Significance
Measurements of daily energy expenditure indicate that primates, including humans, expend only half of the calories expected for mammals of similar body size. As energy expenditure is central to organismal biology, these results hold important implications for life history, evolutionary biology, and foraging ecology for primates and other mammals. Specifically, we show that primates’ remarkably low metabolic rates account for their distinctively slow rates of growth, reproduction, and aging.
Keywords: metabolism, evolution, ecology
Abstract
Humans and other primates are distinct among placental mammals in having exceptionally slow rates of growth, reproduction, and aging. Primates’ slow life history schedules are generally thought to reflect an evolved strategy of allocating energy away from growth and reproduction and toward somatic investment, particularly to the development and maintenance of large brains. Here we examine an alternative explanation: that primates’ slow life histories reflect low total energy expenditure (TEE) (kilocalories per day) relative to other placental mammals. We compared doubly labeled water measurements of TEE among 17 primate species with similar measures for other placental mammals. We found that primates use remarkably little energy each day, expending on average only 50% of the energy expected for a placental mammal of similar mass. Such large differences in TEE are not easily explained by differences in physical activity, and instead appear to reflect systemic metabolic adaptation for low energy expenditures in primates. Indeed, comparisons of wild and captive primate populations indicate similar levels of energy expenditure. Broad interspecific comparisons of growth, reproduction, and maximum life span indicate that primates’ slow metabolic rates contribute to their characteristically slow life histories.
The pace at which organisms grow, reproduce, and age must ultimately reflect their physiological energy expenditure; growth of new tissue (self or offspring) and the maintenance and repair of the body all require metabolic investment. In principle, either the total energy budget, also called “total energy expenditure” (TEE) (kilocalories per day), or allocation within the energy budget could change over evolutionary time to fuel changes in life history schedules. Studies of mammalian life history have generally focused on variation in allocation (1–6), in part because of the lack of evidence correlating gross measures of energy expenditure with life history. The basal metabolic rate (BMR) (kilocalories per day), often used as an index of the total energy budget, is unrelated to rates of growth, reproduction, or aging among placental mammals when accounting for the effects of body mass and phylogenetic relatedness (7–9). The focus on allocation is also consistent with evidence, albeit mixed, for evolved tradeoffs among metabolically expensive organs (10, 11) and between metabolically expensive organs and reproductive output (12).
Variation in allocation undoubtedly affects life history schedules, but the use of BMR as a measure of the energy budget may obscure the complementary role of variation in energy throughput. For example, senescence due to the production of free radicals and other metabolic damage is a consequence of TEE, not only the portion expended on BMR (7). Further, because BMR accounts for less than half of TEE for most mammals (13), analyses of BMR do not reflect the full amount of energy potentially available for growth and reproduction. Indeed, the relationship between BMR and TEE is quite variable, with the ratio of TEE:BMR ranging from less than two to more than seven among mammals (13).
In this study, we examined TEE among primates and other placental mammals to test the hypothesis that evolved differences in the size of the energy budget contribute to the exceptionally slow life histories of primates. Primates are important points of comparison in life history analyses because they have the longest lifespans and the slowest rates of growth and reproduction of any eutherian Order (1, 2). Previous analyses have shown that haplorhine primates (apes, monkeys, and tarsiers) have BMRs similar to other placental mammals, whereas strepsirrhine primates (lemurs and lorisiform primates) have BMRs that are marginally lower (14). BMR does not explain primates’ low rates of growth or senescence (7–9), and the slow life histories of primates, particularly of humans and other apes, are instead thought to reflect an evolved reduction in energy allocation to growth and reproduction among primates (1, 2). Before this study there were insufficient data on primate TEE to test an alternative hypothesis: that slow life histories among primates reflect smaller energy budgets.
We measured TEE using the doubly labeled water (DLW) technique (15) (Methods and SI Text, section 1) in chimpanzees (Pan troglodytes), bonobos (Pan paniscus), Western lowland gorillas (Gorilla gorilla), an Allen’s swamp monkey (Allenopithecus nigroviridis), common marmosets (Callithrix jacchus), ring-tailed lemurs (Lemur catta), and diademed sifakas (Propithecus diadema), and combined these with published measurements (16–25) for 11 other primate species, including our recent studies of orangutans and humans (Table 1, Table S1); this primate dataset is taxonomically diverse and captures the full range of body size for the Order. We then compared primate TEE to similar measures in other placental mammals (n = 67 species, Table S2) and examined the relationships between TEE, life history traits, and BMR (26).
Table 1.
Primate TEE data
| Species | Population | N | Mass, kg | TEE, kcal/d | Percent expected* | Refs. |
| Microcebus murinus | Wild | 18 | 0.064 | 28 | 113 | 16 |
| Lepilemur ruficaudatus | Wild | 9 | 0.77 | 121 | 70 | 19 |
| Eulemur sp. | Wild | 11 | 1.84 | 146 | 43 | 17 |
| Lemur catta | Wild | 11 | 2.24 | 146 | 37 | 17 |
| Propithecus diadema | Wild | 6 | 4.90 | 346 | 48 | — |
| Alouatta palliata | Wild | 5 | 7.12 | 602 | 62 | 18 |
| Papio cynocephalus | Wild | 6 | 12.0 | 813 | 56 | 25 |
| Homo sapiens | Hadza foragers | 30 | 46.6 | 2,212 | 53 | 23 |
| Callithrixjacchus | Laboratory | 5 | 0.45 | 51 | 45 | — |
| Lemurcatta | Research station | 5 | 2.21 | 217 | 56 | — |
| Macaca radiata | Laboratory | 5 | 4.20 | 251 | 39 | 21 |
| Allenopithecus nigroviridis | Zoo | 1 | 7.90 | 524 | 50 | — |
| Macaca mulatta | Laboratory | 11 | 14.4 | 607 | 36 | 22 |
| Papio anubis | Research station | 8 | 16.2 | 832 | 45 | 20 |
| Panpaniscus | Sanctuary | 4 | 38.0 | 1,767 | 49 | — |
| Pantroglodytes | Sanctuary and zoo | 10 | 57.1 | 2,386 | 49 | — |
| Homo sapiens | Westerners | 195 | 72.2 | 2,482 | 42 | 23 |
| Pongo pygmaeus | Zoo | 3 | 74.8 | 1,984 | 33 | 24 |
| Gorillagorilla | Zoo | 5 | 123.7 | 3,160 | 35 | — |
Results and Discussion
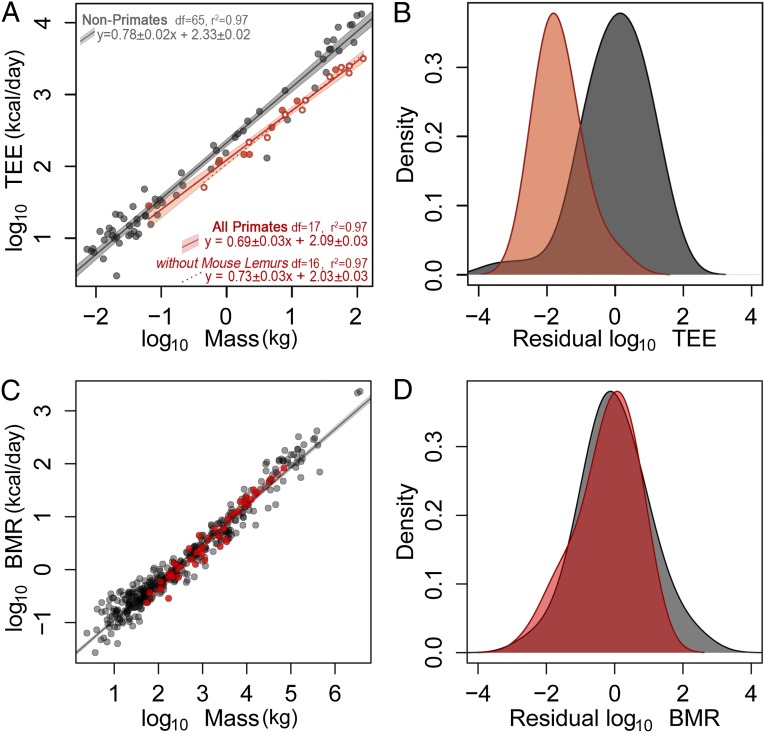
Primate TEE was only half of that expected for their body mass (mean: 50.4 ± 17.8%; Table 1), substantially less than other placental mammals [F(1,82) = 35.3, P < 0.001; Fig. 1]. Phylogenetically controlled and traditional statistical analyses indicate that primate TEE is significantly lower than other eutherian mammals (SI Text, section 2). When analysis of covariance (ANCOVA) models are run assuming parallel slopes for primate and nonprimate eutherians, the difference in intercept between primates and nonprimates is significant using both phylogenetic (P = 0.015) or non-phylogenetic models (P < 0.001; Fig. 1 and Fig. S1). By contrast, primate BMR was similar to that of other placental mammals (Fig. 1), although as in previous studies we found marginally lower BMR among strepsirrhine primates (SI Text, section 2 and Fig. S1).
Fig. 1.
Primate TEE and BMR. (A) TEE vs. body mass for primates (red, n = 17 species, 19 populations) and nonprimate eutherian mammals (gray, n = 67); shaded areas indicate 95% confidence regions for the ordinary least squares (OLS) regressions. The dotted line represents primate OLS regression excluding mouse lemurs. Open primate symbols indicate captive primate populations. (B) Density plots of standardized residuals (Z scores) from the nonprimate regression. ANCOVA and residuals show a significant shift in TEE for primates (SI Text, section 2 and Fig. S1). (C) BMR vs. body mass; symbols as in A (n = 43 primates, 407 nonprimates). (D) Standardized residuals (Z scores) from the nonprimate BMR regression.
The difference in TEE between primates and other placental mammals tends to increase with body size (Fig. 1), but the difference in TEE:Mass slopes between primates and nonprimates did not achieve statistical significance (phylogenetically controlled analysis: P = 0.182; non-phylogenetic analysis: P = 0.104; SI Text, section 2) and is driven largely by the smallest primate in our sample. When mouse lemurs (the only primate species with TEE above the placental trendline) are removed, the difference in TEE:Mass slopes between primate and nonprimate samples does not approach significance (phylogenetically controlled analysis: P = 0.581; non-phylogenetic analysis: P = 0.519; Fig. 1 and SI Text, section 2). We note that mouse lemurs are also the only primate in our sample that regularly undergoes torpor (16), but this lower metabolic state was excluded from our analyses (SI Text, section 1). Excluding torpid TEE is a conservative approach in our analyses testing for decreased primate TEE. Future analyses might examine how the use of torpor affects average TEE over longer time periods in this species.
Some of the primate populations included here are captive, but the reduction in TEE is simply too great to be explained by differences in physical activity. With TEE only 50% that of other mammals, primates in this sample would need to increase their activity to levels unseen among mammals to approach the habitual energy throughput of other species: traditional Hadza hunter–gatherers would need to run an additional 45 km each day (equivalent to a daily marathon); chimpanzees in our sample would need to travel an additional 48 km/d, more than 10 times the average daily travel distance for wild chimpanzees (SI Text, section 3, Table S3). Moreover, TEE measurements among captive and wild populations do not indicate a decrease in TEE for populations raised in captivity, at least in our primate sample. For example, captive lemurs average 20% greater TEE than their wild counterparts (Table 1; P = 0.003, t test), which is consistent with the similarity in TEE among hunter–gatherer and Western human populations reported previously (23), the similarity in TEE among US zoo-living chimpanzees and those in large seminatural African sanctuaries, and with similarities in TEE among captive and wild populations of other mammals (SI Text, section 3). Further, long-term studies of food intake among wild populations of mountain gorillas, chimpanzees, orangutans, baboons, and spider monkeys indicate daily energy expenditures similar to measures of TEE in our primate sample (Fig. S2, Table S4, and SI Text, section 3). And finally, within our primate sample there was no difference in TEE between captive and wild populations [ANCOVA with Mass: F(1,16) = 0.43, P = 0.52]. Rather than low levels of physical activity, the magnitude of difference in primate TEE suggests a systemic reduction in cellular metabolism.
Residual TEE and BMR were correlated among species (SI Text, section 2 and Fig. S3), but BMR and TEE did not show the same pattern of variation for primates and nonprimates (Fig. 1 and Figs. S1 and S2). Primates have greater BMR, relative to TEE, than other placental mammals (Fig. S3), which may reflect the metabolic cost of their larger brains. A systemic decrease in cellular metabolic rates would be expected to reduce both TEE and BMR among primates, and indeed both TEE and BMR are lower in less-encephalized strepsirrhine primates than in nonprimate placental mammals (ref. 14; Fig. 1 and Fig. S1). The increase in brain size in haplorhine primates (apes and monkeys) may have subsequently increased their BMR to the level seen in other placental mammals. Regardless, BMR, like physical activity, does not explain primates’ large reduction in TEE. Instead, some third component of TEE, separate from BMR and physical activity, may be influencing metabolic differences between primates and other mammals. One hypothesis is that variation in the circadian fluctuation in cellular metabolic rates (27, 28) leads to variation in TEE that is independent of BMR (which is measured at the nadir of metabolic activity) and physical activity. This view is consistent with the wide range of TEE:BMR ratios observed among mammals (13).
Low TEE accounts for much of primates’ slow life histories. Current life history frameworks (refs. 1–6; SI Text, section 4) model an organism’s rate of production (either growth or reproduction) as a power-law function of its size,
where M is body mass, t is time, a is relatively invariant across species, and the exponent c is generally ∼0.75. As noted above, relative to body size (M) primate life histories are slower than in other mammals (Fig. 2 A–C). However, Eq. 1 can be rewritten in terms of TEE as
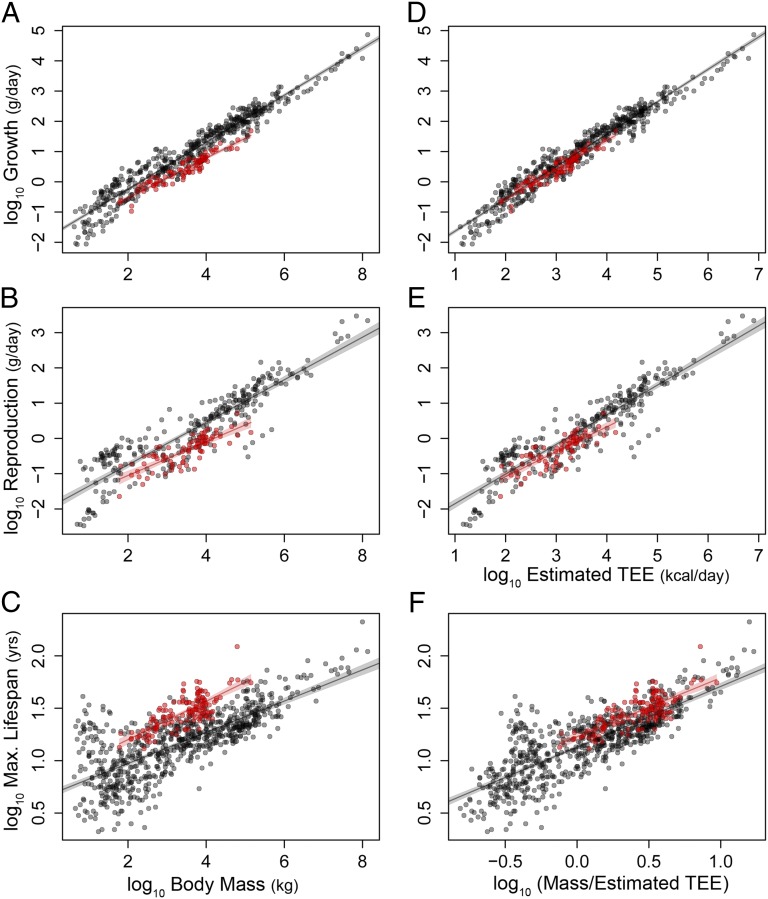
where TEE is a power-law function of body mass such that TEE = b⋅Mc, h is the percentage of TEE allocated to production, and σ is a constant relating tissue growth to energy investment, (grams per kilocalories). In this case a = σ⋅h⋅b. Reformulating Eq. 1 this way allows us to consider the effects of allocation (h) and energy throughput (TEE) separately. Following Eq. 2, growth (grams per day) and reproductive output (grams per day) are expected to increase with TEE. Both predictions are borne out; primate reproductive output and growth rate are similar to those of other eutherian mammals when plotted against estimated TEE (Fig. 2). Differences in TEE also account for long primate lifespans. If senescence is a function of accumulated metabolic damage (3, 7), then mortality rate should increase with the cellular metabolic rate. Assuming the number of cells per gram of body mass is essentially constant across species, cellular metabolic rate (kilocalories per cell per day), and thus mortality rate, should be proportional to estimated TEE/M. Conversely, maximum life span would be expected to increase with M/TEE (i.e., the inverse of mortality rate), consistent with our findings (Fig. 2).
Fig. 2.
Primate life history and TEE. Life history traits (9) for primates (red) and other eutherian mammals (gray) vs. mass (A–C), estimated TEE (D and E) or mass/estimated TEE (F); see Fig. 1 for estimation equations. Shaded areas indicate 95% confidence regions for the OLS regression.
Our results do not diminish the importance of variation in allocation (h in Eq. 2) in shaping life histories. TEE is correlated with growth, reproduction, and senescence among mammals after controlling for body mass, but only the relationship with reproduction remains significant after controlling for phylogenetic relatedness (Figs. S4 and S5, SI Text, section 5). This lack of a strong correlation between TEE and life history traits likely reflects variation in energy allocated to production and maintenance (7, 29). That is, the grade shift in primate life histories may reflect a similar shift in throughput (TEE), whereas variation at finer phylogenetic scales (e.g., between species) may be largely driven by differences in allocation (h). Results here underscore the need to integrate TEE and allocation in models of mammalian life history evolution. Such an approach has already proven useful in studies of tropical birds, whose slow life histories are reflected in lower metabolic rates and increased investment in somatic maintenance (30).
Primates’ remarkably low TEE holds broad implications for their ecology (3), and untangling proximate physiological mechanisms and ultimate evolutionary causes involved will require additional work. Initial work on orangutans suggested that reduced TEE was an evolved strategy to reduce the risk of starvation in unpredictable environments (24, 31), but it is unclear whether this explanation holds more broadly for the Order. Humans fit the primate pattern of low TEE (Table 1), consistent with our species’ slow growth and long lifespans. Results here indicate intriguing variation in TEE for humans and apes (percent expected values in Table 1), but additional measurements of ape TEE are needed to test hypotheses regarding the evolution of energy expenditure and life history with adequate statistical power. Previous work in hominoid energetics has focused on evolutionary changes in allocation (10–12), but changes in throughput may have also been critical in shaping our lineage.
Methods
TEE for healthy, adult, nonpregnant, nonlactating adults was calculated using the DLW method (15) over periods of 7–14 d. Institutional Animal Care and Use Committee (IACUC) approvals (Hunter College, Lincoln Park Zoo, Tchimpounga Sanctuary, Lola Ya Bonobo Sanctuary, Duke University, Duke Lemur Center, University of Zurich, St. Louis Zoo, McGill University) were obtained before data collection. Subjects were dosed with sufficient 2H2O and H218O to achieve recommended initial enrichments for their body mass (15). Doses for chimpanzees, bonobos, gorillas, and the Allen’s swamp monkey were administered orally (24). Doses for marmosets and diademed sifakas were injected s.c. For most subjects, urine samples (ad libitum; one before dosing and two to four postdose) were pipetted from clean, dry collection surfaces and frozen (−5 °C) until analysis using gas-isotope mass spectrometry or cavity ring-down spectroscopy; in some cases blood or saliva samples were used. The slope-intercept method was used to calculate the rates of 2H and 18O depletion, and the rate of CO2 production was calculated using equation 17.15 in ref. 15. TEE was then calculated using estimated food quotients (FQs) of 0.95 for gorillas, chimpanzees, and the Allen’s swamp monkey; this estimate is based on similarity with diets of known FQs for marmosets (0.94, this study) and orangutans (0.95, ref. 24). An FQ of 0.90 was used for diademed sifakas following values for other strepsirrhines (17). See SI Text, section 1 for additional details.
Supplementary Material
Acknowledgments
We thank Maureen Leahy, Kathy Wagner, and the Regenstein Center for African Apes keepers (Lincoln Park Zoo); Randall Junge with the sifaka project; Bernard Moumbaka (Tchimpounga Chimpanzee Rehabilitation Center); David Brewer, Bobby Schopler, and Sarah Zehr (Duke Lemur Center) for their assistance administering doses and collecting samples for analysis; Rebeca Atencia (Director, Tchimpounga Chimpanzee Rehabilitation Center) and Claudine Andre (Director, Lola ya Bonobo) for supporting this project; and Kevin Stacy for assisting with efforts at the Lincoln Park Zoo. Work at Tchimpounga and Lola Ya Bonobo was performed under the authority of the Ministry of Research and the Ministry of Environment in the Democratic Republic of Congo (research permit #MIN.RS/SG/004/2009) and the Ministry of Scientific Research and Technical Innovation in the Congo Republic (research permit 009/MRS/DGRST/DMAST), with samples imported under CITES permits 09US223466/9 and 09US207589/9. Funding was provided by the Wenner–Gren Foundation (Grant 7981), the National Science Foundation (BCS-0850815), National Geographic, Washington University, University of Arizona, University of Zurich, the Claire Garber Goodman Fund, and Hunter College. This is publication 1260 of the Duke Lemur Center.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1316940111/-/DCSupplemental.
References
- 1.Charnov EL. Life History Invariants. Oxford: Oxford Univ Press; 1993. [Google Scholar]
- 2.Charnov EL, Berrigan D. Why do primates have such long life spans and so few babies? Evol Anthropol. 1993;1(6):191–194. [Google Scholar]
- 3.Brown JH, Gillooly JF, Allen AP, Savage VM, West BG. Toward a metabolic theory of ecology. Ecology. 2004;85(7):1771–1789. [Google Scholar]
- 4.Kozłowski J, Weiner J. Interspecific allometries are byproducts of body size optimization. Am Nat. 1997;149(2):352–380. [Google Scholar]
- 5.West GB, Brown JH, Enquist BJ. A general model for ontogenetic growth. Nature. 2001;413(6856):628–631. doi: 10.1038/35098076. [DOI] [PubMed] [Google Scholar]
- 6.Stearns SC. The Evolution of Life Histories. Oxford: Oxford Univ Press; 1992. [Google Scholar]
- 7.Speakman JR. Body size, energy metabolism and lifespan. J Exp Biol. 2005;208(Pt 9):1717–1730. doi: 10.1242/jeb.01556. [DOI] [PubMed] [Google Scholar]
- 8.Lovegrove BG. Age at first reproduction and growth rate are independent of basal metabolic rate in mammals. J Comp Physiol B. 2009;179(4):391–401. doi: 10.1007/s00360-008-0322-4. [DOI] [PubMed] [Google Scholar]
- 9.de Magalhães JP, Costa J, Church GM. An analysis of the relationship between metabolism, developmental schedules, and longevity using phylogenetic independent contrasts. J Gerontol A Biol Sci Med Sci. 2007;62(2):149–160. doi: 10.1093/gerona/62.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navarrete A, van Schaik CP, Isler K. Energetics and the evolution of human brain size. Nature. 2011;480(7375):91–93. doi: 10.1038/nature10629. [DOI] [PubMed] [Google Scholar]
- 11.Aiello LC, Wheeler P. The expensive tissue hypothesis. Curr Anthropol. 1995;36(2):199–221. [Google Scholar]
- 12.Isler K, van Schaik CP. The Expensive Brain: A framework for explaining evolutionary changes in brain size. J Hum Evol. 2009;57(4):392–400. doi: 10.1016/j.jhevol.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Westerterp KR, Speakman JR. Physical activity energy expenditure has not declined since the 1980s and matches energy expenditures of wild mammals. Int J Obes (Lond) 2008;32(8):1256–1263. doi: 10.1038/ijo.2008.74. [DOI] [PubMed] [Google Scholar]
- 14.Snodgrass JJ, Leonard WR, Robertson ML. Primate bioenergetics: An evolutionary perspective. In: Ravosa MJ, Dagosto M, editors. Primate Origins: Adaptations and Evolution. New York: Springer; 2007. pp. 703–737. [Google Scholar]
- 15.Speakman JR. Doubly Labelled Water: Theory and Practice. London: Chapman & Hall; 1997. [Google Scholar]
- 16.Schmid J, Speakman JR. Daily energy expenditure of the grey mouse lemur (Microcebus murinus): A small primate that uses torpor. J Comp Physiol B. 2000;170(8):633–641. doi: 10.1007/s003600000146. [DOI] [PubMed] [Google Scholar]
- 17.Simmen B, et al. Total energy expenditure and body composition in two free-living sympatric lemurs. PLoS ONE. 2010;5(3):e9860. doi: 10.1371/journal.pone.0009860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagy KA, Milton K. Energy metabolism and food consumption by wild howler monkeys (Alouatta palliata) Ecology. 1979;60(3):475–480. [Google Scholar]
- 19.Drack S, et al. 1999. Field metabolic rate and the cost of ranging of the red-tailed sportive lemur (Lepilemur ruficaudatus). New Directions in Lemur Studies, eds Rakotosamimanana B. et al. (Plenum, New York), pp 83–91.
- 20.Rosetta L, Lee PC, Garcia C. Energetics during reproduction: A doubly labeled water study of lactating baboons. Am J Phys Anthropol. 2011;144(4):661–668. doi: 10.1002/ajpa.21475. [DOI] [PubMed] [Google Scholar]
- 21.Rising R, Signaevsky M, Rosenblum LA, Kral JG, Lifshitz F. Energy expenditure in chow-fed female non-human primates of various weights. Nutr Metab (Lond) 2008;5:32. doi: 10.1186/1743-7075-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blanc S, et al. Energy expenditure of rhesus monkeys subjected to 11 years of dietary restriction. J Clin Endocrinol Metab. 2003;88(1):16–23. doi: 10.1210/jc.2002-020405. [DOI] [PubMed] [Google Scholar]
- 23.Pontzer H, et al. Hunter-gatherer energetics and human obesity. PLoS ONE. 2012;7(7):e40503. doi: 10.1371/journal.pone.0040503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pontzer H, Raichlen DA, Shumaker RW, Ocobock C, Wich SA. Metabolic adaptation for low energy throughput in orangutans. Proc Natl Acad Sci USA. 2010;107(32):14048–14052. doi: 10.1073/pnas.1001031107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayes M, et al. Low physical activity levels of modern Homo sapiens among free-ranging mammals. Int J Obes (Lond) 2005;29(1):151–156. doi: 10.1038/sj.ijo.0802842. [DOI] [PubMed] [Google Scholar]
- 26.de Magalhães JP, Costa J. A database of vertebrate longevity records and their relation to other life-history traits. J Evol Biol. 2009;22(8):1770–1774. doi: 10.1111/j.1420-9101.2009.01783.x. [DOI] [PubMed] [Google Scholar]
- 27.Ravussin E, Burnand B, Schutz Y, Jéquier E. Twenty-four-hour energy expenditure and resting metabolic rate in obese, moderately obese, and control subjects. Am J Clin Nutr. 1982;35(3):566–573. doi: 10.1093/ajcn/35.3.566. [DOI] [PubMed] [Google Scholar]
- 28.Bass J. Circadian topology of metabolism. Nature. 2012;491(7424):348–356. doi: 10.1038/nature11704. [DOI] [PubMed] [Google Scholar]
- 29.Csiszar A, et al. Testing the oxidative stress hypothesis of aging in primate fibroblasts: Is there a correlation between species longevity and cellular ROS production? J Gerontol A Biol Sci Med Sci. 2012;67(8):841–852. doi: 10.1093/gerona/glr216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams JB, Miller RA, Harper JM, Wiersma P. Functional linkages for the pace of life, life-history, and environment in birds. Integr Comp Biol. 2010;50(5):855–868. doi: 10.1093/icb/icq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sibly RM, Brown JH. Effects of body size and lifestyle on evolution of mammal life histories. Proc Natl Acad Sci USA. 2007;104(45):17707–17712. doi: 10.1073/pnas.0707725104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.