β stimulation, and it is of great interest to identify additional molecules or mechanisms responsible for the negative regulation of TNFα and IL-1β signaling.
TRIM38 belongs to the TRIM protein family that is involved in various biological processes, including cell proliferation and apoptosis, innate immunity, and inflammatory response (21). Previously, it has been shown that TRIM38 acts as a suppressor in TOLL-like receptor (TLR)-mediated IFN-β induction by promoting degradation of TRAF6 and NAP1 through the ubiquitin-proteasome system in mouse macrophages (22, 23), whereas another study demonstrates that TRIM38 induces proteasomal degradation of TRIF, thereby inhibiting TLR3-mediated IFN-β induction (24). Whether and how TRIM38 plays a role in TNFα- and IL-1β–triggered signaling remains unknown.
In this study, we identified TRIM38 as a negative regulator in TNFα- and IL-1β–triggered activation of NF-κB and MAPKs. TRIM38 promoted the degradation of TAB2/3 through a lysosomal-dependent pathway, which required its C-terminal PRY-SPRY but not the RING domain. In addition, TRIM38 deficiency resulted in increased recruitment of TAK1 to upstream adaptors RIP1 and TRAF6, which was associated with elevated activation of NF-κB and MAPKs and induction of downstream cytokines. Our findings thus identified a previously undiscovered mechanism for regulation of TNFα and IL-1β signaling and a strategy for the host to control excessive inflammatory responses induced by TNFα or IL-1β.
Results
Overexpression of TRIM38 Inhibits TNFα- and IL-1β–Triggered Signaling.
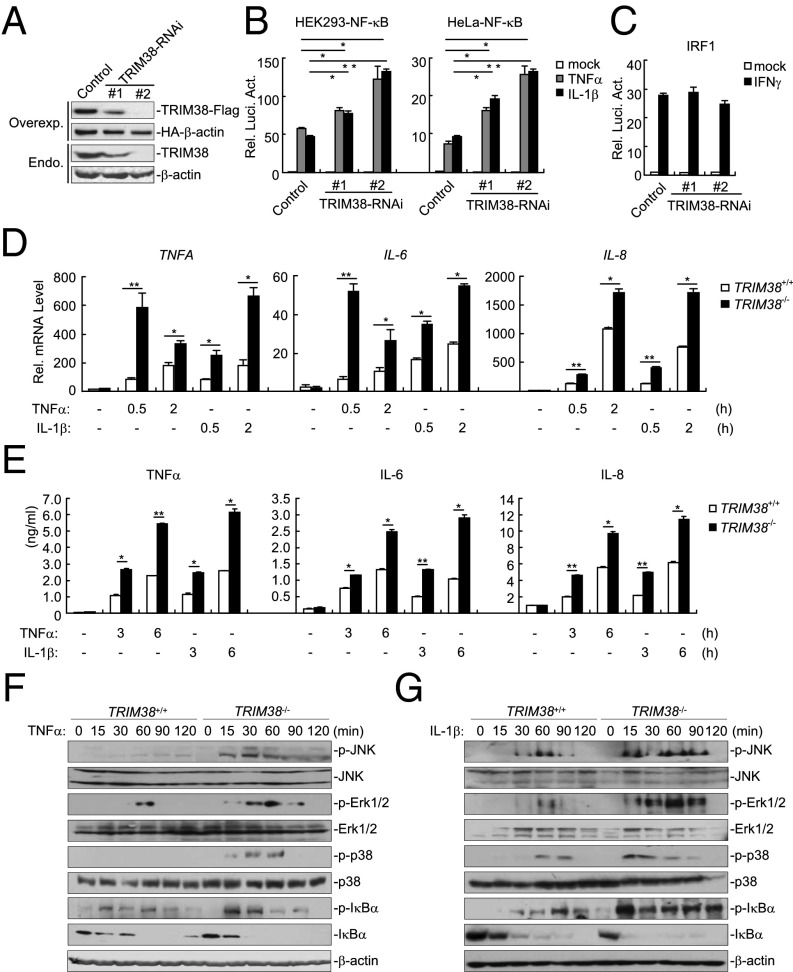
In an attempt to identify additional proteins regulating TNFα and IL-1β signaling, we screened ∼10,000 independent human cDNA expression plasmids for their abilities to regulate TNFα- and IL-1β–triggered activation of NF-κB by reporter assays. This effort led to the identification of TRIM38, a member of the TRIM family. To confirm that TRIM38 regulates TNFα- and IL-1β–triggered signaling, we constructed an HA-tagged TRIM38 expression plasmid and performed additional reporter assays. As shown in Fig. 1A, overexpression of TRIM38 inhibited TNFα- and IL-1β–triggered NF-κB activation dose-dependently in HEK293 cells. In addition, TRIM38 also inhibited TNFα- and IL-1β–triggered activation of NF-κB in HCT116 and HeLa cells (Fig. 1B). In similar reporter assays, TRIM38 did not inhibit IFNγ-triggered activation of IRF1 reporter (Fig. 1C). To confirm these results, we established stable cell lines that were transduced with either an empty vector or the HA-TRIM38 plasmid and performed quantitative PCR (qPCR) and ELISA analysis with these cell lines. As shown in Fig. 1 D and E, ectopic expression of TRIM38 inhibited TNFα- and IL-1β–induced expression of cytokines including TNFα, IL-6, and IL-8 at both the mRNA and the protein level. In contrast, IFNγ-induced expression of the IRF1 gene was comparable between the cell lines transduced with empty vector and HA-TRIM38 (Fig. 1F). These data together suggest that overexpression of TIRM38 inhibits NF-κB activation and cytokine induction after TNFα or IL-1β stimulation.
Fig. 1.
Overexpression of TRIM38 inhibits TNFα- and IL-1β–triggered signaling. (A) Effects of TRIM38 on TNFα- and IL-1β–triggered NF-κB activation in HEK293 cells. HEK293 cells (1 × 105) were transfected with the NF-κB luciferase plasmid (0.01 μg) and an HA-TRIM38 plasmid (0.2 or 0.4 μg). Twenty hours after transfection, cells were treated with TNFα (10 ng/mL) or IL-1β (10 ng/mL) or left untreated for 10 h before luciferase assays were performed. Expression of transfected TRIM38 in each unstimulated sample was examined by immunoblot analysis. (B) Effects of TRIM38 on TNFα- and IL-1β–triggered NF-κB activation in HCT116 and HeLa cells. The experiments were performed as in A. (C) Effects of TRIM38 on IFNγ-induced activation of the IRF1 promoter. The experiments were performed as in A except that the IRF1 promoter reporter plasmid was used and transfected cells were treated with IFNγ (100 ng/mL). (D) Effects of TRIM38 on TNFα- and IL-1β–induced transcription of TNFA, IL-6, and IL-8 genes. HEK293 cells were transduced with either an empty vector or an HA-TRIM38 plasmid to establish stable cell lines. Cells (4 × 105) from both stable cell lines were treated with TNFα or IL-1β for the indicated times, and then total RNA was prepared for qPCR analysis. Expression of TRIM38 in the stable cell lines was examined by immunoblot analysis (Right). (E) Effects of TRIM38 on TNFα- and IL-1β–induced cytokine of TNFα, IL-6, and IL-8. HEK293 cells were transduced with either an empty vector or an HA-TRIM38 plasmid to establish stable cell lines. Cells (4 × 105) from both stable cell lines were treated with TNFα or IL-1β for the indicated times, and then the medium was collected for ELISA analysis. (F) Effects of TRIM38 on IFNγ-induced transcription of IRF1 gene. Cells (4 × 105) were left untreated or treated with IFNγ (100 ng/mL) for the indicated times, and total RNA was extracted for qPCR analysis. Graphs show mean ± SD; n = 3. *P < 0.05; **P < 0.01.
Knockdown or Knockout of TRIM38 Potentiates TNFα- and IL-1β–Triggered Signaling.
We next examined whether endogenous TRIM38 was involved in negative regulation of TNFα or IL-1β signaling. We constructed two TRIM38-RNAi plasmids, both of which could inhibit expression of overexpressed or endogenous TRIM38 in HEK293 cells (Fig. 2A). In reporter assays, knockdown of TRIM38 potentiated TNFα- and IL-1β–triggered activation of NF-κB but not IFNγ-triggered activation of IRF1 promoter (Fig. 2 B and C). Consistent with these observations, TNFα- and IL-1β–induced expression of TNFA, IL-6, and IL-8, but not IFNγ-induced expression of IRF1, was increased by knockdown of TRIM38 (Fig. S1). In these experiments, the degrees of positive regulation of NF-κB activation and cytokine production were correlated with the knockdown efficiencies of the respective RNAi plasmids, indicating critical negative regulatory roles of TRIM38 in TNFα and IL-1β signaling.
Fig. 2.
Knockdown or knockout of TRIM38 potentiates TNFα- and IL-1β–triggered signaling. (A) Efficiencies of TRIM38-RNAi plasmids on TRIM38 levels. (Upper) HEK293 cells (4 × 105) were transfected with expression plasmids for TRIM38-Flag and HA-β-actin (0.1 μg each) and the indicated RNAi plasmids (1 μg each). Twenty-four hours after transfection, cell lysates were analyzed by immunoblot with anti-Flag or anti-HA. (Lower) HEK293 cells (1 × 107) were transfected with control or the indicated TRIM38-RNAi plasmids (10 μg each) for 36 h. Cell lysates were analyzed by immunoblot with anti-TRIM38 or anti–β-actin. (B) Effects of TRIM38-RNAi on TNFα- and IL-1β–triggered NF-κB activation in HEK293 and HeLa cells. The cells (1 × 105) were transfected with RNAi plasmids (1 μg each) along with the NF-κB reporter plasmid (0.01 μg). Thirty-six hours after transfection, cells were left untreated or treated with TNFα (10 ng/mL) or IL-1β (10 ng/mL) for 10 h before luciferase assays were performed. (C) Effects of TRIM38-RNAi on IFNγ-induced IRF1 promoter activation. Reporter assays were performed as in B except that cells were transfected with IRF1 promoter reporter plasmid and treated with IFNγ (100 ng/mL). (D) Effects of TRIM38 deficiency on TNFα and IL-1β–induced transcription of TNFA, IL-6, and IL-8 genes. The indicated cells (4 × 105) were treated with TNFα (10 ng/mL) or IL-1β (10 ng/mL) for the indicated times, and then total RNA was extracted for qPCR analysis. (E) Effects of TRIM38 deficiency on TNFα and IL-1β–induced cytokine production. The indicated cells (4 × 105) were treated with TNFα (10 ng/mL) or IL-1β (10 ng/mL) for the indicated times, and then the medium was collected for ELISA analysis. (F) TRIM38 deficiency potentiates TNFα-triggered MAPK activation. The indicated cells (1 × 107) were left untreated or treated with TNFα (10 ng/mL) for the indicated times. Cells were lyzed and immunoblot analysis was performed with the indicated antibodies. (G) TRIM38 deficiency potentiates IL-1β–triggered MAPK activation. The experiments were performed as in E, except that cells were treated with IL-1β (10 ng/mL). Graphs show mean ± SD; n = 3. *P < 0.05; **P < 0.01.
To further confirm these results, we generated TRIM38-deficient HCT116 cells by deleting a part of exon 3 of the TRIM38 gene in both alleles following a previously described gene knockout strategy (Fig. S2A). The deletion of TRIM38 was confirmed at both DNA and protein levels (Fig. S2B). In qPCR and ELISA analysis, induction of TNFα, IL-6, and IL-8 was substantially increased in TRIM38−/− compared with TRIM38+/+ cells following TNFα or IL-1β stimulation (Fig. 2 D and E). Consistently, activation of NF-κB and MAPKs was significantly enhanced in TRIM38−/− compared with TRIM38+/+ cells following TNFα or IL-1β stimulation, indicating that TRIM38 functioned upstream of MAPKs and IKK (Fig. 2 F and G). In contrast, IFNγ-induced expression of IRF1 or phosphorylation of STAT1 was comparable between TRIM38−/− and TRIM38+/+ cells (Fig. S2 C and D). These results suggest that endogenous TRIM38 is required to negatively regulate TNFα and IL-1β signaling.
TRIM38 Interacts with TAB2/3 Through Its C-Terminal PRY-SPRY Domain.
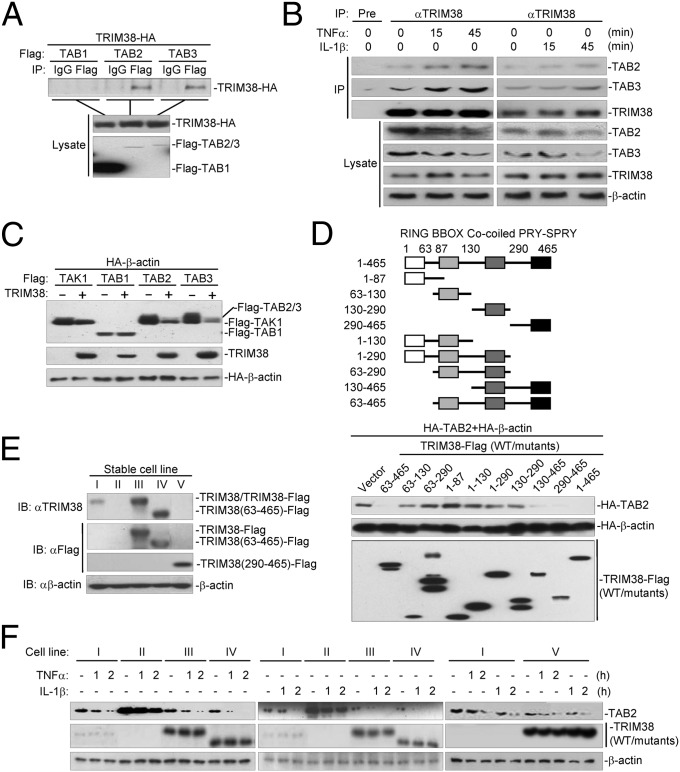
The results above indicate that TRIM38 functions upstream of MAPKs and NF-κB in both TNFα- and IL-1β–triggered signaling pathways. Considering that TNFα- and IL-1β–triggered signaling pathways converge at the TAK1-TAB1-TAB2/3 complex, which is upstream of MAPKs and IKK (4), we thus hypothesized that TRIM38 functioned at the level of the TAK1-TAB1-TAB2/3 complex. Interestingly, TAB2-, TAB3-, TRAF6-, and RIP1-, but not TAK1-, IKKβ-, or p65-mediated activation of NF-κB, was substantially enhanced in TRIM38−/− cells compared with that in TRIM38+/+ cells in reporter assays (Fig. S2E). In addition, results from transient transfection and coimmunoprecipitation experiments suggested that TRIM38 interacted with TAB2 and TAB3 but not with TAB1 (Fig. 3A), supporting the notion that TRIM38 functioned at the level of TAB2/3. Furthermore, we found that TRIM38 interacted with TAB2 and TAB3 constitutively, and the association was enhanced following TNFα or IL-1β treatment (Fig. 3B). Because TAB2 shares a highly homologous amino acid sequence and functions redundantly with TAB3, we next focused on the regulation of TAB2 by TRIM38. Domain mapping analysis indicated that the C-terminal PRY-SPRY domain of TRIM38 (amino acids 290–645) was associated with TAB2, whereas the middle fragment of TAB2 (amino acids 361–553) was responsible for its association with TRIM38 (Fig. S3 A and B). These data collectively suggest that TRIM38 interacts with TAB2 through its C-terminal PRY-SPRY domain.
Fig. 3.
TRIM38 interacts with and destabilizes TAB2 through its C-terminal PRY-SPRY domain. (A) TRIM38 interacts with TAB2 and TAB3 in mammalian overexpression system. HEK293 cells (1 × 107) were transfected with the indicated plasmids for 24 h. Coimmunoprecipitation and immunoblots were performed with the indicated antibodies. (B) Endogenous TRIM38 interacts with TAB2/3. HEK293 cells (3 × 107) were left untreated or treated with TNFα (Left) or IL-1β (Right) for the indicated times. Endogenous coimmunoprecipitation and immunoblots were performed with the indicated antibodies. (C) TRIM38 specifically destabilizes TAB2/3. HEK293 (4 × 105) cells were transfected with the indicated plasmids for 24 h, and then immunoblots were performed with the indicated antibodies. (D) Effects of TRIM38 truncation mutants on destabilization of TAB2. HEK293 (4 × 105) cells were transfected with the indicated plasmids for 24 h before immunoblots were performed with the indicated antibodies. (E) Analysis of TRIM38 expression in TRIM38−/− cells stably transduced with an empty vector (II), TRIM38-Flag (III), TRIM38(63-465)-Flag (IV), or TRIM38(290-465)-Flag (V), respectively and in TRIM38+/+ cells stably transduced with an empty vector (I). Cells (1 × 107) (I, II, III, IV, V) were harvested and lysed. Immunoblot analysis was performed with the indicated antibodies. (F) Reconstitution of TRIM38 or TRIM38 mutant (63–465) into TRIM38-deficient cells leads to down-regulation of TAB2. Cells (1 × 107) (I, II, III, IV, V) were left untreated or treated with TNFα or IL-1β for the indicated times. Immunoblot analysis was performed with the indicated antibodies.
TRIM38 Destabilizes TAB2/3 Through Its C-Terminal PRY-SPRY Domain.
When we examined TRIM38-TAB2/3 association in transient transfection and coimmunoprecipitation experiments, we routinely found that TRIM38 markedly down-regulated the expression level of TAB2/3, but not of TAK1 and TAB1 (Fig. 3 A and C). Interestingly, the C-terminal PRY-SPRY domain of TRIM38 was sufficient to down-regulate the expression level of TAB2 (Fig. 3D) as well as to inhibit TNFα- and IL-1β–triggered activation of NF-κB (Fig. S3C), indicating that TRIM38 down-regulated the expression level of TAB2 independently of its RING domain or its E3 ubiquitin ligase activity. Furthermore, TRIM38 promoted down-regulation of endogenous TAB2 at the protein level without altering the mRNA expression level of TAB2 (Fig. S3D), indicating that TRIM38 destabilized TAB2 at the protein level.
To further confirm the results, we reconstituted TRIM38−/− cells with empty vector (II), Flag-tagged TRIM38 (III), TRIM38(63–465) (IV), or TRIM38(290–465) (V) by retroviral transduction and examined TNFα- or IL-1β–triggered signaling in these cells. In these experiments, TRIM38+/+ cells reconstituted with empty vector (I) were used as a control (Fig. 3E). The results indicated that reconstitution of TRIM38, TRIM38(63–465), or TRIM38(290–465) could potently inhibit TNFα- or IL-1β–induced transcription of TNFA, IL-6, and IL-8 (Fig. S4A), but had no effect on IFNγ-induced expression of IRF1 (Fig. S4B). In addition, the protein level of TAB2 was significantly higher in TRIM38−/− than in TRIM38+/+ cells without stimulation, and TAB2 protein was more resistant to TNFα- or IL-1β–induced degradation in TRIM38−/− cells compared with TRIM38+/+ cells (Fig. 3F). Reconstitution of TRIM38(1–465, 63–465, or 290–465) into TRIM38−/− cells markedly down-regulated the protein levels of TAB2 and accelerated TNFα- or IL-1β–induced down-regulation of TAB2 (Fig. 3F). Taken together, these data suggest that TRIM38 mediates destabilization of TAB2 in rest cells as well as after TNFα or IL-1β stimulation, which depends on the PRY-SPRY domain and is independent of its RING domain.
TRIM38 Mediates Lysosome-Dependent Degradation of TAB2.
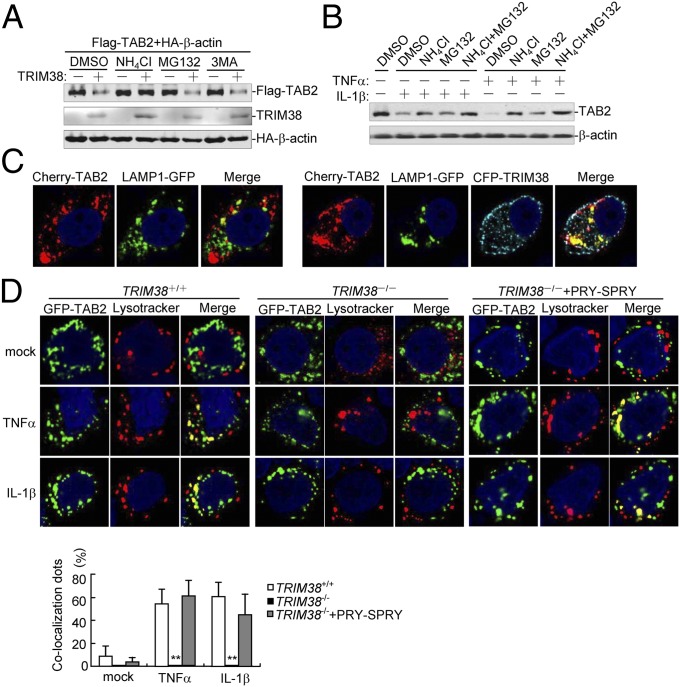
Protein degradation is one of the main strategies involved for turning off protein functions in biological processes. At least three systems exist for protein degradation, including the ubiquitin-proteasome, lysosomal, and autophagosome pathways. Because TRIM38 mediated degradation of TAB2 independently of its RING domain, we speculated that TRIM38 might induce proteolysis of TAB2 in a manner independent of the ubiquitin-proteasome system. Consistent with our hypothesis, TRIM38 did not catalyze ubiquitination of TAB2 (Fig. S5). In addition, TRIM38-mediated degradation of TAB2 was completely inhibited by the lysosomal inhibitor NH4Cl but not MG132 or 3MA, which are inhibitors for the proteasome and autophagosome pathways, respectively (Fig. 4A). These results indicate that TRIM38 mediated degradation of TAB2 through the lysosomal pathway. Actually, we found that TNFα- or IL-1β–induced down-regulation of TAB2 was partially restored by NH4Cl or MG132 alone but completely restored by a combination of NH4Cl and MG132 (Fig. 4B), indicating that TNFα- or IL-1β–induced down-regulation of TAB2 is mediated through both the proteasome- and lysosome-dependent degradation pathways. In this context, it has been reported that RBCK1 induces proteasomal degradation of TAB2 after TNFα or IL-1β treatment (10). Confocal microcopy analysis indicated that a fraction of TAB2 did localize in lysosomes in unstimulated cells, and an increased amount of TAB2 was found to localize in lysosomes when cotransfected with TRIM38 (Fig. 4C), indicating that TRIM38 promoted translocation of TAB2 to the lysosomes. In addition, TNFα or IL-1β treatment induced colocalization of TAB2 with the LysoTracker in wild-type cells, which was almost completely absent in TRIM38−/− cells (Fig. 4D). As mentioned above, the PRY-SPRY domain of TRIM38 is sufficient and necessary for inhibition of TAB2. Consistently, we found that the PRY-SPRY domain was also sufficient to rescue TAB2 recruitment to lysosomes in TRIM38−/− cells following TNFα or IL-1β stimulation. These results demonstrate that the TRIM38-mediated lysosomal degradation pathway is involved in TNFα- and IL-1β–induced proteolysis of TAB2.
Fig. 4.
TRIM38 mediates lysosomal degradation of TAB2. (A) Effects of inhibitors on TRIM38-mediated destabilization of TAB2. HEK293 cells (4 × 105) were transfected with the indicated plasmids. Fourteen hours after transfection, the cells were treated with the indicated inhibitors for 6 h before immunoblot analysis was performed. (B) Effects of NH4Cl and MG132 on down-regulation of TAB2 triggered by TNFα and IL-1β. HEK293 (1 × 107) cells were treated with NH4Cl or MG132 for 4 h and then further treated with TNFα and IL-1β for 2 h before immunoblot analysis was performed. (C) TRIM38 promotes translocation of TAB2 to the lysosome. HEK293 cells (1 × 105) were transfected with Cherry-TAB2 and GFP-LAMP1 (Left) or CFP-TRIM38 (Right). Twenty hours after transfection, cells were fixed with 4% (wt/vol) paraformaldehyde and subjected for confocal microscopy. (D) Effect of TRIM38 deficiency on TNFα- or IL-1β–induced colocalization of TAB2 with the lysosomes. TRIM38+/+, TRIM38−/−, or TRIM38−/− cells reconstituted with the PRY-SPRY domain (1 × 105) were transfected with GFP-TAB2. Twenty hours after transfection, cells were stained with Red Lysotracker (200 nM) for 2 h and treated with TNFα (20 ng/mL) or IL-1β (20 ng/mL) for 1 h and then fixed with 4% (wt/vol) paraformaldehyde and subjected to confocal microscopy. A random 10 cells in each sample were used for calculating the colocalization dots that were normalized to the total lysosome-red dots. Graphs show mean ± SD; n = 3. **P < 0.01.
TRIM38 Promotes Translocation of TAB2 to Endosomes/Lysosomes.
To explore the detail of the mechanism of how TAB2 is delivered to the lysosome for degradation by TRIM38, we analyzed the subcellular localizations of these two proteins by cell fractionation assays. In these experiments, a small fraction of TRIM38 was found in the membrane fraction in unstimulated cells, and TNFα or IL-1β stimulation promoted translocation of TRIM38 from the cytosol to the membrane fraction. Interestingly, the majority of TAB2 was found in the membrane fraction in the absence of TNFα or IL-1β and was dissociated from the membrane fraction into the cytosol upon TNFα or IL-1β stimulation (Fig. S6 A and B). In this context, it has been reported that dissociation of TAB2 from the membrane to the cytosol is required for the recruitment of TAK1 to the upstream adaptors (25). Interestingly, however, the association of TAB2 and TRIM38 was markedly increased both in the cytosol and the membrane fractions following TNFα or IL-1β stimulation (Fig. S6B). The simplest explanation for this is that TAB2 undergoes a stepwise regulation after TNFα or IL-1β stimulation. First, upon TNFα or IL-1β stimulation, TAB2 is disassociated from the plasma membrane to the cytosol to form the TAK1-TAB1-TAB2 complex, which is recruited to polyubiquitinated RIP1 or TRAF6, leading to activation of TAK1. Second, TAB2 in the TAK1-TAB1-TAB2 complex is immediately captured and translocated from the cytosol to certain membrane fractions for proteolysis by TRIM38 to prevent persistent activation of TAK1.
To determine to which membrane TRIM38 and TAB2 may attach, we performed confocal microscopy experiments. Results from confocal microscopy analysis indicated that a fraction of TRIM38 was colocalized with EEA1 (endosome marker), but not with BID (mitochondrial marker), LC3 (autophagesome marker), Sec61β (ER marker), or LAMP1 (lysosome marker) (Fig. S7A). Furthermore, a small fraction of TAB2 protein was also found to colocalize with EEA1 (Fig. S7B). Coexpression of TRIM38 markedly increased translocation of TAB2 to the EEA1-labeled subcellular compartments (Fig. S7C). In addition, TRIM38 and TAB2 were found to colocalize with EEA1 (Fig. S7C), but not with LAMP1 (Fig. 4C). These observations together suggest that TRIM38 interacts with TAB2 in both the cytosol and the endosome membrane fractions and promotes the translocation of TAB2 to the endosomes/lysosomes for proteolysis.
TRIM38 Deficiency Leads to Increased Recruitment of TAK1 to Upstream Adaptors.
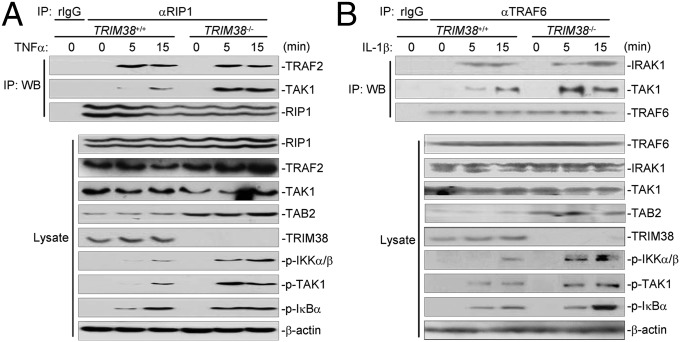
TAB2 is a chaperone protein critical for TAK1 recruitment to upstream adaptors such as RIP1 and TRAF6 following TNFα or IL-1β stimulation, respectively. So we examined whether TAK1 recruitment would be affected in TRIM38-deficient cells after TNFα or IL-1β stimulation. In coimmunoprecipitation assays, TAK1-RIP1 and TAK1-TRAF6 associations were substantially increased in TRIM38−/− cells compared with those in TRIM38+/+ cells following TNFα or IL-1β stimulation, respectively. In contrast, TNFα- or IL-1β–induced TRAF2-RIP1 or IRAK1-TRAF6 association was comparable between TRIM38+/+ and TRIM38−/− cells (Fig. 5 A and B). These data suggest that TRIM38 deficiency results in increased recruitment of TAK1 to the upstream adaptors, which is probably due to increased cellular abundance of TAB2 in TRIM38−/− cells. In support of this notion, we observed that phosphorylation of TAK1 and IKKα/β was enhanced in TRIM38−/− compared with TRIM38+/+ cells following TNFα or IL-1β stimulation (Fig. 5 A and B).
Fig. 5.
TRIM38 deficiency leads to increased recruitment of TAK1 to the upstream adaptors. (A) TRIM38 deficiency potentiates TAK1 recruitment to RIP1 upon TNFα stimulation. TRIM38+/+ and TRIM38−/− cells (3 × 107) were left untreated or treated with TNFα (20 ng/mL) for the indicated times. Coimmunoprecipitation and immnoblot analysis were performed with the indicated antibodies. (B) TRIM38 deficiency potentiates TAK1 recruitment to TRAF6 upon IL-1β stimulation. The experiment was performed as in A, except that IL-1β (20 ng/mL) and the TRAF6 antibody were used.
Discussion
It has been well documented that the TAK1-TAB2/3 complex plays crucial roles in TNFα and IL-1β signaling. In this study, we found that TRIM38 negatively regulated TNFα- and IL-1β–triggered signaling by promoting TAB2 proteolysis in a lysosome-dependent manner. Deficiency of TRIM38 resulted in increased recruitment of TAK1 to the upstream adaptors RIP1 and TRAF6 and potentiated activation of TAK1 following TNFα or IL-1β treatment. Consistently, TNFα- or IL-1β–induced activation of IKKα/β and MAPKs and the subsequent expression of cytokines were increased in TRIM38-deficient cells compared with wild-type cells following TNFα or IL-1β treatment. These results together suggest that TRIM38 is required for the restriction of TNFα- and IL-1β–triggered signaling.
It has been reported that TRIM38 negatively regulates TLR-triggered NF-κB activation and type I IFN induction by mediating degradation of TRAF6, NAP1, and TRIF via the ubiquitin-proteasome–dependent pathway (22–24). However, it is unlikely that TRIM38 inhibits TNFα or IL-1β signaling via a similar mechanism for the following reasons. First, TRIM38 did not mediate ubiquitination of TAB2 when coexpressed in HEK293 cells, indicating that TRIM38 was not an E3 for TAB2. Second, the C-terminal PRY-SPRY domain of TRIM38, which lacks the N-terminal RING domain, was sufficient to interact with TAB2 and promote TAB2 degradation, indicating that the E3 ubiquitin ligase activity was not required for TRIM38-mediated degradation of TAB2. Third, TRIM38-mediated proteolysis of TAB2 was dramatically inhibited by the lysosomal inhibitor NH4Cl but not the proteasome inhibitor MG132, supporting the notion that TRIM38 promoted lysosomal proteolysis of TAB2. Fourth, TNFα or IL-1β stimulation resulted in the translocation of TAB2 to the lysosomes, a process depending on TRIM38, indicating that TAB2 could be degraded through the lysosomal pathway. Although it has been shown that overexpression of TRIM38 alone could activate NF-κB in reporter assays (21), our studies suggest that endogenous TRIM38 suppresses TNFα- or IL-1β–triggered signaling via the lysosomal degradation pathway.
In cell fractionation assays and confocal microscopy experiments, we found that TRIM38 promoted translocation of TAB2 to the endosomes/lysosomes for degradation. How endosome-located TAB2 was degraded in lysosomes is by far less clear. The simplest hypothesis is that the TAB2-containing endosomes are fused with prelysosomes or undergo acidization to form acid lysosomes, where TRIM38 is disassociated and TAB2 remains to be degraded. It is possible that other proteins are involved in regulating this process in addition to TRIM38. In this context, it has been shown that TRIM5α promotes TAB2 proteolysis in a lysosome-dependent manner (11). Whether and how TRIM38 and TRIM5α function redundantly or cooperatively in mediating lysosomal degradation of TAB2 requires further investigations.
TNFα and IL-1β are two proinflammatory cytokines that are involved in many human diseases, including rheumatoid arthritis, infectious and inflammatory diseases, and cancers. Thus, our identification of TRIM38 as a negative regulator of TNFα and IL-1β signaling provides a therapeutic target for disease control or treatment in the future.
Materials and Methods
Reagents and Antibodies.
Recombinant human TNFα, IL-1β, and IFNγ (R&D Systems); mouse monoclonal antibodies against Flag (Sigma), HA (Covance), and β-actin (Sigma); mouse anti-TAK1, p-TAK1, p-IKKα/β; rabbit anti-JNK, p-JNK, p38, p-p38, Erk1/2, p-Erk1/2 (CST); rabbit anti-TRAF6, RIP1, IRAK1, TRAF2 (Santa Cruz Biotechnology); rabbit anti-TAB3 (Epitomics); LysoTracker (Invitrogene); and ELISA kits for human TNFα, IL-6, and IL-8 (BOSTER) were purchased from the indicated manufacturers. Mouse anti-TRIM38 and rabbit anti-TRIM38 were raised against human TRIM38. Mouse anti-IκBα antibodies were raised against human IκBα. Mouse anti-TAB2 was previously described (10).
Constructs.
Mammalian expression plasmids for Flag- or HA-tagged RIP-1, TRAF6, TAB2 and its mutants, TAB3, TRIM38 and its mutants, GFP-tagged TRIM38 and TAB2, and Cherry-tagged TRIM38 and TAB2 were constructed by standard molecular biology techniques. NF-κB luciferase reporter plasmid was kindly provided by Gary Johnson (University of Colorado Health Sciences Center, Denver). Organelle markers are kindly provided by Li Yu (Tsinghua University, Beijing, China).
RNAi Experiments.
Double-strand oligonucleotides corresponding to the target sequences were cloned into the pSuper.Retro RNAi plasmid (Oligoengine Inc.). The following sequences were targeted for human TRIM38 cDNA: #1—5′-GGATCCAGATACAGCTCAT-3′; #2—5′-GGAAAGAGAAGGTACAGAT-3.
Fluorescent Confocal Microscopy.
HEK293, HeLa, and HCT116 cells were transfected with the indicated plasmids by lipofectamine 2000 (Invitrogen). At 24 h after transfection, the cells were treated with TNFα or IL-1β for the indicated time points followed by fixation with 4% (wt/vol) paraformaldehyde for 15 min at 4 °C. The cells were then observed with an Olympus confocal microscope under a 100× oil objective.
Other Materials and Methods.
Other materials and methods used in this study are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Shu Li, Shuai Wang, and Shang-Ze Li (Wuhan University) for technical help and stimulating discussions. This study was supported by the Ministry of Science and Technology of China (2012CB910201, 2014CB542600) and the National Natural Science Foundation of China (31221061, 31130020, 3171427, and 91029302).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1318227111/-/DCSupplemental.
References
- 1.Hayden MS, Ghosh S. NF-κB in immunobiology. Cell Res. 2011;21(2):223–244. doi: 10.1038/cr.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayden MS, Ghosh S. NF-κB, the first quarter-century: Remarkable progress and outstanding questions. Genes Dev. 2012;26(3):203–234. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Razani B, Cheng G. NF-kappaB: Much learned, much to learn. Sci Signal. 2010;3(138):pe29. doi: 10.1126/scisignal.3138pe29. [DOI] [PubMed] [Google Scholar]
- 4.Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-κB signaling pathways. Nat Immunol. 2011;12(8):695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- 5.Verstrepen L, et al. TLR-4, IL-1R and TNF-R signaling to NF-kappaB: Variations on a common theme. Cell Mol Life Sci. 2008;65(19):2964–2978. doi: 10.1007/s00018-008-8064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen ZJ. Ubiquitin signalling in the NF-kappaB pathway. Nat Cell Biol. 2005;7(8):758–765. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weber A, Wasiliew P, Kracht M. Interleukin-1 (IL-1) pathway. Sci Signal. 2010;3(105):cm1. doi: 10.1126/scisignal.3105cm1. [DOI] [PubMed] [Google Scholar]
- 8.Chen R, Li M, Zhang Y, Zhou Q, Shu HB. The E3 ubiquitin ligase MARCH8 negatively regulates IL-1β-induced NF-κB activation by targeting the IL1RAP coreceptor for ubiquitination and degradation. Proc Natl Acad Sci USA. 2012;109(35):14128–14133. doi: 10.1073/pnas.1205246109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahoney DJ, et al. Both cIAP1 and cIAP2 regulate TNFalpha-mediated NF-kappaB activation. Proc Natl Acad Sci USA. 2008;105(33):11778–11783. doi: 10.1073/pnas.0711122105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian Y, et al. RBCK1 negatively regulates tumor necrosis factor- and interleukin-1-triggered NF-kappaB activation by targeting TAB2/3 for degradation. J Biol Chem. 2007;282(23):16776–16782. doi: 10.1074/jbc.M701913200. [DOI] [PubMed] [Google Scholar]
- 11.Gong J, Shen XH, Qiu H, Chen C, Yang RG. Rhesus monkey TRIM5α represses HIV-1 LTR promoter activity by negatively regulating TAK1/TAB1/TAB2/TAB3-complex-mediated NF-κB activation. Arch Virol. 2011;156(11):1997–2006. doi: 10.1007/s00705-011-1097-6. [DOI] [PubMed] [Google Scholar]
- 12.Heyninck K, Beyaert R. The cytokine-inducible zinc finger protein A20 inhibits IL-1-induced NF-kappaB activation at the level of TRAF6. FEBS Lett. 1999;442(2–3):147–150. doi: 10.1016/s0014-5793(98)01645-7. [DOI] [PubMed] [Google Scholar]
- 13.He X, et al. USP2a negatively regulates IL-1β- and virus-induced NF-κB activation by deubiquitinating TRAF6. J Mol Cell Biol. 2013;5(1):39–47. doi: 10.1093/jmcb/mjs024. [DOI] [PubMed] [Google Scholar]
- 14.Xiao N, et al. Ubiquitin-specific protease 4 (USP4) targets TRAF2 and TRAF6 for deubiquitination and inhibits TNFα-induced cancer cell migration. Biochem J. 2012;441(3):979–986. doi: 10.1042/BJ20111358. [DOI] [PubMed] [Google Scholar]
- 15.Yasunaga J, Lin FC, Lu X, Jeang KT. Ubiquitin-specific peptidase 20 targets TRAF6 and human T cell leukemia virus type 1 tax to negatively regulate NF-kappaB signaling. J Virol. 2011;85(13):6212–6219. doi: 10.1128/JVI.00079-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trompouki E, et al. CYLD is a deubiquitinating enzyme that negatively regulates NF-kappaB activation by TNFR family members. Nature. 2003;424(6950):793–796. doi: 10.1038/nature01803. [DOI] [PubMed] [Google Scholar]
- 17.Saito K, et al. The CAP-Gly domain of CYLD associates with the proline-rich sequence in NEMO/IKKgamma. Structure. 2004;12(9):1719–1728. doi: 10.1016/j.str.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Fan YH, et al. USP4 targets TAK1 to downregulate TNFα-induced NF-κB activation. Cell Death Differ. 2011;18(10):1547–1560. doi: 10.1038/cdd.2011.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133(4):693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 20.Zheng H, et al. The dual-specificity phosphatase DUSP14 negatively regulates tumor necrosis factor- and interleukin-1-induced nuclear factor-κB activation by dephosphorylating the protein kinase TAK1. J Biol Chem. 2013;288(2):819–825. doi: 10.1074/jbc.M112.412643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uchil PD, et al. TRIM protein-mediated regulation of inflammatory and innate immune signaling and its association with antiretroviral activity. J Virol. 2013;87(1):257–272. doi: 10.1128/JVI.01804-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao W, et al. Tripartite motif-containing protein 38 negatively regulates TLR3/4- and RIG-I-mediated IFN-β production and antiviral response by targeting NAP1. J Immunol. 2012;188(11):5311–5318. doi: 10.4049/jimmunol.1103506. [DOI] [PubMed] [Google Scholar]
- 23.Zhao W, Wang L, Zhang M, Yuan C, Gao C. E3 ubiquitin ligase tripartite motif 38 negatively regulates TLR-mediated immune responses by proteasomal degradation of TNF receptor-associated factor 6 in macrophages. J Immunol. 2012;188(6):2567–2574. doi: 10.4049/jimmunol.1103255. [DOI] [PubMed] [Google Scholar]
- 24.Xue Q, et al. TRIM38 negatively regulates TLR3-mediated IFN-β signaling by targeting TRIF for degradation. PLoS ONE. 2012;7(10):e46825. doi: 10.1371/journal.pone.0046825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang Z, Ninomiya-Tsuji J, Qian Y, Matsumoto K, Li X. Interleukin-1 (IL-1) receptor-associated kinase-dependent IL-1-induced signaling complexes phosphorylate TAK1 and TAB2 at the plasma membrane and activate TAK1 in the cytosol. Mol Cell Biol. 2002;22(20):7158–7167. doi: 10.1128/MCB.22.20.7158-7167.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.